Influenza and Pneumococcal Vaccination and the Risk of COVID-19: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Study Selection and Study Characteristics
3.2. The Association between Influenza Vaccination and SARS-CoV-2 Infection
3.3. The Association between Influenza Vaccination and COVID19 Clinical Outcomes
3.4. The Association between Pneumococcal Vaccination with SARS-CoV-2 Infection and Its Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Maier, H.J.; Bickerton, E.; Britton, P. Preface. Coronaviruses. Methods Mol. Biol. 2015, 1282. [Google Scholar]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic Characterisation and Epidemiology of 2019 Novel Coronavirus: Implications for Virus Origins and Receptor Binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- WHO. Origin of SARS-CoV-2. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/332197/WHO-2019-nCoV-FAQ-Virus_origin-2020.1-eng.pdf (accessed on 20 May 2022).
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26 (Suppl. 4), D49–D53. [Google Scholar] [CrossRef]
- WHO. Influenza (Seasonal). 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 6 May 2022).
- Jiang, C.; Yao, X.; Zhao, Y.; Wu, J.; Huang, P.; Pan, C.; Liu, S.; Pan, C. Comparative review of respiratory diseases caused by coronaviruses and influenza A viruses during epidemic season. Microbes Infect. 2020, 22, 236–244. [Google Scholar] [CrossRef] [PubMed]
- WHO. Pneumonia. Available online: https://www.who.int/news-room/fact-sheets/detail/pneumonia (accessed on 11 November 2022).
- Xing, Q.-S.; Li, G.-J.; Xing, Y.-H.; Chen, T.; Li, W.-J.; Ni, W.; Deng, K.; Gao, R.-Q.; Chen, C.-Z.; Gao, Y.; et al. Precautions are Needed for COVID-19 Patients with Coinfection of Common Respiratory Pathogens. medRxiv 2020. [Google Scholar] [CrossRef]
- Basta, N.; Moodie, E. COVID-19 Vaccine Tracker. Available online: https://covid19.trackvaccines.org/ (accessed on 2 March 2022).
- Amirlak, L.; Haddad, R.; Hardy, J.D.; Khaled, N.S.; Chung, M.H.; Amirlak, B. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum. Vaccines Immunother. 2021, 7, 1956228. [Google Scholar] [CrossRef]
- Joy, M.; Malavika, B.; Asirvatham, E.S.; Sudarsanam, T.D.; Jeyaseelan, L. Is BCG associated with reduced incidence of COVID-19? A meta-regression of global data from 160 countries. Clin. Epidemiol. Glob. Health 2021, 9, 202–203. [Google Scholar] [CrossRef]
- Dolgikh, S. Hospitalization Data Supports Correlation of Lower COVID-19 Severity vs. Universal BCG Immunization in the Early Phase of the Pandemic. medRxiv 2021. [Google Scholar] [CrossRef]
- Mosaddeghi, P.; Shahabinezhad, F.; Dorvash, M.; Goodarzi, M.; Negahdaripour, M. Harnessing the non-specific immunogenic effects of available vaccines to combat COVID-19. Hum. Vaccines Immunother. 2021, 17, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
- Kissling, E.; Hooiveld, M.; Brytting, M.; Vilcu, A.M.; de Lange, M.; Martínez-Baz, I.; Sigerson, D.; Enkirch, T.; Belhillil, S.; Meijer, A.; et al. Absence of association between 2019–2020 influenza vaccination and COVID-19: Results of the European I-MOVE-COVID-19 primary care project, March–August 2020. Influenza Other Respir. Viruses 2021, 15, 429–438. [Google Scholar] [CrossRef]
- Li, Q.; Tang, B.; Bragazzi, N.L.; Xiao, Y.; Wu, J. Modeling the impact of mass influenza vaccination and public health interventions on COVID-19 epidemics with limited detection capability. Math. Biosci. 2020, 325, 108378. [Google Scholar] [CrossRef] [PubMed]
- Marotz, C.; Belda-Ferre, P.; Ali, F.; Das, P.; Huang, S.; Cantrell, K.; Jiang, L.; Martino, C.; Diner, R.E.; Rahman, G. SARS-CoV-2 detection status associates with bacterial community composition in patients and the hospital environment. Microbiome 2021, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, A.; Anand, P.; Lenehan, P.J.; Suratekar, R.; Raghunathan, B.; Niesen, M.J.; Soundararajan, V. Omicron variant of SARS-CoV-2 harbors a unique insertion mutation of putative viral or human genomic origin. J. Autoimmun. 2021, 126, 102779. [Google Scholar]
- PubMed. 2005. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 16 April 2022).
- medRxiv. Available online: https://www.medrxiv.org/ (accessed on 26 April 2022).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.C.; Costlow, M.R.; Graff, J.S.; Dubois, R.W. Standards and guidelines for observational studies: Quality is in the eye of the beholder. J. Clin. Epidemiol. 2016, 71, 3–10. [Google Scholar] [CrossRef]
- Forero, D.A.; Lopez-Leon, S.; González-Giraldo, Y.; Bagos, P.G. Ten simple rules for carrying out and writing meta-analyses. PLoS Comput. Biol. 2019, 15, e1006922. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- StataCorp. Stata 13 Base Reference Manual; Stata Press: College Station, TX, USA, 2013. [Google Scholar]
