Lichen Sclerosus: A Current Landscape of Autoimmune and Genetic Interplay
Abstract
1. Introduction
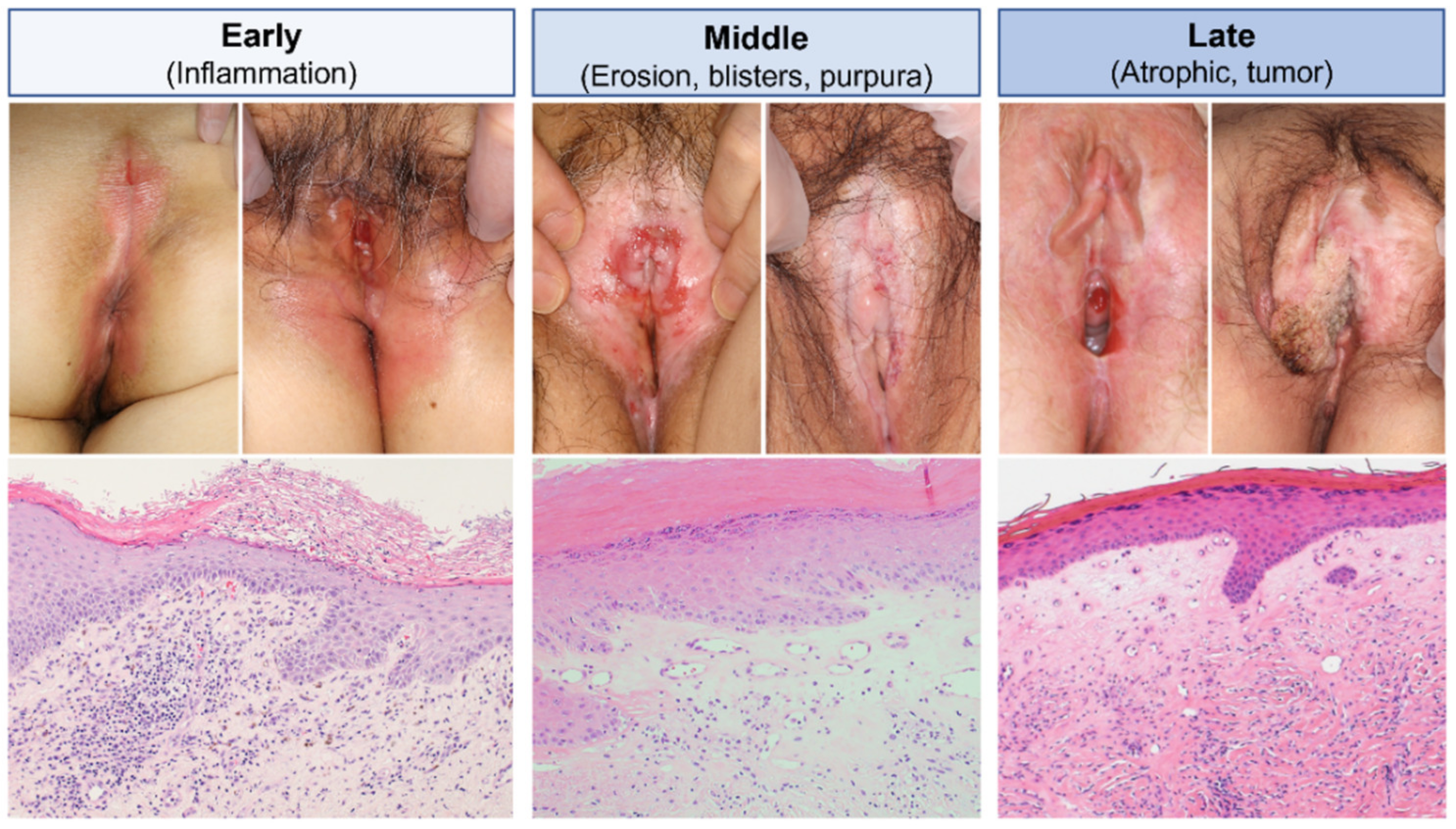
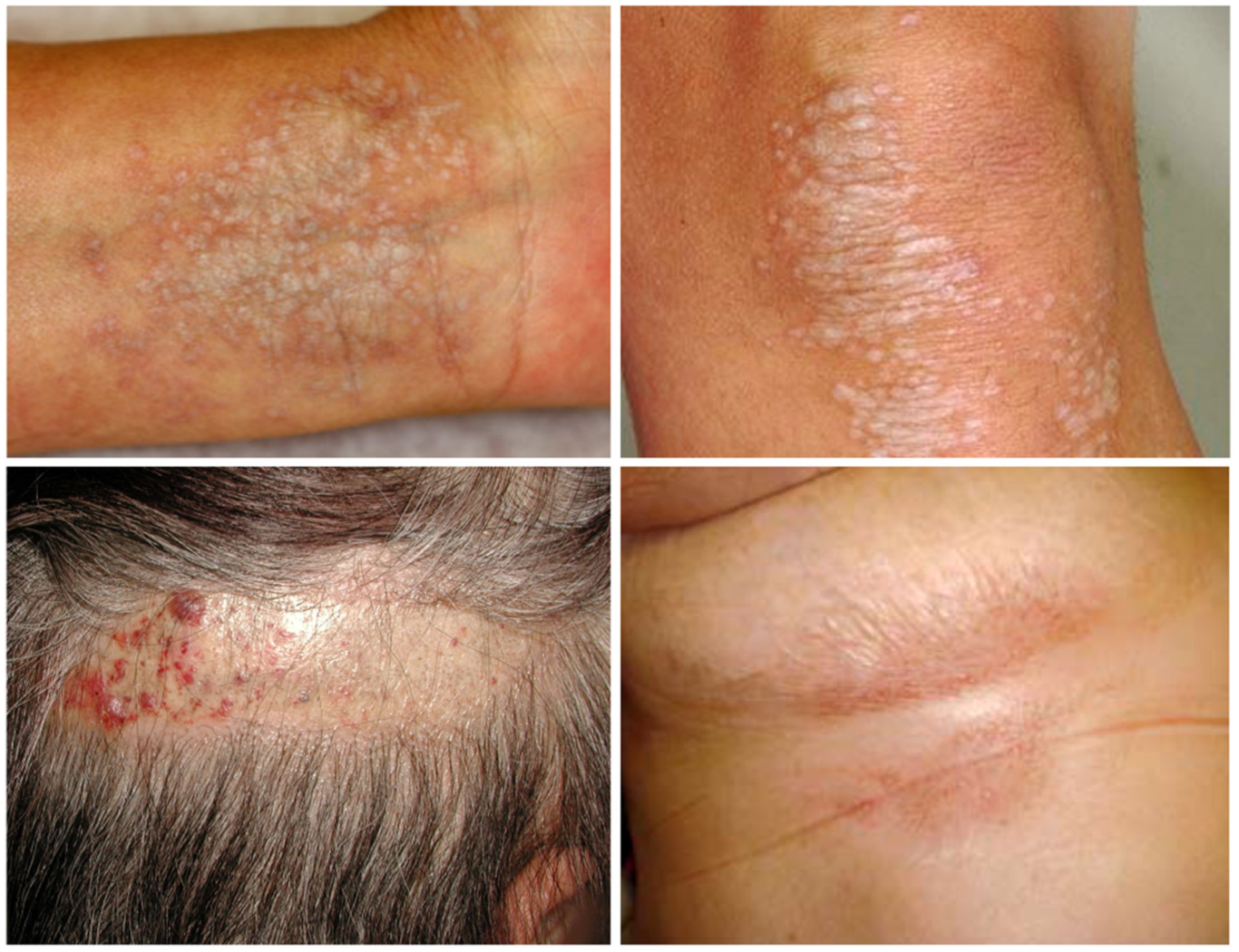
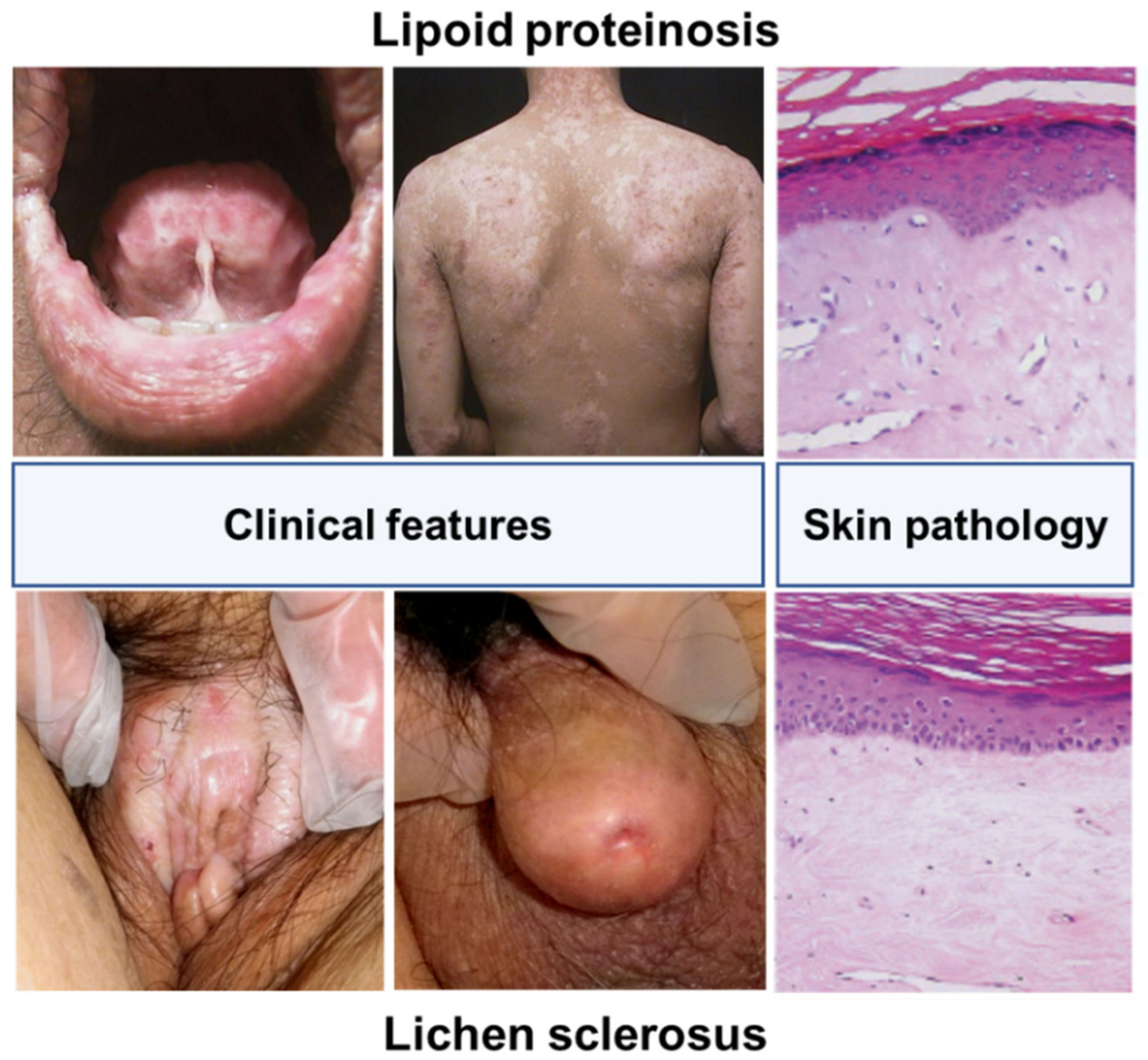
2. Clinical Characteristics of LS
2.1. Clinical Features
2.2. Epidemiology and Etiology
2.3. Histopathological Features
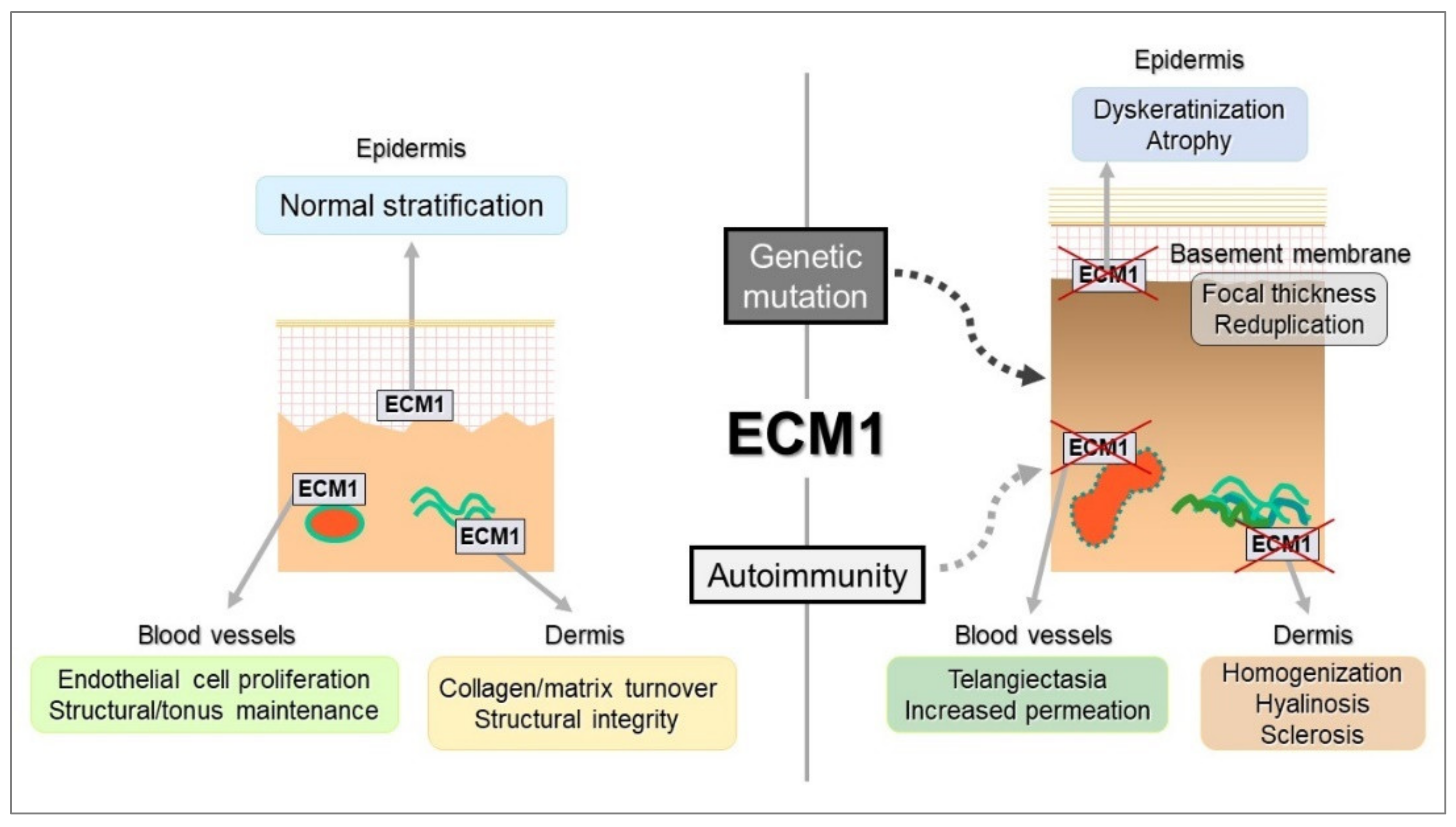
3. Molecular Characteristics of ECM1: A Secretory Glycoprotein
3.1. Historical Background for the Discovery of the ECM1 Gene: What It Means
3.2. Gene Structure and Variants
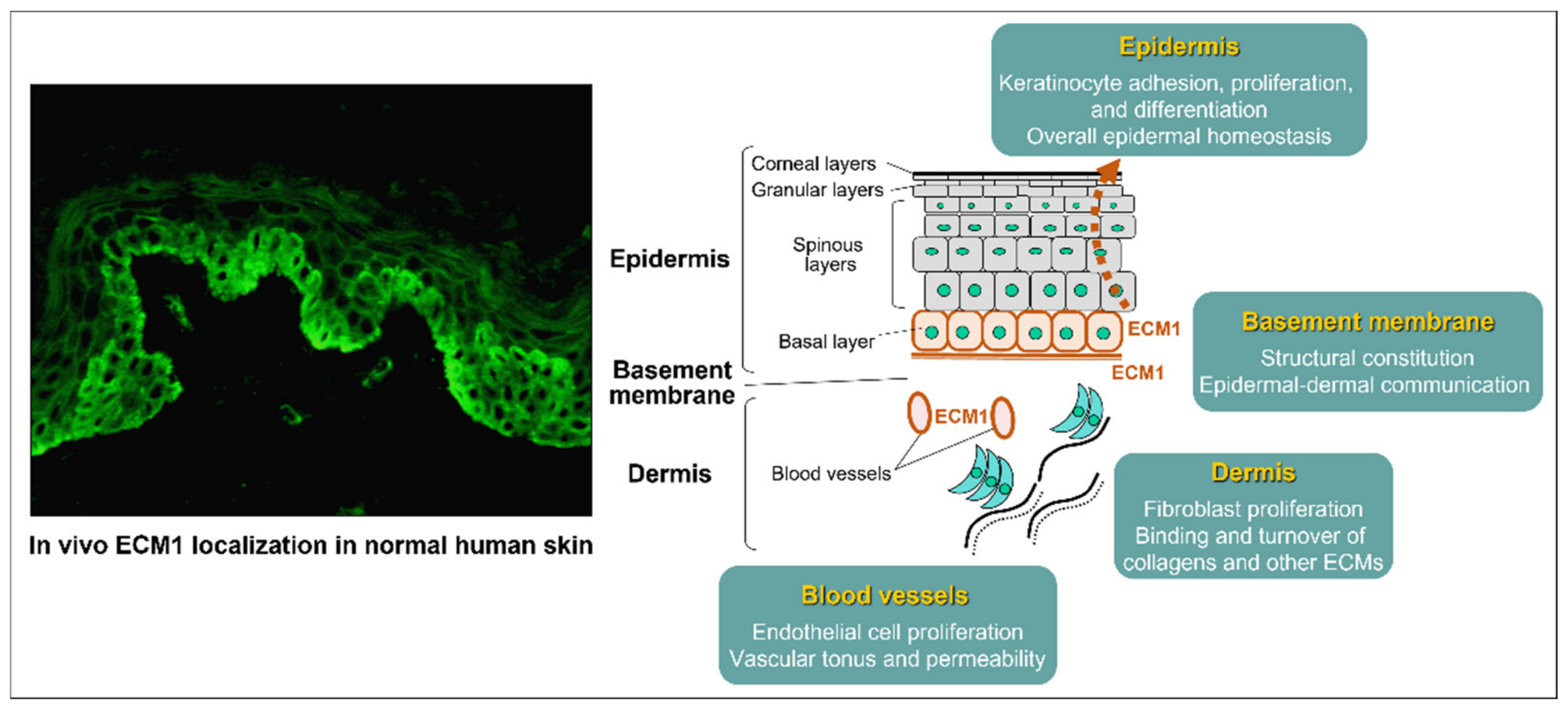
3.3. Protein Structure and Function
4. Autoimmune Response in LS
4.1. Etiological Scenario for Autoimmunity to ECM1 in LS
4.2. Identification of IgG Autoantibodies Reactive with ECM1 in LS
5. Issues that Need to be Addressed to Better Understand ECM1 Autoimmunity in LS
5.1. Lack of In Vivo-Bound Anti-ECM1 IgG in the LS Skin
5.2. Difficulty in the Establishment of Mouse Models for LS and LiP
5.3. Establishment of ECM1-Knockdown Human Dermal Fibroblasts
6. Lessens from Novel ECM1 Function in Other Animal Models
6.1. Th2 Cell-Dependent Allergic Response in the Airway
6.2. Macrophage Polarization in Inflammatory Bowel Diseases (IBDs)
6.3. Miscellaneous
6.3.1. Multiple Sclerosis
6.3.2. B Cell Function
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldstein, A.T.; Marinoff, S.C.; Christopher, K.; Srodon, M. Prevalence of Vulvar Lichen Sclerosus in a General Gynecology Practice. J. Reprod. Med. 2005, 50, 477–480. [Google Scholar] [PubMed]
- Edmonds, E.V.J.; Hunt, S.; Hawkins, D.; Dinneen, M.; Francis, N.; Bunker, C.B. Clinical Parameters in Male Genital Lichen Sclerosus: A Case Series of 329 Patients. J. Eur. Acad. Dermatology Venereol. 2012, 26, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, M.; Buxmeyer, B.; Schmitt, Y.; Bauknecht, T. Survey of Genital Lichen Sclerosus in Women and Men. Arch. Gynecol. Obstet. 2002, 266, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Kirtschig, G.; Becker, K.; Günthert, A.; Jasaitiene, D.; Cooper, S.; Chi, C.C.; Kreuter, A.; Rall, K.K.; Aberer, W.; Riechardt, S.; et al. Evidence-Based (S3) Guideline on (Anogenital) Lichen Sclerosus. J. Eur. Acad. Dermatol. Venereol. 2015, 29, e1–e43. [Google Scholar] [PubMed]
- Bleeker, M.C.G.; Visser, P.J.; Overbeek, L.I.H.; Van Beurden, M.; Berkhof, J. Lichen Sclerosus: Incidence and Risk of Vulvar Squamous Cell Carcinoma. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1224–1230. [Google Scholar] [CrossRef]
- Davick, J.; Samuelson, M.; Krone, J.; Stockdale, C. The Prevalence of Lichen Sclerosus in Patients with Vulvar Squamous Cell Carcinoma. Int. J. Gynecol. Pathol. 2017, 36, 305–309. [Google Scholar] [CrossRef]
- Micheletti, L.; Preti, M.; Radici, G.; Boveri, S.; Di Pumpo, O.; Privitera, S.S.; Ghiringhello, B.; Benedetto, C. Vulvar Lichen Sclerosus and Neoplastic Transformation: A Retrospective Study of 976 Cases. J. Low. Genit. Tract. Dis. 2016, 20, 180–183. [Google Scholar] [CrossRef]