- Martinez-Baz, I.; Trobajo-Sanmartin, C.; Arregui, I.; Navascues, A.; Adelantado, M.; Indurain, J.; Fresan, U.; Ezpeleta, C.; Castilla, J. Influenza Vaccination and Risk of SARS-CoV-2 Infection in a Cohort of Health Workers. Vaccines 2020, 8, 611. [Google Scholar] [CrossRef]
- Massoudi, N.; Mohit, B. A Case-Control Study of the 2019 Influenza Vaccine and Incidence of COVID-19 among Healthcare Workers. J. Clin. Immunol. 2021, 41, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Belingheri, M.; Paladino, M.E.; Latocca, R.; De Vito, G.; Riva, M.A. Association between seasonal flu vaccination and COVID-19 among healthcare workers. Occup. Med. 2020, 70, 665–671. [Google Scholar] [CrossRef]
- Rivas, M.N.; Ebinger, J.E.; Wu, M.; Sun, N.; Braun, J.; Sobhani, K.; Van Eyk, J.E.; Cheng, S.; Arditi, M. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J. Clin. Investig. 2021, 131, JCI145157. [Google Scholar] [CrossRef] [PubMed]
- Erismis, B.; Karabela, S.N.; Eksi, F.; Karandere, F.; Dogan, B.; Okay, F.; Filiz, M.; Kocoglu, H.; Issever, H.; Hursitoglu, M.; et al. Annual influenza vaccination effect on the susceptibility to COVID-19 infection. Cent. Eur. J. Public Health 2021, 29, 14–17. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz Conty, M.L.; Encinas Pardilla, M.B.; Garcia Sanchez, M.; Gonzalez Rodriguez, L.; Muner-Hernando, M.L.; Royuela Vicente, A.; Pintado Recarte, P.; Martinez Varea, A.; Martinez Diago, C.; Cruz Melguizo, S.; et al. Impact of Recommended Maternal Vaccination Programs on the Clinical Presentation of SARS-CoV-2 Infection: A Prospective Observational Study. Vaccines 2021, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Azzi, Y.; Parides, M.; Alani, O.; Loarte-Campos, P.; Bartash, R.; Forest, S.; Colovai, A.; Ajaimy, M.; Liriano-Ward, L.; Pynadath, C.; et al. COVID-19 infection in kidney transplant recipients at the epicenter of pandemics. Kidney Int. 2020, 98, 1559–1567. [Google Scholar] [CrossRef]
- Caratozzolo, S.; Zucchelli, A.; Turla, M.; Cotelli, M.S.; Fascendini, S.; Zanni, M.; Bianchetti, A.; Psy, M.P.; Rozzini, R.; Boffelli, S.; et al. The impact of COVID-19 on health status of home-dwelling elderly patients with dementia in East Lombardy, Italy: Results from COVIDEM network. Aging Clin. Exp. Res. 2020, 32, 2133–2140. [Google Scholar] [CrossRef]
- Huang, K.; Lin, S.W.; Sheng, W.H.; Wang, C.C. Influenza vaccination and the risk of COVID-19 infection and severe illness in older adults in the United States. Sci. Rep. 2021, 11, 11025. [Google Scholar] [CrossRef]
- Noale, M.; Trevisan, C.; Maggi, S.; Antonelli Incalzi, R.; Pedone, C.; Di Bari, M.; Adorni, F.; Jesuthasan, N.; Sojic, A.; Galli, M.; et al. The Association between Influenza and Pneumococcal Vaccinations and SARS-Cov-2 Infection: Data from the EPICOVID19 Web-Based Survey. Vaccines 2020, 8, 471. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, R.; Villani, L.; La Milia, D.I.; Ieraci, R.; Chini, F.; Volpe, E.; Barca, A.; Fusco, D.; Laurenti, P.; Ricciardi, W.; et al. Influenza and pneumococcal vaccinations are not associated to COVID-19 outcomes among patients admitted to a university hospital. Vaccine 2021, 39, 3493–3497. [Google Scholar] [CrossRef] [PubMed]
- Ragni, P.; Marino, M.; Formisano, D.; Bisaccia, E.; Scaltriti, S.; Bedeschi, E.; Grilli, R. Association between Exposure to Influenza Vaccination and COVID-19 Diagnosis and Outcomes. Vaccines 2020, 8, 675. [Google Scholar] [CrossRef] [PubMed]
- Vila-Córcoles, A.; Ochoa-Gondar, O.; Satué-Gracia, E.M.; Torrente-Fraga, C.; Gomez-Bertomeu, F.; Vila-Rovira, A.; Hospital-Guardiola, I.; De Diego-Cabanes, C.; Bejarano-Romero, F.; Basora-Gallisà, J. Influence of prior comorbidities and chronic medications use on the risk of COVID-19 in adults: A population-based cohort study in Tarragona, Spain. BMJ Open 2020, 10, e041577. [Google Scholar] [CrossRef]
- Green, I.; Ashkenazi, S.; Merzon, E.; Vinker, S.; Golan-Cohen, A. The association of previous influenza vaccination and coronavirus disease-2019. Hum. Vaccines Immunother. 2021, 17, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Conlon, A.; Ashur, C.; Washer, L.; Eagle, K.A.; Bowman, M.A.H. Impact of the influenza vaccine on COVID-19 infection rates and severity. Am. J. Infect. Control. 2021, 49, 694–700. [Google Scholar] [CrossRef]
- Bozek, A.; Kozłowska, R.; Galuszka, B.; Grzanka, A. Impact of influenza vaccination on the risk of SARS-CoV-2 infection in a middle-aged group of people. Hum. Vaccin. Immunother. 2021, 17, 3126–3130. [Google Scholar] [CrossRef]
- Kowalska, M.; Niewiadomska, E.; Barański, K.; Kaleta-Pilarska, A.; Brożek, G.; Zejda, J.E. Association between Influenza Vaccination and Positive SARS-CoV-2 IgG and IgM Tests in the General Population of Katowice Region, Poland. Vaccines 2021, 9, 415. [Google Scholar] [CrossRef]
- Fernández-Prada, M.; García-González, P.; García-Morán, A.; Ruiz-Álvarez, I.; Ramas-Diez, C.; Calvo-Rodríguez, C. Personal and vaccination history as factors associated with SARS-CoV-2 infection. Med. Clin. 2021, 157, 226–233. [Google Scholar] [CrossRef]
- King, J.P.; McLean, H.Q.; Belongia, E.A. Risk of symptomatic severe acute respiratory syndrome coronavirus 2 infection not associated with influenza vaccination in the 2019–2020 season. Influ. Other Respir. Viruses 2021, 15, 697–700. [Google Scholar] [CrossRef]
- Pawlowski, C.; Puranik, A.; Bandi, H.; Venkatakrishnan, A.J.; Agarwal, V.; Kennedy, R.; O’Horo, J.C.; Gores, G.J.; Williams, A.W.; Halamka, J.; et al. Exploratory analysis of immunization records highlights decreased SARS-CoV-2 rates in individuals with recent non-COVID-19 vaccinations. Sci. Rep. 2021, 11, 4741. [Google Scholar] [CrossRef] [PubMed]
- Zein, G.J.; Whelan, G.; Erzurum, S.C. Safety of influenza vaccine during COVID-19. J. Clin. Transl. Sci. 2021, 5. [Google Scholar] [CrossRef]
- Debisarun, P.A.; Struycken, P.; Domínguez-Andrés, J.; Moorlag, S.J.C.F.M.; Taks, E.; Gössling, K.L.; Ostermann, P.N.; Müller, L.; Schaal, H.; ten Oever, J.; et al. The effect of influenza vaccination on trained immunity: Impact on COVID-19. medRxiv 2020. [Google Scholar]
- Xiang, Y.; Wong, K.C.-Y.; So, H.-C. Exploring Drugs and Vaccines Associated with Altered Risks and Severity of COVID-19: A UK Biobank Cohort Study of All ATC Level-4 Drug Categories Reveals Repositioning Opportunities. Pharmaceutics 2021, 13, 1514. [Google Scholar] [CrossRef]
- Alkathlan, M.; Khalil, R.; Alhemaidani, M.F.; Alaed, G.H.; Almutairi, S.M.; A Almalki, H.; Alghofaili, R.H.; Al-Wutayd, O. Trends, Uptake, and Predictors of Influenza Vaccination among Healthcare Practitioners during the COVID-19 Pandemic Flu Season (2020) and the Following Season (2021) in Saudi Arabia. J. Multidiscip. Healthc. 2021, 14, 2527–2536. [Google Scholar] [CrossRef]
- Pedote, P.D.; Termite, S.; Gigliobianco, A.; Lopalco, P.L.; Bianchi, F.P. Influenza Vaccination and Health Outcomes in COVID-19 Patients: A Retrospective Cohort Study. Vaccines 2021, 9, 358. [Google Scholar] [CrossRef]
- Gobbato, M.; Calagnan, E.; Burba, I.; Rizzi, L.; Grassetti, L.; Del Zotto, S.; Maso, L.D.; Serraiono, D.; Tonutti, G. Clinical, demographical characteristics and hospitalisation of 3010 patients with COVID-19 in Friuli Venezia Giulia Region (Northern Italy). A multivariate, population-based, statistical analysis. Epidemiol. Prev. 2020, 44, 226–234. [Google Scholar]
- Yang, M.-J.; Rooks, B.J.; Le, T.-T.T.; Santiago, I.O.; Diamond, J.; Dorsey, N.L.; Mainous, A.G. Influenza Vaccination and Hospitalizations among COVID-19 Infected Adults. J. Am. Board Fam. Med. 2021, 34, S179–S182. [Google Scholar] [CrossRef]
- Greco, S.; Bella, A.; Bonsi, B.; Fabbri, N.; Califano, A.; Morrone, S.; Chessa, P.; Pistolesi, C.; Zuliani, G.; De Motoli, F.; et al. SARS-CoV-2 infection and H1N1 vaccination: Does a relationship between the two factors really exist? A retrospective analysis of a territorial cohort in Ferrara, Italy. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2795–2801. [Google Scholar]
- Massari, M.; Spila-Alegiani, S.; Fabiani, M.; Belleudi, V.; Trifirò, G.; Kirchmayer, U.; Poggi, F.R.; Mancuso, P.; Menniti-Ippolito, F.; Gini, R.; et al. Association of Influenza Vaccination and Prognosis in Patients Testing Positive to SARS-CoV-2 Swab Test: A Large-Scale Italian Multi-Database Cohort Study. Vaccines 2021, 9, 716. [Google Scholar] [CrossRef]
- Wilcox, C.R.; Islam, N.; Dambha-Miller, H. Association between influenza vaccination and hospitalisation or all-cause mortality in people with COVID-19: A retrospective cohort study. BMJ Open Respir. Res. 2021, 8, e000857. [Google Scholar] [CrossRef]
- Ilic, I.; Zdravkovic, M.; Timcic, S.; Stojanovic, D.U.; Bojic, M.; Loncar, G. Pneumonia in medical professionals during COVID-19 outbreak in cardiovascular hospital. Int. J. Infect. Dis. 2021, 103, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Taghioff, S.M.; Slavin, B.R.; Holton, T.; Singh, D. Examining the potential benefits of the influenza vaccine against SARS-CoV-2: A retrospective cohort analysis of 74,754 patients. PLoS ONE 2021, 16, e0255541. [Google Scholar] [CrossRef] [PubMed]
- Fink, G.; Orlova-Fink, N.; Schindler, T.; Grisi, S.; Ferrer, A.P.S.; Daubenberger, C.; Brentani, A. Inactivated trivalent influenza vaccination is associated with lower mortality among patients with COVID-19 in Brazil. BMJ Evid.-Based Med. 2021, 26, 192–193. [Google Scholar] [CrossRef] [PubMed]
- Demkina, A.E.; Morozov, S.P.; Vladzymyrskyy, A.V.; Kljashtorny, V.G.; Guseva, O.I.; Pugachev, P.S.; Artemova, O.R.; Reshetnikov, R.V.; Gombolevskiy, V.A.; Ryabinina, M.N. Risk factors for outcomes of COVID-19 patients: An observational study of 795,572 patients in Russia. medRxiv 2020. [Google Scholar]
- Candelli, M.; Pignataro, G.; Torelli, E.; Gullì, A.; Nista, E.C.; Petrucci, M.; Saviano, A.; Marchesini, D.; Covino, M.; Ojetti, V.; et al. Effect of influenza vaccine on COVID-19 mortality: A retrospective study. Intern. Emerg. Med. 2021, 16, 1849–1855. [Google Scholar] [CrossRef]