- Leis, M.; Singh, A.; Li, C.; Ahluwalia, R.; Fleming, P.; Lynde, C.W. Risk of Vulvar Squamous Cell Carcinoma in Lichen Sclerosus and Lichen Planus: A Systematic Review. J. Obstet. Gynaecol. Canada 2022, 44, 182–192. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ishikawa, O.; Asano, Y.; Sato, S.; Jinnin, M.; Takehara, K.; Fujimoto, M.; Yamamoto, T.; Ihn, H. Diagnostic Criteria, Severity Classification and Guidelines of Lichen Sclerosus et Atrophicus. J. Dermatol. 2018, 45, 891–897. [Google Scholar] [CrossRef]
- Neill, S.M.; Lewis, F.M.; Tatnall, F.M.; Cox, N.H. British Association of Dermatologists’ Guidelines for the Management of Lichen Sclerosus 2010. Br. J. Dermatol. 2010, 163, 672–682. [Google Scholar] [CrossRef]
- Funaro, D.; Lovett, A.; Leroux, N.; Powell, J. A Double-Blind, Randomized Prospective Study Evaluating Topical Clobetasol Propionate 0.05% versus Topical Tacrolimus 0.1% in Patients with Vulvar Lichen Sclerosus. J. Am. Acad. Dermatol. 2014, 71, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Bulbul Baskan, E.; Turan, H.; Tunali, S.; Toker, S.C.; Saricaoglu, H. Open-Label Trial of Cyclosporine for Vulvar Lichen Sclerosus. J. Am. Acad. Dermatol. 2007, 57, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Virgili, A.; Corazza, M.; Bianchi, A.; Mollica, G.; Califano, A. Open Study of Topical 0.025% Tretinoin in the Treatment of Vulvar Lichen Sclerosus: One Year of Therapy. J. Reprod. Med. Obstet. Gynecol. 1995, 40, 614–618. [Google Scholar]
- Terras, S.; Gambichler, T.; Moritz, R.K.C.; Stücker, M.; Kreuter, A. UV-A1 Phototherapy vs Clobetasol Propionate, 0.05%, in the Treatment of Vulvar Lichen Sclerosus: A Randomized Clinical Trial. JAMA Dermatol. 2014, 150, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, E.; Panagiotidou, D.; Ioannidis, D. An Open Trial of 5-Aminolevulinic Acid Photodynamic Therapy for Vulvar Lichen Sclerosus. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 141, 187–188. [Google Scholar] [CrossRef]
- Thomas, R.H.M.; Kennedy, C.T.C. The Development of Lichen Sclerosus et Atrophicus in Monozygotic Twin Girls. Br. J. Dermatol. 1986, 114, 377–379. [Google Scholar] [CrossRef]
- Sahn, E.E.; Bluestein, E.L.; Oliva, S. Familial Lichen Sclerosus et Atrophicus in Childhood. Pediatr. Dermatol. 1994, 11, 160–163. [Google Scholar] [CrossRef]
- Azurdia, R.M.; Luzzi, G.A.; Byren, I.; Welsh, K.; Wojnarowska, F.; Marren, P.; Edwards, A. Lichen Sclerosus in Adult Men: A Study of HLA Associations and Susceptibility to Autoimmune Disease. Br. J. Dermatol. 1999, 140, 79–83. [Google Scholar] [CrossRef]
- Marren, P.; Jell, J.; Charnock, F.M.; Bunce, M.; Welsh, K.; Wojnarowska, F. The Association between Lichen Sclerosus and Antigens of the HLA System. Br. J. Dermatol. 1995, 132, 197–203. [Google Scholar] [CrossRef]
- Gao, X.H.; Barnardo, M.C.M.N.; Winsey, S.; Ahmad, T.; Cook, J.; Agudelo, J.D.; Zhai, N.; Powell, J.J.; Fuggle, S.V.; Wojnarowska, F. The Association between HLA DR, DQ Antigens, and Vulval Lichen Sclerosus in the UK: HLA DRB112 and Its Associated DRB112/DQB10301/04/09/010 Haplotype Confers Susceptibility to Vulval Lichen Sclerosus, and HLA DRB10301/04 and Its Associated DRB10301/04/DQB10201/02/03 Haplotype Protects from Vulval Lichen Sclerosus. J. Investig. Dermatol. 2005, 125, 895–899. [Google Scholar]