- Ibáñez, J.M.F.; Ballesteros, M.D.C.M.; Anguita, M.J.F.; Andúgar, M.G.; Arias, A.; Barberá-Farré, J.R. Influence of influenza vaccine and comorbidity on the evolution of hospitalized COVID-19 patients. Med. Clin. 2022, 158, 603–607. [Google Scholar] [CrossRef]
- Giannoglou, D.; Meimeti, E.; Provatopoulou, X.; Stathopoulos, K.; Roukas, I.K.; Galanis, P. Predictors of mortality in hospitalized COVID-19 patients in Athens, Greece. medRxiv 2020. [Google Scholar]
- Ortiz-Prado, E.; Simbaña-Rivera, K.; Barreno, L.G.; Diaz, A.M.; Barreto, A.; Moyano, C.; Arcos, V.; Vásconez-González, E.; Paz, C.; Simbaña-Guaycha, F.; et al. Epidemiological, socio-demographic and clinical features of the early phase of the COVID-19 epidemic in Ecuador. PLOS Negl. Trop. Dis. 2021, 15, e0008958. [Google Scholar] [CrossRef]
- Wang, R.; Liu, M.; Liu, J. The Association between Influenza Vaccination and COVID-19 and Its Outcomes: A Systematic Review and Meta-Analysis of Observational Studies. Vaccines 2021, 9, 529. [Google Scholar] [CrossRef]
- Bujani, M.Z.; Behnampour, M.; Rahimi, N.; Safari, T.; Feizabad, A.K.; Sarbazi, A.H.; Baniasadi, M.; Rezaei, N.; Moghaddam, A.A. The Effect of Influenza Vaccination on COVID-19 Morbidity, Severity and Mortality: Systematic Review and Meta-Analysis. Malays. J. Med. Sci. 2021, 28, 20–31. [Google Scholar] [CrossRef]
- Jehi, L.; Ji, X.; Milinovich, A.; Erzurum, S.; Rubin, B.P.; Gordon, S.; Young, J.B.; Kattan, M.W. Individualizing Risk Prediction for Positive Coronavirus Disease 2019 Testing: Results From 11,672 Patients. Chest 2020, 158, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Caban-Martinez, A.J.; Schaefer-Solle, N.; Santiago, K.; Louzado-Feliciano, P.; Brotons, A.; Gonzalez, M.; Issenberg, S.B.; Kobetz, E. Epidemiology of SARS-CoV-2 antibodies among firefighters/paramedics of a US fire department: A cross-sectional study. Occup. Environ. Med. 2020, 77, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; Zou, M.; Clarke, Q.; Chambers, C.; Dickinson, A.J.; Sabaiduc, S.; Olsha, R.; Gubbay, J.B.; Drews, S.J.; Charest, H.; et al. Influenza Vaccine Does Not Increase the Risk of Coronavirus or Other Noninfluenza Respiratory Viruses: Retrospective Analysis from Canada, 2010–2011 to 2016–2017. Clin. Infect. Dis. 2020, 71, 2285–2288. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Camilloni, B.; Esposito, S. ESCMID Vaccine Study Group Influenza immunization policies: Which could be the main reasons for differences among countries? Hum. Vaccines Immunother. 2018, 14, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.K.; Yu, Q.; Salvador, C.E.; Melani, I.; Kitayama, S. Mandated Bacillus Calmette-Guérin (BCG) vaccination predicts flattened curves for the spread of COVID-19. Sci. Adv. 2020, 6, eabc1463. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.; Reandelar, M.J.; Fasciglione, K.; Roumenova, V.; Li, Y.; Otazu, G.H. Correlation between universal BCG vaccination policy and reduced mortality for COVID-19. medrXiv 2020. [Google Scholar]
- Singh, S.; Khera, D.; Chugh, A.; Khasbage, S.; Khera, P.S.; Chugh, V.K. BCG vaccination impact on mortality and recovery rates in COVID-19: A meta-analysis. Monaldi Arch. Chest Dis. 2021. [Google Scholar] [CrossRef]
- Covián, C.; Fernández-Fierro, A.; Retamal-Díaz, A.; Díaz, F.E.; Vasquez, A.; Lay, M.K.; Riedel, C.; Gonzalez, P.A.; Bueno, S.M.; Kalergis, A.M. BCG-Induced Cross-Protection and Development of Trained Immunity: Implication for Vaccine Design. Front. Immunol. 2019, 10, 2806. [Google Scholar] [CrossRef]
- Long, B.R.; Michaelsson, J.; Loo, C.; Ballan, W.M.; Vu, B.-A.N.; Hecht, F.M.; Lanier, L.L.; Chapman, J.M.; Nixon, D. Elevated Frequency of Gamma Interferon-Producing NK Cells in Healthy Adults Vaccinated against Influenza Virus. Clin. Vaccine Immunol. 2008, 15, 120–130. [Google Scholar] [CrossRef]
- Endrich, M.M.; Blank, P.R.; Szucs, T.D. Influenza vaccination uptake and socioeconomic determinants in 11 European countries. Vaccine 2009, 27, 4018–4024. [Google Scholar] [CrossRef] [PubMed]
- Rollston, R.; Galea, S. COVID-19 and the Social Determinants of Health. Am. J. Health Promot. 2020, 34, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Nielsen, F.; Badiani, A.; Assi, S.; Unadkat, V.; Patel, B.; Ravindrane, R.; Wardle, H. Poverty, inequality and COVID-19: The forgotten vulnerable. Public Health 2020, 183, 110–111. [Google Scholar] [CrossRef] [PubMed]
- Wachtler, B.; Michalski, N.; Nowossadeck, E.; Diercke, M.; Wahrendorf, M.; Santos-Hövener, C.; Lampert, T.; Hoebel, J. Socioeconomic inequalities in the risk of SARS-CoV-2 infection–First results from an analysis of surveillance data from Germany. J. Health Monit. 2020, 5, 18. [Google Scholar]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Feldman, C.; Anderson, R. The role of co-infections and secondary infections in patients with COVID-19. Pneumonia 2021, 13, 5. [Google Scholar] [CrossRef]
- Joseph, C.; Togawa, Y.; Shindo, N. Bacterial and viral infections associated with influenza. Influ. Other Respir. Viruses 2013, 7, 105–113. [Google Scholar] [CrossRef]
- Im, H.; Ser, J.; Sim, U.; Cho, H. Promising Expectations for Pneumococcal Vaccination during COVID-19. Vaccines 2021, 9, 1507. [Google Scholar] [CrossRef]
- Smith, A.P.; Williams, E.P.; Plunkett, T.R.; Selvaraj, M.; Lane, L.C.; Zalduondo, L.; Xue, Y.; Vogel, P.; Channappanavar, R.; Jonsson, C.B.; et al. Time-Dependent Increase in Susceptibility and Severity of Secondary Bacterial Infection during SARS-CoV-2 Infection. bioRxiv 2022. [Google Scholar]
- Bersanelli, M.; Giannarelli, D.; De Giorgi, U.; Pignata, S.; Di Maio, M.; Verzoni, E.; Clemente, A.; Guadalupi, V.; Signorelli, D.; Tiseo, M.; et al. Symptomatic COVID-19 in advanced-cancer patients treated with immune-checkpoint inhibitors: Prospective analysis from a multicentre observational trial by FICOG. Ther. Adv. Med. Oncol. 2020, 12. [Google Scholar] [CrossRef]
- Oliveira, L.M.d.; Tiyo, B.T.; da Silva, L.T.; Fonseca, L.A.M.; Rocha, R.C.; Santos, V.A.d.; Ceneviva, C.; Bedin, A.A.; de Almeida, A.; Duarte, A.J.d.; et al. Prevalence of anti-SARS-CoV-2 antibodies in outpatients of a large public university hospital in Sao Paulo, Brazil. Rev. Inst. Med. Trop. São Paulo 2020, 62. [Google Scholar] [CrossRef]
- Kindgen-Milles, D.; Brandenburger, T.; Braun, J.F.W.; Cleff, C.; Moussazadeh, K.; Mrosewski, I.; Timm, J.; Wetzchewald, D. Prevalence of SARS-COV-2 positivity in 516 German intensive care and emergency physicians studied by seroprevalence of antibodies National Covid Survey Germany (NAT-COV-SURV). PLoS ONE 2021, 16, e0248813. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Zamora, E.; Trujillo, X.; Huerta, M.; Ríos-Silva, M.; Mendoza-Cano, O. Male gender and kidney illness are associated with an increased risk of severe laboratory-confirmed coronavirus disease. BMC Infect. Dis. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Angulo-Zamudio, U.A.; Martínez-Villa, F.M.; Leon-Sicairos, N.; Flores-Villaseñor, H.; Velazquez-Roman, J.; Campos-Romero, A.; Alcántar-Fernández, J.; Urrea, F.; Muro-Amador, S.; Medina-Serrano, J.; et al. Analysis of Epidemiological and Clinical Characteristics of COVID-19 in Northwest Mexico and the Relationship Between the Influenza Vaccine and the Survival of Infected Patients. Front. Public Health 2021, 9, 570098. [Google Scholar] [CrossRef] [PubMed]
- Alamdari, N.M.; Afaghi, S.; Rahimi, F.S.; Tarki, F.E.; Tavana, S.; Zali, A.; Fathi, M.; Besharat, S.; Bagheri, L.; Pourmotahari, F.; et al. Mortality Risk Factors among Hospitalized COVID-19 Patients in a Major Referral Center in Iran. Tohoku J. Exp. Med. 2020, 252, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, C.; Salinas-Aguirre, J.E.; Rodríguez-Muñoz, L.; Rodríguez-Sánchez, R.; Díaz-Castaño, A.; Bernal-Gómez, R. History of influenza immunization in COVID-19 patients: Impact on mortality. Gac. Med. Mex. 2021, 157, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Poblador-Plou, B.; Carmona-Pírez, J.; Ioakeim-Skoufa, I.; Poncel-Falcó, A.; Bliek-Bueno, K.; Pozo, M.C.-D.; Gimeno-Feliú, L.A.; González-Rubio, F.; Aza-Pascual-Salcedo, M.; Bandrés-Liso, A.C.; et al. Baseline Chronic Comorbidity and Mortality in Laboratory-Confirmed COVID-19 Cases: Results from the PRECOVID Study in Spain. Int. J. Environ. Res. Public Health 2020, 17, 5171. [Google Scholar] [CrossRef]
- Sardinha, D.M.; Lobato, D.D.C.; Ferreira, A.L.D.S.; Lima, K.V.B.; Guimarães, R.J.D.P.S.E.; Lima, L.N.G.C. Analysis of 472,688 Severe Cases of COVID-19 in Brazil Showed Lower Mortality in Those Vaccinated against Influenza. World J. Vaccines 2021, 11, 28–32. [Google Scholar] [CrossRef]
- Marín-Hernández, D.; Schwartz, R.E.; Nixon, D.F. Epidemiological evidence for association between higher influenza vaccine uptake in the elderly and lower COVID-19 deaths in Italy. J. Med. Virol. 2020, 93, 64–65. [Google Scholar] [CrossRef]
- Zanettini, C.; Omar, M.; Dinalankara, W.; Imada, E.L.; Colantuoni, E.; Parmigiani, G.; Marchionni, L. Influenza Vaccination and COVID19 Mortality in the USA. medRxiv 2020. [Google Scholar]
- Cocco, P.; Meloni, F.; Coratza, A.; Schirru, D.; Campagna, M.; De Matteis, S. Vaccination against seasonal influenza and socio-economic and environmental factors as determinants of the geographic variation of COVID-19 incidence and mortality in the Italian elderly. Prev. Med. 2021, 143, 106351. [Google Scholar] [CrossRef] [PubMed]
- Arokiaraj, M.C. Considering Interim Interventions to Control COVID-19 Associated Morbidity and Mortality—Perspectives. Front. Public Health 2020, 8, 444. [Google Scholar] [CrossRef]