- Khan Mohammad Beigi, P. The Immunogenetics of Morphea and Lichen Sclerosus. Adv. Exp. Med. Biol. 2022, 1367, 155–172. [Google Scholar] [PubMed]
- Guarneri, F.; Giuffrida, R.; Di Bari, F.; Cannavò, S.P.; Benvenga, S. Thyroid Autoimmunity and Lichen. Front. Endocrinol. 2017, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- García-Bravo, B.; Sánchez-Pedreño, P.; Rodríguez-Pichardo, A.; Camacho, F. Lichen Sclerosus et Atrophicus. A Study of 76 Cases and Their Relation to Diabetes. J. Am. Acad. Dermatol. 1988, 19, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Harrington, C.I.; Dunsmore, I.R. An Investigation into the Incidence of Auto-immune Disorders in Patients with Lichen Sclerosus and Atrophicus. Br. J. Dermatol. 1981, 104, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.H.M.; Ridley, C.M.; McGibbon, D.H.; Black, M.M. Lichen Sclerosus et Atrophicus and Autoimmunity—a Study of 350 Women. Br. J. Dermatol. 1988, 118, 41–46. [Google Scholar] [CrossRef]
- Baldo, M.; Bailey, A.; Bhogal, B.; Groves, R.W.; Ogg, G.; Wojnarowska, F. T Cells Reactive with the NC16A Domain of BP180 Are Present in Vulval Lichen Sclerosus and Lichen Planus. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 186–190. [Google Scholar] [CrossRef]
- Walsh, M.L.; Leonard, N.; Shawki, H.; Bell, H.K. Lichen Sclerosus and Immunobullous Disease. J. Low. Genit. Tract Dis. 2012, 16, 468–470. [Google Scholar] [CrossRef]
- Hamada, T.; McLean, W.H.I.; Ramsay, M.; Ashton, G.H.S.; Nanda, A.; Jenkins, T.; Edelstein, I.; South, A.P.; Bleck, O.; Wessagowit, V.; et al. Lipoid Proteinosis Maps to 1q21 and Is Caused by Mutations in the Extracellular Matrix Protein 1 Gene (ECM1). Hum. Mol. Genet. 2002, 11, 833–840. [Google Scholar] [CrossRef]
- Oyama, N.; Chan, I.; Neill, S.M.; Hamada, T.; South, A.P.; Wessagowit, V.; Wojnarowska, F.; D’Cruz, D.; Hughes, G.J.; Black, M.M.; et al. Autoantibodies to Extracellular Matrix Protein 1 in Lichen Sclerosus. Lancet 2003, 362, 118–123. [Google Scholar] [CrossRef]
- Oyama, N.; Chan, I.; Neill, S.M.; South, A.P.; Wojnarowska, F.; Kawakami, Y.; D’Cruz, D.; Mepani, K.; Hughes, G.J.; Bhogal, B.S.; et al. Development of Antigen-Specific ELISA for Circulating Autoantibodies to Extracellular Matrix Protein 1 in Lichen Sclerosus. J. Clin. Investig. 2004, 113, 1550–1559. [Google Scholar] [CrossRef][Green Version]
- Li, Z.; Zhang, Y.; Liu, Z.; Wu, X.; Zheng, Y.; Tao, Z.; Mao, K.; Wang, J.; Lin, G.; Tian, L.; et al. ECM1 Controls T(H)2 Cell Egress from Lymph Nodes through Re-Expression of S1P(1). Nat. Immunol. 2011, 12, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Donahue, A.; Smith, S.; McGrath, J.A.; Oyama, N.; Uitto, J. Zebrafish Model of Lipoid Proteinosis. J. Investig. Dermatol. 2011, 131, S70. [Google Scholar]