- Amato, M.; Werba, J.P.; Frigerio, B.; Coggi, D.; Sansaro, D.; Ravani, A.; Ferrante, P.; Veglia, F.; Tremoli, E.; Baldassarre, D. Relationship between Influenza Vaccination Coverage Rate and COVID-19 Outbreak: An Italian Ecological Study. Vaccines 2020, 8, 535. [Google Scholar] [CrossRef] [PubMed]



| First Author’s Name | Year of Publication | Country | Identification of COVID-19 | Sample Size | Effect Size | Adjusted Estimate |
|---|---|---|---|---|---|---|
| M. Noale et al. [38] | 2020 | Italy | rt-PCR | 6061 | OR | 0.85 (95% CI: 0.74–0.98) |
| M. Noale et al. [38] | 2020 | Italy | rt-PCR | 619 | OR | 0.87 (95% CI: 0.59–1.28) |
| I. Martínez-Baz et al. [29] | 2020 | Spain | rt-PCR or Antibody Rapid test | 9745 | OR | 1.07(95% CI: 0.92–1.24) |
| P. Ragni et al. [40] | 2020 | Italy | rt-PCR | 17,608 | OR | 0.89 (95% CI: 0.80–0.99) |
| M. Belingheri et al. [31] | 2020 | Italy | rt-PCR | 3520 | OR | 0.41 (95% CI: 0.07–2.39) |
| A. Vila-Córcoles et al. [41] | 2020 | Spain | rt-PCR | 79,083 | HR | 1.02 (95% CI: 0.79–1.32) |
| I. Green et al. [42] | 2020 | Israel | rt-PCR | 19,089 | OR | 0.79 (95% CI: 0.67–0.98) |
| E. Kissling et al. [15] | 2021 | Europe | rt-PCR | 2147 | OR | 0.93 (95% CI: 0.66–1.32) |
| A. Conlon et al. [43] | 2021 | USA | rt-PCR | 27,201 | OR | 0.76 (95% CI: 0.68–0.86) |
| B. Erismis et al. [33] | 2021 | Turkey | Not specified | 203 | RR | 0.83 (95% CI: 0.75–0.93) |
| A. Bozek et al. [44] | 2021 | Poland | rt-PCR | 2558 | HR | 0.74 (95% CI: 0.54–0.89) |
| M. Kowalska et al. [45] | 2021 | Poland | IgG antibodies | 5376 | OR | 0.68 (95% CI: 0.55–0.83) |
| M. Fernández-Prada et al. [46] | 2021 | Spain | rt-PCR | 188 | OR | 1.70 (95% CI: 0.97–3.25) |
| K. Huang et al. [37] | 2021 | USA | Not specified | 55,667.997 | OR | 0.76 (95% CI: 0.75–0.77) |
| J. P. King et al. [47] | 2021 | USA | rt-PCR | 1356 | OR | 0.83 (95% CI: 0.63–1.10) |
| C. Pawlowski et al. [48] | 2021 | USA | rt-PCR | 12,791 | RR | 0.85 (95% CI: 0.75–0.96) |
| S. Caratozzolo et al. [36] | 2020 | Italy | rt-PCR/or infection according to WHO definition | 848 | OR | 0.47 (95% CI: 0.29–0.74) |
| M. N. Rivas et al. [32] | 2021 | USA | IgG antibodies | 6087 | OR | 1.84 (95% CI: 0.57–11.3) |
| J. G. Zein et al. [49] | 2020 | USA | Not specified | 13,220 | OR | 0.79 (95% CI: 0.62–1.00) |
| P. A. Debisarun et al. [50] | 2020 | Netherlands | rt-PCR | 6856 | RR | 0.61 (95% CI: 0.46–0.82) |
| Y. Xiang et al. [51] | 2020 | UK | Laboratory confirmed | 30,835 | OR | 0.60 (95% CI: 0.53–0.68) |
| M. Alkathlan et al. [52] | 2021 | Saudi Arabia | Laboratory confirmed | 424 | OR | 0.83 (95% CI: 0.51–1.35) |
| First Author’s Name | Year of Publication | Country | Identification of COVID-19 | Sample Size | Effect Size | Adjusted Estimate |
|---|---|---|---|---|---|---|
| Hospitalization | ||||||
| P. D. Pedote et al. [53] | 2021 | Italy | rt-PCR | 662 | OR | 1.20 (95% CI: 0.70–1.90) |
| R. Pastorino et al. [39] | 2021 | Italy | rt-PCR | 741 | OR | 1.03 (95% CI: 0.66–1.62) |
| C. Pawlowski et al. [48] | 2021 | USA | rt-PCR | 959 | RR | 1.10 (95% CI: 0.83–1.50) |
| M. Gobbato et al. [54] | 2020 | Italy | Laboratory confirmed | 3010 | OR | 0.78 (95% CI: 0.61–1.01) |
| M-J. Yang et al. [55] | 2020 | USA | Laboratory confirmed | 2005 | OR | 0.41 (95% CI: 0.28–0.59) |
| S. Greco et al. [56] | 2021 | Italy | rt-PCR | 952 | OR | 1.44 (95% CI: 1.01–2.05) |
| A. Bozek et al. [44] | 2021 | Poland | rt-PCR | 151 | HR | 0.48 (95% CI: 0.36–0.77) |
| K. Huang et al. [37] | 2021 | USA | Not specified | 55,667,977 | OR | 0.76 (95% CI: 0.75–0.77) |
| M. Massari et al. [57] | 2021 | Italy | rt-PCR | 115,945 | RR | 0.87 (95% CI: 0.86–0.88) |
| A. Conlon et al. [43] | 2021 | USA | rt-PCR | 1218 | OR | 0.58 (95% CI: 0.46–0.73) |
| P. Ragni et al. [40] | 2020 | Italy | rt-PCR | 4485 | HR | 1.00 (95% CI: 0.84–1.19) |
| C. R. Wilcox et al. [58] | 2021 | UK | rt-PCR | 6921 | HR | 0.85 (95% CI: 0.75–0.97) |
| J. G. Zein et al. [49] | 2020 | USA | Not specified | 1434 | OR | 1.29 (95% CI: 0.72–2.31) |
| I. Ilic et al. [59] | 2020 | Serbia | rt-PCR | 107 | OR | 1.31 (95% CI: 0.54–3.17) |
| S. M. Taghioff et al. [60] | 2021 | Netherlands | Not specified | 74,754 | OR | 1.07 (95% CI: 0.96–1.18) |
| Mechanical Ventilation/Invansive Respiratory Support | ||||||
| G. Fink et al. [61] | 2020 | Brazil | rt-PCR | 39,745 | OR | 0.83 (95% CI: 0.77–0.89) |
| A. Conlon et al. [43] | 2021 | USA | rt-PCR | 1218 | OR | 0.45 (95% CI: 0.27–0.78) |
| A. E. Demkina et al. [62] | 2020 | Russia | Clinically confirmed/ rt-PCR | 214,751 | OR | 0.74 (95% CI: 0.54–1.01) |
| A. Bozek et al. [44] | 2021 | Poland | rt-PCR | 21 | OR | 0.42 (95% CI: 0.02–9.96) |
| Intensive Care | ||||||
| M-J. Yang et al. [55] | 2021 | USA | rt-PCR | 2005 | OR | 0.31 (95% CI: 0.07–0.85) |
| M. Massari et al. [57] | 2021 | Italy | rt-PCR | 111,740 | RR | 1.01 (95% CI: 0.99–1.04) |
| R. Pastorino et al. [39] | 2021 | Italy | rt-PCR | 99 | OR | 1.26 (95% CI: 0.74–2.21) |
| A. Conlon et al. [43] | 2021 | USA | rt-PCR | 1218 | OR | 0.64 (95% CI: 0.41–1.00) |
| C. Pawlowski et al. [48] | 2021 | USA | rt-PCR | 959 | RR | 1.10 (95% CI: 0.56–2.20) |
| G. Fink et al. [61] | 2020 | Brazil | rt-PCR | 39,156 | OR | 0.93 (95% CI: 0.87–0.99) |
| A. E. Demkina et al. [62] | 2020 | Russia | Clinically confirmed/ rt-PCR | 214,751 | OR | 0.76 (95% CI: 0.59–0.97) |
| M. Candelli et al. [63] | 2021 | Italy | rt-PCR | 602 | OR | 0.73 (95% CI: 0.35–1.56) |
| M. L. de la Cruz Contyet al. [34] | 2021 | Spain | rt-PCR | 206 | OR | 1.92 (95% CI: 0.36–10.3) |
| J. G. Zein et al. [49] | 2020 | USA | Not specified | 1434 | OR | 0.65 (95% CI: 0.22–1.79) |
| S. M. Taghioff et al. [60] | 2021 | Netherlands | Not specified | 74,754 | OR | 1.18 (95% CI: 1.00–1.39) |
| Mortality | ||||||
| S. Greco et al. [56] | 2021 | Italy | rt-PCR | 952 | OR | 1.06 (95% CI: 0.60–1.88) |
| P. D. Pedote et al. [53] | 2021 | Italy | rt-PCR | 662 | OR | 1.60 (95% CI: 0.80–3.20) |
| R. Pastorino et al. [39] | 2021 | Italy | rt-PCR | 97 | OR | 1.33 (95% CI: 0.77–2.31) |
| M. Massari et al. [57] | 2021 | Italy | rt-PCR | 115,945 | RR | 1.04 (95% CI: 1.01–1.06) |
| J. M. Fernadez. Ibánez et al. [64] | 2021 | Spain | rt-PCR | 410 | OR | 1.55 (95% CI: 0.96–2.48) |
| A. Bozek et al. [44] | 2021 | Poland | rt-PCR | 2558 | HR | 0.74 (95% CI: 0.03–20.8) |
| M. Candelli et al. [63] | 2021 | Italy | rt-PCR | 602 | OR | 0.20 (95% CI: 0.08–0.51) |
| A. Conlon et al. [43] | 2021 | USA | rt-PCR | 1218 | HR | 0.94 (95% CI: 0.61–1.47) |
| P. Ragni et al. [40] | 2020 | Italy | rt-PCR | 4872 | HR | 1.14 (95% CI: 0.95–1.37) |
| C. R Wilcox et al. [58] | 2021 | UK | rt-PCR | 6368 | OR | 0.76 (95% CI: 0.64–0.90) |
| G. Fink et al. [61] | 2020 | Brazil | rt-PCR | 53,752 | OR | 0.84 (95% CI: 0.78–0.91) |
| A. E. Demkina et al. [62] | 2020 | Russia | Clinically confirmed/ rt-PCR | 117,346 | HR | 0.78 (95% CI: 0.63–0.95) |
| Y. Azzi et al. [35] | 2020 | USA | rt-PCR | 229 | OR | 0.88 (95% CI: 0.70–0.96) |
| M. Gobbato et al. [54] | 2020 | Italy | Laboratory confirmed | 3010 | OR | 0.78 (95% CI: 0.61–1.01) |
| J. G. Zein et al. [49] | 2020 | USA | Not specified | 14,654 | OR | 0.98 (95% CI: 0.39–2.43) |
| D. Giannoglou et al. [65] | 2020 | Greece | Not specified | 512 | OR | 0.38 (95% CI: 0.17–0.81) |
| E. Ortiz-Prado et al. [66] | 2020 | Ecuador | rt-PCR | 9468 | RR | 1.40 (95% CI: 0.46–4.28) |
| S. M. Taghioff et al. [57] | 2021 | Netherlands | Not specified | 74,754 | OR | 0.89 (95% CI: 0.77–1.03) |
| First Author’s Name | Year of Publication | Country | Identification of COVID-19 | Sample Size | Effect Size | Adjusted Estimate |
|---|---|---|---|---|---|---|
| Infection | ||||||
| A. Vila-Córcoles et al. [41] | 2020 | Spain | rt-PCR | 79,083 | HR | 1.02 (95% CI0.78–1.33) |
| M. Noale et al. [38] | 2020 | Italy | rt-PCR | 6061 | OR | 0.61 (95% CI0.41–0.91) |
| M. Noale et al. [38] | 2020 | Italy | rt-PCR | 619 | OR | 0.56 (95% CI0.33–0.95) |
| C. Pawlowski et al. [48] | 2021 | USA | rt-PCR | 4693 | RR | 0.72 (95% CI0.56–0.92) |
| C. Pawlowski et al. [48] | 2021 | USA | rt-PCR | 4636 | RR | 1.06 (95% CI0.81–137) |
| M. N. Rivas et al. [32] | 2021 | USA | IgG antibodies | 6083 | OR | 0.99 (95% CI0.71–1.36) |
| M. Fernández-Prada et al. [46] | 2021 | Spain | rt-PCR | 188 | OR | 0.40 (95% CI0.17–1.01) |
| M. Fernández-Prada et al. [46] | 2021 | Spain | rt-PCR | 187 | OR | 0.70 (95% CI: 0.28–2.10) |
| M. Fernández-Prada et al. [46] | 2021 | Spain | rt-PCR | 188 | OR | 0.20 (95% CI: 0.06–1.18) |
| Y. Xiang et al. [51] | 2020 | UK | Laboratory confirmed | 30,835 | OR | 0.5 (95% CI: 0.31–0.82) |
| Hospitalization | ||||||
| M. Gobbato et al. [54] | 2020 | Italy | Laboratory confirmed | 3010 | OR | 1.53 (95% CI:1.91–1.97) |
| R. Pastorino et al. [39] | 2021 | Italy | rt-PCR | 741 | OR | 0.96 (95% CI: 0.53–1.78) |
| C. Pawlowski et al. [48] | 2021 | USA | rt-PCR | 277 | RR | 1.30 (95% CI: 0.86–2.10) |
| C. Pawlowski et al. [48] | 2021 | USA | rt-PCR | 282 | RR | 1.20 (95% CI: 0.71–2.20) |
| Intensive Care Unit | ||||||
| R. Pastorino et al. [39] | 2021 | Italy | rt-PCR | 741 | OR | 0.75 (95% CI: 0.35–1.61) |
| C. Pawlowski et al. [48] | 2021 | USA | rt-PCR | 254 | RR | 1.40 (95% CI: 0.48–3.90) |
| C. Pawlowski et al. [48] | 2021 | USA | rt-PCR | 236 | RR | 1.10 (95% CI: 0.46–2.40) |
| Outcomes | No. of Studies | Adjusted Estimates (95% CI) | p-Value | I2 Value (%) | Bias (p-Value) |
|---|---|---|---|---|---|
| SARS-CoV-2 infection | 22 | 0.80 (0.75–0.86) | <0.01 | 70.1 | Begg’s test: 0.82 Egger’s test: 0.19 |
| Hospitalization | 15 | 0.88 (0.81–0.95) | <0.01 | 95.1 | Begg’s test: 0.55 Egger’s test: 0.62 |
| Mechanical Ventilation/invasive respiratory support | 4 | 0.73 (0.58–0.92) | 0.01 | 42.4 | Begg’s test: 0.73 Egger’s test: 0.21 |
| Intensive care unit admission | 11 | 0.96 (0.88–1.06) | 0.40 | 86.0 | Begg’s test: 0.76 Egger’s test: 0.14 |