- He, L.; Gu, W.; Wang, M.; Chang, X.; Sun, X.; Zhang, Y.; Lin, X.; Yan, C.; Fan, W.; Su, P.; et al. Extracellular Matrix Protein 1 Promotes Follicular Helper T Cell Differentiation and Antibody Production. Proc. Natl. Acad. Sci. USA 2018, 115, 8621–8626. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.A.; Tremelling, M.; Anderson, C.A.; Gwilliam, R.; Bumpstead, S.; Prescott, N.J.; Nimmo, E.R.; Massey, D.; Berzuini, C.; Johnson, C.; et al. Genetic Determinants of Ulcerative Colitis Include the ECM1 Locus and Five Loci Implicated in Crohn’s Disease. Nat. Genet. 2008, 40, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Luo, Z.; Ma, L.; Zhu, S.; Wang, Z.; Wen, J.; Cheng, S.; Gu, W.; Lian, Q.; et al. ECM1 Is an Essential Factor for the Determination of M1 Macrophage Polarization in IBD in Response to LPS Stimulation. Proc. Natl. Acad. Sci. USA 2020, 117, 3083–3092. [Google Scholar] [CrossRef]
- Sugimoto, N.; Oida, T.; Hirota, K.; Nakamura, K.; Nomura, T.; Uchiyama, T.; Sakaguchi, S. Foxp3-Dependent and -Independent Molecules Specific for CD25+CD4+ Natural Regulatory T Cells Revealed by DNA Microarray Analysis. Int. Immunol. 2006, 18, 1197–1209. [Google Scholar] [CrossRef]
- Abdelbaky, A.M.; Aluru, P.; Keegan, P.; Greene, D.R. Development of Male Genital Lichen Sclerosus in Penile Reconstruction Skin Grafts after Cancer Surgery: An Unreported Complication. BJU Int. 2012, 109, 776–779. [Google Scholar] [CrossRef]
- Virgili, A.; Borghi, A.; Toni, G.; Minghetti, S.; Corazza, M. Prospective Clinical and Epidemiologic Study of Vulvar Lichen Sclerosus: Analysis of Prevalence and Severity of Clinical Features, Together with Historical and Demographic Associations. Dermatology 2014, 228, 145–151. [Google Scholar] [CrossRef]
- Kawakami, Y.; Oyama, N.; Hanami, Y.; Kimura, T.; Kishimoto, K.; Yamamoto, T. A Case of Lichen Sclerosus of the Scalp Associated with Autoantibodies to Extracellular Matrix Protein 1. Arch. Dermatol. 2009, 145, 1458–1460. [Google Scholar] [CrossRef]
- Khatib, J.; Wargo, J.J.; Krishnamurthy, S.; Travers, J.B. Hemorrhagic Bullous Lichen Sclerosus: A Case Report. Am. J. Case Rep. 2020, 21, e919353-1. [Google Scholar] [CrossRef]
- Al-Niaimi, F.; Lyon, C. Peristomal Lichen Sclerosus: The Role of Occlusion and Urine Exposure? Br. J. Dermatol. 2013, 168, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Lansdorp, C.A.; Van Den Hondel, K.E.; Korfage, I.J.; Van Gestel, M.J.; Van Der Meijden, W.I. Quality of Life in Dutch Women with Lichen Sclerosus. Br. J. Dermatol. 2013, 168, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.J. Lichen Sclerosus et Atrophicus. Trans. St. Johns. Hosp. Dermatol. Soc. 1971, 57, 9–30. [Google Scholar] [PubMed]
- Powell, J.; Wojnarowska, F. Childhood Vulvar Lichen Sclerosus: An Increasingly Common Problem. J. Am. Acad. Dermatol. 2001, 44, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.M.; Peterson, A.C. Lichen Sclerosus: Epidemiological Distribution in an Equal Access Health Care System. J. Urol. 2011, 185, 522–525. [Google Scholar] [CrossRef]
- Kizer, W.S.; Prarie, T.; Morey, A.F. Balanitis Xerotica Obliterans: Epidemiologic Distribution in an Equal Access Health Care System. South. Med. J. 2003, 96, 9–11. [Google Scholar] [CrossRef]
- West, D.S.; Papalas, J.A.; Selim, M.A.; Vollmer, R.T. Dermatopathology of the Foreskin: An Institutional Experience of over 400 Cases. J. Cutan. Pathol. 2013, 40, 11–18. [Google Scholar] [CrossRef]
- Leibovitz, A.; Kaplun, V.; Saposhnicov, N.; Habot, B. Vulvovaginal Examinations in Elderly Nursing Home Women Residents. Arch. Gerontol. Geriatr. 2000, 31, 1–4. [Google Scholar] [CrossRef]
- Vittrup, G.; Westmark, S.; Riis, J.; Mørup, L.; Heilesen, T.; Jensen, D.; Melgaard, D. The Impact of Psychosexual Counseling in Women With Lichen Sclerosus: A Randomized Controlled Trial. J. Low. Genit. Tract Dis. 2022, 26, 258–264. [Google Scholar] [CrossRef]
- Haefner, H.K.; Aldrich, N.Z.; Dalton, V.K.; Gagné, H.M.; Marcus, S.B.; Patel, D.A.; Berger, M.B. The Impact of Vulvar Lichen Sclerosus on Sexual Dysfunction. J. Women’s Health 2014, 23, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Halonen, P.; Jakobsson, M.; Heikinheimo, O.; Riska, A.; Gissler, M.; Pukkala, E. Lichen Sclerosus and Risk of Cancer. Int. J. Cancer 2017, 140, 1998–2002. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Bradford, J.; Fischer, G. Long-Term Management of Adult Vulvar Lichen Sclerosus: A Prospective Cohort Study of 507 Women. JAMA Dermatol. 2015, 151, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Regauer, S.; Liegl, B.; Reich, O. Early Vulvar Lichen Sclerosus: A Histopathological Challenge. Histopathology 2005, 47, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Niamh, L.; Naveen, S.; Hazel, B. Diagnosis of Vulval Inflammatory Dermatoses: A Pathological Study with Clinical Correlation. Int. J. Gynecol. Pathol. 2009, 28, 554–558. [Google Scholar] [CrossRef]
- Smits, P.; Ni, J.; Feng, P.; Wauters, J.; Van Hul, W.; El Boutaibi, M.; Dillon, P.J.; Merregaert, J. The Human Extracellular Matrix Gene 1 (ECM1): Genomic Structure, CDNA Cloning, Expression Pattern, and Chromosomal Localization. Genomics 1997, 45, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.R.; Wilkin, D.J.; Vos, H.L.; Ortiz De Luna, R.I.; Dehejia, A.M.; Polymeropoulos, M.H.; Francomano, C.A. Characterization of the Human Extracellular Matrix Protein 1 Gene on Chromosome 1q21. Matrix Biol. 1997, 16, 289–292. [Google Scholar] [CrossRef]
- Mathieu, E.; Meheus, L.; Raymackers, J.; Merregaert, J. Characterization of the Osteogenic Stromal Cell Line MN7: Identification of Secreted MN7 Proteins Using Two-Dimensional Polyacrylamide Gel Electrophoresis, Western Blotting, and Microsequencing. J. Bone Miner. Res. 1994, 9, 903–913. [Google Scholar] [CrossRef]
- Smits, P.; Bhalerao, J.; Merregaert, J. Molecular Cloning and Characterization of the Mouse Ecm1 Gene and Its 5’ Regulatory Sequences. Gene 1999, 226, 253–261. [Google Scholar] [CrossRef]
- Sercu, S.; Oyama, N.; Merregaert, J. Importance of Extracellular Matrix Protein 1 (ECM1) in Maintaining the Functional Integrity of the Human Skin. Undefined 2009, 3, 44–51. [Google Scholar] [CrossRef]