| Mortality | 18 | 0.90 (0.81–1.01) | 0.07 | 78.2 | Begg’s test: 0.82 Egger’s test: 0.07 |
| Subgroup Variables | No. of Studies | Adjusted Estimate (95% CI) | p-Value | I2 Value (%) |
|---|---|---|---|---|
| Study Design | ||||
| cross sectional study | 5 | 0.76 (0.75–0.77) | <0.01 | 0.00 |
| retrospective cohort study | 10 | 0.80 (0.75–0.86) | <0.01 | 41.5 |
| prospective cohort study | 4 | 0.80 (0.56–1.08) | 0.14 | 90.9 |
| case-control study | 3 | 0.99 (0.76–1.23) | 0.93 | 51.7 |
| Study Population | ||||
| adults-general population | 13 | 0.80 (0.74–0.87) | <0.01 | 68.4 |
| older adults | 3 | 0.72 (0.56–0.92) | 0.01 | 53.8 |
| health workers | 6 | 0.84 (0.68–1.04) | 0.12 | 66.6 |
| Effect Size | ||||
| OR | 17 | 0.80 (0.74–0.87) | <0.01 | 72.5 |
| RR/HR | 5 | 0.82 (0.73–0.92) | <0.01 | 46.4 |
| Adjustment Factors | ||||
| age/gender/age and gender | 5 | 0.86 (0.62–1.21) | 0.40 | 63.5 |
| age, gender and comorbidities | 9 | 0.82 (0.73–0.92) | <0.01 | 78.4 |
| age, gender, comorbidities, and socioeconomic status, education or residence | 6 | 0.87 (0.68–1.11) | 0.26 | 81.8 |
| COVID-19 test | ||||
| rt-PCR | 15 | 0.84 (0.77–0.92) | <0.01 | 57.7 |
| IgG/IgM antibodies | 2 | 0.83 (0.38–1.82) | 0.64 | 37.9 |
| Laboratory confirmed (not specified) | 5 | 0.74 (0.66–0.83) | <0.01 | 74.9 |
| Continent | ||||
| Europe | 13 | 0.81 (0.70–0.93) | <0.01 | 79.7 |
| N. America | 6 | 0.76 (0.75–0.77) | <0.01 | 0.00 |
| Asia | 3 | 0.83 (0.77–0.90) | <0.01 | 0.00 |
| Subgroup Variables | No. of Studies | Adjusted Estimate (95% CI) | p-Value | I2 Value (%) |
|---|---|---|---|---|
| Hospitalization | ||||
| Effect Size | ||||
| OR | 10 | 0.92 (0.78–1.02) | 0.40 | 86.6 |
| RR/HR | 5 | 0.87 (0.71–0.99) | 0.03 | 71.0 |
| Adjusted Factors | ||||
| age/gender/age and gender | 1 | 1.44 (1.00–2.07) | 0.05 | 89.8 |
| age, gender and comorbidities | 7 | 0.85 (0.71–1.03) | 0.49 | 66.3 |
| COVID-19 test | ||||
| rt-PCR | 10 | 0.89 (0.78–1.02) | 0.09 | 74.6 |
| Laboratory confirmed/ (not specified) | 5 | 0.88 (0.67–1.12) | 0.30 | 90.0 |
| Continent | ||||
| Europe | 10 | 0.93 (0.83–1.04) | 0.21 | 75.7 |
| N. America | 5 | 0.80 (0.63–1.02) | 0.07 | 73.1 |
| Intensive Care Unit | ||||
| Effect Size | ||||
| OR | 9 | 0.91 (0.76–1.08) | 0.26 | 55.9 |
| HR | 2 | 1.01 (0.99–1.04) | 0.43 | 0.0 |
| COVID-19 test | ||||
| rt-PCR | 9 | 0.93 (0.84–1.03) | 0.14 | 58.5 |
| Laboratory confirmed/ (not specified) | 2 | 1.12 (0.80–1.57) | 0.52 | 14.1 |
| Adjusted Factors | ||||
| age, gender and comorbidities | 4 | 0.87 (0.65–1.17) | 0.35 | 17.6 |
| Continent | ||||
| Europe | 5 | 1.05 (0.95–1.16) | 0.32 | 20.6 |
| N. America | 4 | 0.69 (0.47–1.02) | 0.06 | 10.3 |
| S. America | 1 | 0.93 (0.87–0.99) | 0.02 | - |
| Asia | 1 | 0.76 (0.59–0.98) | 0.03 | - |
| Mortality | ||||
| Effect Size | ||||
| OR | 12 | 0.87 (0.76–0.99) | 0.03 | 60.5 |
| HR | 6 | 0.99 (0.86–1.12) | 0.82 | 46.3 |
| COVID-19 test | ||||
| rt-PCR | 14 | 0.93 (0.82–1.06) | 0.27 | 80.3 |
| Laboratory confirmed/ (not specified) | 4 | 0.81 (0.65–1.00) | 0.05 | 37.1 |
| Adjusted Factors | ||||
| age/gender/age and gender | 3 | 1.07 (0.75–1.53) | 0.70 | 59.2 |
| age, gender and comorbidities | 8 | 0.82 (0.61–1.10) | 0.19 | 75.3 |
| Continent | ||||
| Europe | 12 | 0.94 (0.80–1.09) | 0.40 | 75.3 |
| N. America | 3 | 0.89 (0.76–1.03) | 0.12 | 0.0 |
| S. America | 2 | 0.84 (0.78–0.91) | <0.01 | 0.0 |
| Asia | 1 | 0.78 (0.63–0.95) | 0.02 | - |
| Outcomes | No. of Studies | Adjusted Estimates (95% CI) | p-Value | I2 value (%) | Bias (p-Value) |
|---|---|---|---|---|---|
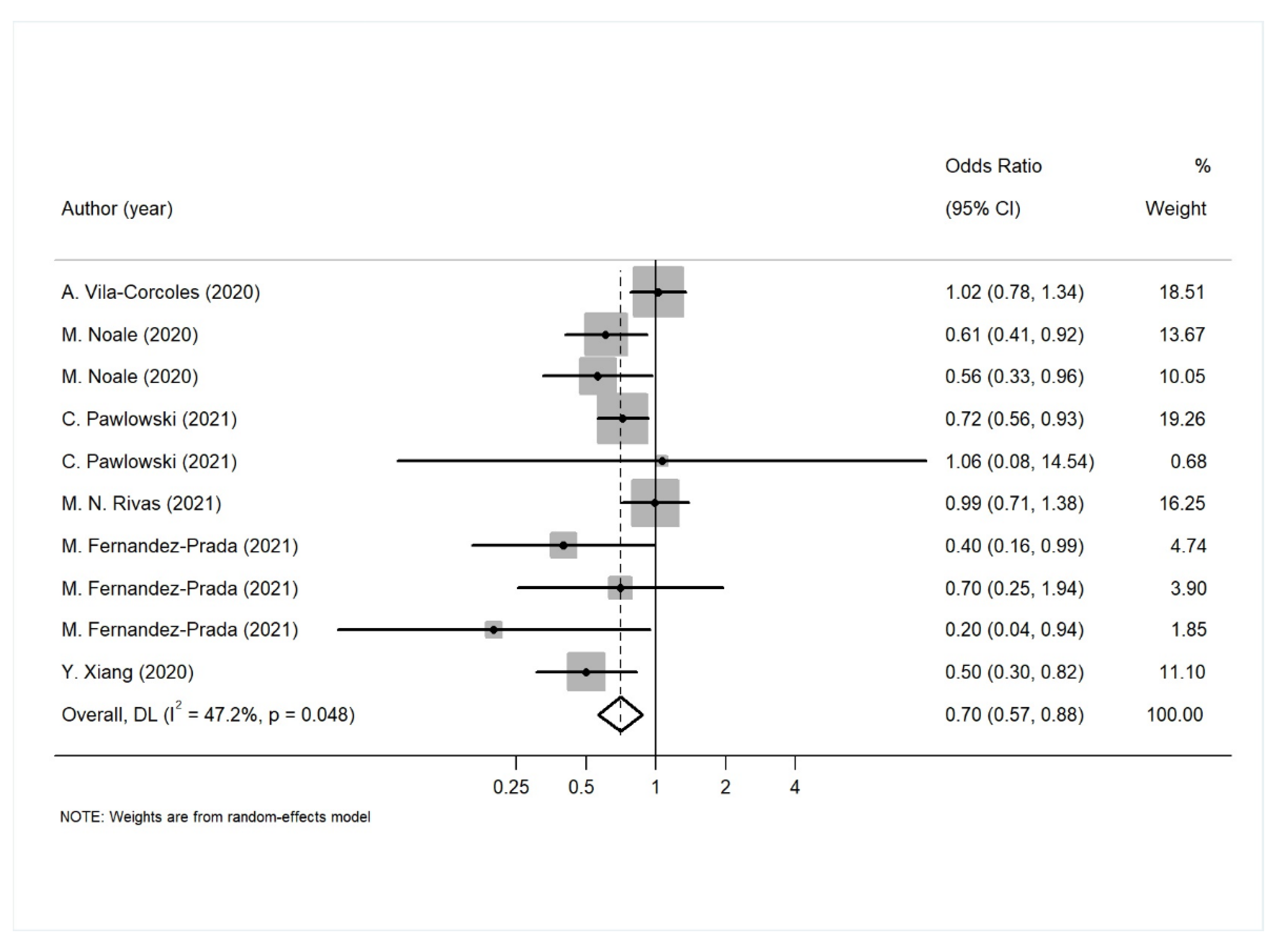
| SARS-CoV-2 infection | 10 | 0.70 (0.57–0.88) | <0.01 | 47.2 | Begg’s test: 0.37 Egger’s test: 0.11 |
| Hospitalization | 4 | 1.47 (1.30–1.67) | <0.01 | 11.4 | Begg’s test: 0.09 Egger’s test: 0.05 |
| Intensive care unit admission | 3 | 0.99 (0.60- 1.64) | 0.96 | 0.0 | Begg’s test: 0.30 Egger’s test: 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapoula, G.V.; Vennou, K.E.; Bagos, P.G. Influenza and Pneumococcal Vaccination and the Risk of COVID-19: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 3086. https://doi.org/10.3390/diagnostics12123086
Kapoula GV, Vennou KE, Bagos PG. Influenza and Pneumococcal Vaccination and the Risk of COVID-19: A Systematic Review and Meta-Analysis. Diagnostics. 2022; 12(12):3086. https://doi.org/10.3390/diagnostics12123086
Chicago/Turabian StyleKapoula, Georgia V., Konstantina E. Vennou, and Pantelis G. Bagos. 2022. "Influenza and Pneumococcal Vaccination and the Risk of COVID-19: A Systematic Review and Meta-Analysis" Diagnostics 12, no. 12: 3086. https://doi.org/10.3390/diagnostics12123086
APA StyleKapoula, G. V., Vennou, K. E., & Bagos, P. G. (2022). Influenza and Pneumococcal Vaccination and the Risk of COVID-19: A Systematic Review and Meta-Analysis. Diagnostics, 12(12), 3086. https://doi.org/10.3390/diagnostics12123086