- Sander, C.S.; Sercu, S.; Ziemer, M.; Hipler, U.C.; Elsner, P.; Thiele, J.; Merregaert, J. Expression of Extracellular Matrix Protein 1 (ECM1) in Human Skin Is Decreased by Age and Increased upon Ultraviolet Exposure. Br. J. Dermatol. 2006, 154, 218–224. [Google Scholar] [CrossRef]
- Mongiat, M.; Fu, J.; Oldershaw, R.; Greenhalgh, R.; Gown, A.M.; Iozzo, R.V. Perlecan Protein Core Interacts with Extracellular Matrix Protein 1 (ECM1), a Glycoprotein Involved in Bone Formation and Angiogenesis. J. Biol. Chem. 2003, 278, 17491–17499. [Google Scholar] [CrossRef]
- Horev, L.; Potikha, T.; Ayalon, S.; Molho-Pessach, V.; Ingber, A.; Gany, M.A.; Edin, B.S.; Glaser, B.; Zlotogorski, A. A Novel Splice-Site Mutation in ECM-1 Gene in a Consanguineous Family with Lipoid Proteinosis. Exp. Dermatol. 2005, 14, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Smits, P.; Poumay, Y.; Karperien, M.; Tylzanowski, P.; Wauters, J.; Huylebroeck, D.; Ponec, M.; Merregaert, J. Differentiation-Dependent Alternative Splicing and Expression of the Extracellular Matrix Protein 1 Gene in Human Keratinocytes. J. Investig. Dermatol. 2000, 114, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Sercu, S.; Zhang, M.; Oyama, N.; Hansen, U.; Ghalbzouri, A.E.L.; Jun, G.; Geentjens, K.; Zhang, L.; Merregaert, J.H. Interaction of Extracellular Matrix Protein 1 with Extracellular Matrix Components: ECM1 Is a Basement Membrane Protein of the Skin. J. Investig. Dermatol. 2008, 128, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Tian, Q.; Guo, F.; Mucignat, M.T.; Perris, R.; Sercu, S.; Merregaert, J.; Di Cesare, P.E.; Liu, C.-J. Interaction between Cartilage Oligomeric Matrix Protein and Extracellular Matrix Protein 1 Mediates Endochondral Bone Growth. Matrix Biol. 2010, 29, 276–286. [Google Scholar] [CrossRef]
- Merregaert, J.; Van Langen, J.; Hansen, U.; Ponsaerts, P.; El Ghalbzouri, A.; Steenackers, E.; Van Ostade, X.; Sercu, S. Phospholipid Scramblase 1 Is Secreted by a Lipid Raft-Dependent Pathway and Interacts with the Extracellular Matrix Protein 1 in the Dermal Epidermal Junction Zone of Human Skin. J. Biol. Chem. 2010, 285, 37823–37837. [Google Scholar] [CrossRef]
- Sercu, S.; Lambeir, A.M.; Steenackers, E.; El Ghalbzouri, A.; Geentjens, K.; Sasaki, T.; Oyama, N.; Merregaert, J. ECM1 Interacts with Fibulin-3 and the Beta 3 Chain of Laminin 332 through Its Serum Albumin Subdomain-like 2 Domain. Matrix Biol. 2009, 28, 160–169. [Google Scholar] [CrossRef]
- Fujimoto, N.; Terlizzi, J.; Brittingham, R.; Fertala, A.; McGrath, J.A.; Uitto, J. Extracellular Matrix Protein 1 Interacts with the Domain III of Fibulin-1C and 1D Variants through Its Central Tandem Repeat 2. Biochem. Biophys. Res. Commun. 2005, 333, 1327–1333. [Google Scholar] [CrossRef]
- Utsunomiya, N.; Utsunomiya, A.; Chino, T.; Hasegawa, M.; Oyama, N. Gene Silencing of Extracellular Matrix Protein 1 (ECM1) Results in Phenotypic Alterations of Dermal Fibroblasts Reminiscent of Clinical Features of Lichen Sclerosus. J. Dermatol. Sci. 2020, 100, 99–109. [Google Scholar] [CrossRef]
- Han, Z.; Ni, J.; Smits, P.; Underhill, C.B.; Xie, B.; Chen, Y.; Liu, N.; Tylzanowski, P.; Parmelee, D.; Feng, P.; et al. Extracellular Matrix Protein 1 (ECM1) Has Angiogenic Properties and Is Expressed by Breast Tumor Cells. FASEB J. 2001, 15, 988–994. [Google Scholar] [CrossRef]
- Deckers, M.M.L.; Smits, P.; Karperien, M.; Ni, J.; Tylzanowski, P.; Feng, P.; Parmelee, D.; Zhang, J.; Bouffard, E.; Gentz, R.; et al. Recombinant Human Extracellular Matrix Protein 1 Inhibits Alkaline Phosphatase Activity and Mineralization of Mouse Embryonic Metatarsals in Vitro. Bone 2001, 28, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Sercu, S.; Zhang, L.; Merregaert, J. The Extracellular Matrix Protein 1: Its Molecular Interaction and Implication in Tumor Progression. Cancer Investig. 2008, 26, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Sherman, V.; McPherson, T.; Baldo, M.; Salim, A.; Gao, X.; Wojnarowska, F. The High Rate of Familial Lichen Sclerosus Suggests a Genetic Contribution: An Observational Cohort Study. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Goolamali, S.K.; Shuster, S.; Barnes, E.W.; Irvine, W.J. Organ-Specific Antibodies in Patients with Lichen Sclerosus. Br. Med. J. 1974, 4, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Irvine, H. Idiopathic Atrophy of the Skin. JAMA 1913, 61, 396–400. [Google Scholar] [CrossRef]
- Chan, I. The Role of Extracellular Matrix Protein 1 in Human Skin. Clin. Exp. Dermatol. 2004, 29, 52–56. [Google Scholar] [CrossRef]
- Bushkell, L.L.; Friedrich, E.G.; Jordon, R.E. An Appraisal of Routine Direct Immunofluorescence in Vulvar Disorders. Acta Derm. Venereol. 1981, 61, 157–161. [Google Scholar]
- Chan, I.; Liu, L.; Hamada, T.; Sethuraman, G.; Mcgrath, J.A. The Molecular Basis of Lipoid Proteinosis: Mutations in Extracellular Matrix Protein 1. Exp. Dermatol. 2007, 16, 881–890. [Google Scholar] [CrossRef]
- Kirby, L.; Gran, S.; Kreuser-Genis, I.; Owen, C.; Simpson, R. Is Urinary Incontinence Associated with Lichen Sclerosus in Females? A Systematic Review and Meta-Analysis. Ski. Health Dis. 2021, 1, e13. [Google Scholar] [CrossRef]
- Sercu, S.; Liekens, J.; Umans, L.; Van De Putte, T.; Beek, L.; Huylebroeck, D.; Zwijsen, A.; Merregaert, J. The Extracellular Matrix 1 Gene Is Essential for Early Mouse Development. FEBS J. 2005, 272, 571. [Google Scholar]
- Liu, Z.; Kim, J.H.; Falo, L.D.; You, Z. Tumor Regulatory T Cells Potently Abrogate Antitumor Immunity. J. Immunol. 2009, 182, 6160–6167. [Google Scholar] [CrossRef]
- Maul, J.; Loddenkemper, C.; Mundt, P.; Berg, E.; Giese, T.; Stallmach, A.; Zeitz, M.; Duchmann, R. Peripheral and Intestinal Regulatory CD4+CD25high T Cells in Inflammatory Bowel Disease. Gastroenterology 2005, 128, 1868–1878. [Google Scholar] [CrossRef] [PubMed]
- Marazuela, M.; García-López, M.A.; Figueroa-Vega, N.; De La Fuente, H.; Alvarado-Sánchez, B.; Monsiváis-Urenda, A.; Sánchez-Madrid, F.; Gonzalez-Amaro, R. Regulatory T Cells in Human Autoimmune Thyroid Disease. J. Clin. Endocrinol. Metab. 2006, 91, 3639–3646. [Google Scholar] [CrossRef] [PubMed]
- Vocanson, M.; Hennino, A.; Cluzel-Tailhardat, M.; Saint-Mezard, P.; Benetiere, J.; Chavagnac, C.; Berard, F.; Kaiserlian, D.; Nicolas, J.F. CD8+ T Cells Are Effector Cells of Contact Dermatitis to Common Skin Allergens in Mice. J. Investig. Dermatol. 2006, 126, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Shapira, E.; Brodsky, B.; Proscura, E.; Nyska, A.; Erlanger-Rosengarten, A.; Wormser, U. Amelioration of Experimental Autoimmune Encephalitis by Novel Peptides: Involvement of T Regulatory Cells. J. Autoimmun. 2010, 35, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bandala-Sanchez, E.; Harrison, L.C. Revisiting Regulatory T Cells in Type 1 Diabetes. Curr. Opin. Endocrinol. Diabetes. Obes. 2012, 19, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 Programs the Development and Function of CD4+CD25+ Regulatory T Cells. Nat. Immunol. 2003, 4, 986–992. [Google Scholar] [CrossRef]
- Prager, M.; Buettner, J.; Buening, C. Genes Involved in the Regulation of Intestinal Permeability and Their Role in Ulcerative Colitis. J. Dig. Dis. 2015, 16, 713–722. [Google Scholar] [CrossRef]
- Adali, G.; Tunali, N.E.; Yorulmaz, E.; Tiryakioǧlu, N.O.; Mungan, S.G.; Ulaşoǧlu, C.; Enç, F.Y.; Tuncer, I. Extracellular Matrix Protein 1 Gene Rs3737240 Single Nucleotide Polymorphism Is Associated with Ulcerative Colitis in Turkish Patients. Turk. J. Gastroenterol. 2017, 28, 254–259. [Google Scholar] [CrossRef]
- Su, P.; Chen, S.; Zheng, Y.H.; Zhou, H.Y.; Yan, C.H.; Yu, F.; Zhang, Y.G.; He, L.; Zhang, Y.; Wang, Y.; et al. Novel Function of Extracellular Matrix Protein 1 in Suppressing Th17 Cell Development in Experimental Autoimmune Encephalomyelitis. J. Immunol. 2016, 197, 1054–1064. [Google Scholar] [CrossRef]
- Lee, K.M.; Nam, K.; Oh, S.; Lim, J.; Kim, R.K.; Shim, D.; Choi, J.H.; Lee, S.J.; Yu, J.H.; Lee, J.W.; et al. ECM1 Regulates Tumor Metastasis and CSC-like Property through Stabilization of β-Catenin. Oncogene 2015, 34, 6055–6065. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, J.; Ni, J.; Xu, X.-M.; Wang, J.; Ning, H.; Pei, X.-F.; Chen, J.; Yang, S.; Underhill, C.B.; et al. Extracellular Matrix Protein 1 (ECM1) Is over-Expressed in Malignant Epithelial Tumors. Cancer Lett. 2003, 200, 57–67. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyama, N.; Hasegawa, M. Lichen Sclerosus: A Current Landscape of Autoimmune and Genetic Interplay. Diagnostics 2022, 12, 3070. https://doi.org/10.3390/diagnostics12123070
Oyama N, Hasegawa M. Lichen Sclerosus: A Current Landscape of Autoimmune and Genetic Interplay. Diagnostics. 2022; 12(12):3070. https://doi.org/10.3390/diagnostics12123070
Chicago/Turabian StyleOyama, Noritaka, and Minoru Hasegawa. 2022. "Lichen Sclerosus: A Current Landscape of Autoimmune and Genetic Interplay" Diagnostics 12, no. 12: 3070. https://doi.org/10.3390/diagnostics12123070
APA StyleOyama, N., & Hasegawa, M. (2022). Lichen Sclerosus: A Current Landscape of Autoimmune and Genetic Interplay. Diagnostics, 12(12), 3070. https://doi.org/10.3390/diagnostics12123070





