Correlation between Hand Grip Strength and Peak Inspiratory Flow Rate in Patients with Stable Chronic Obstructive Pulmonary Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Procedures
2.3. Outcomes
2.4. Statistical Analysis
3. Results
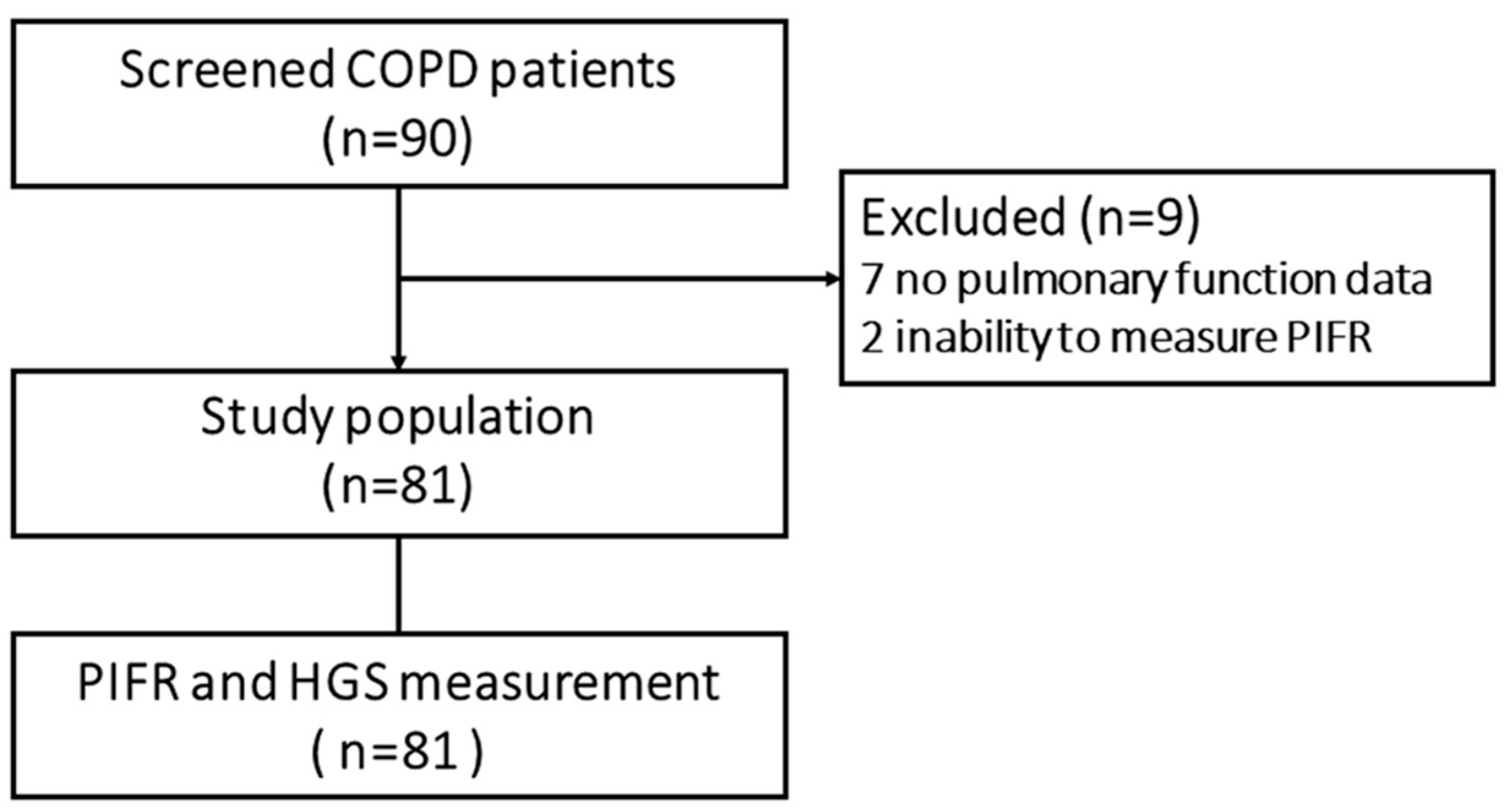
3.1. Participants
3.2. Prevalence of Suboptimal PIFR
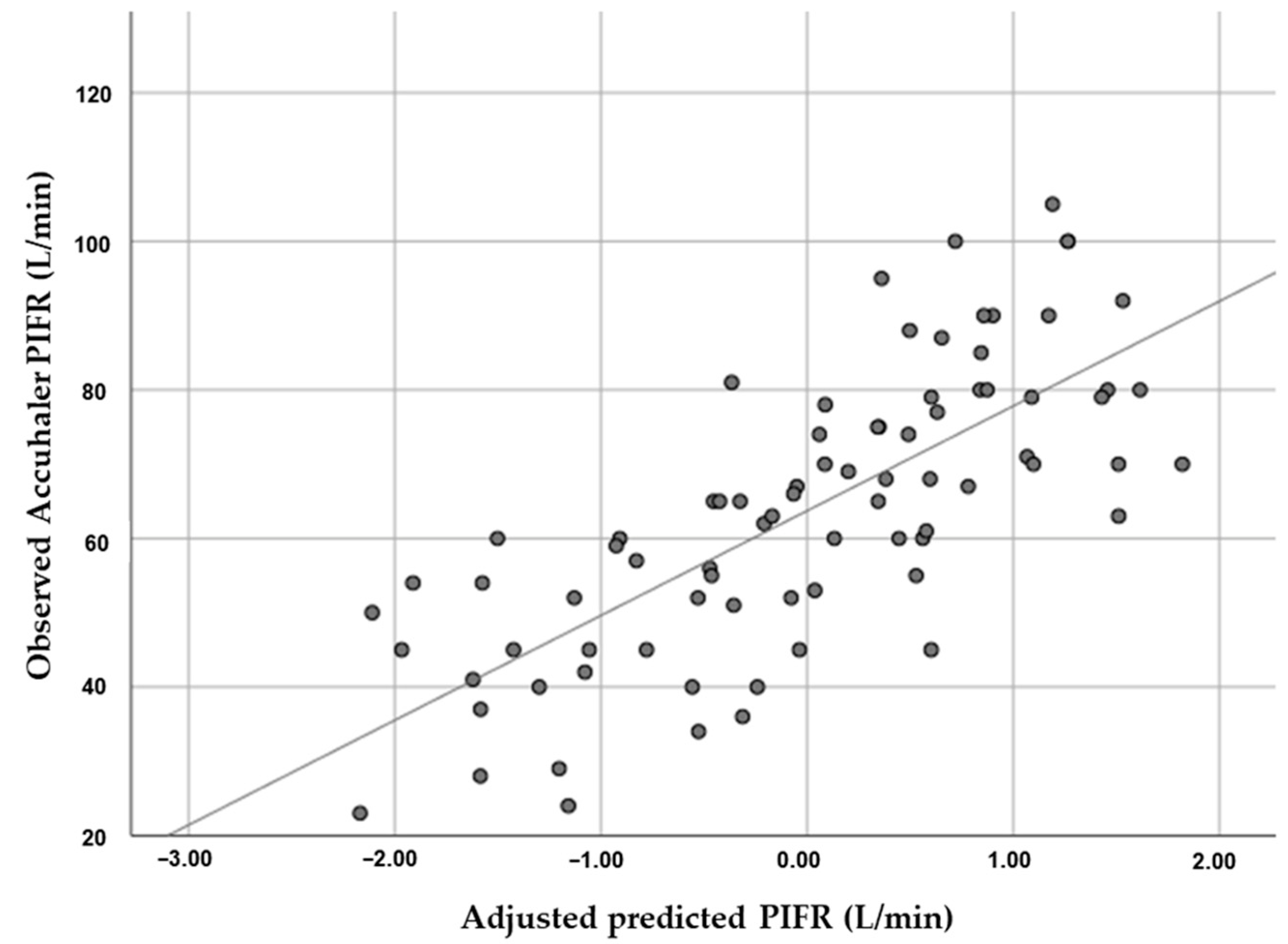
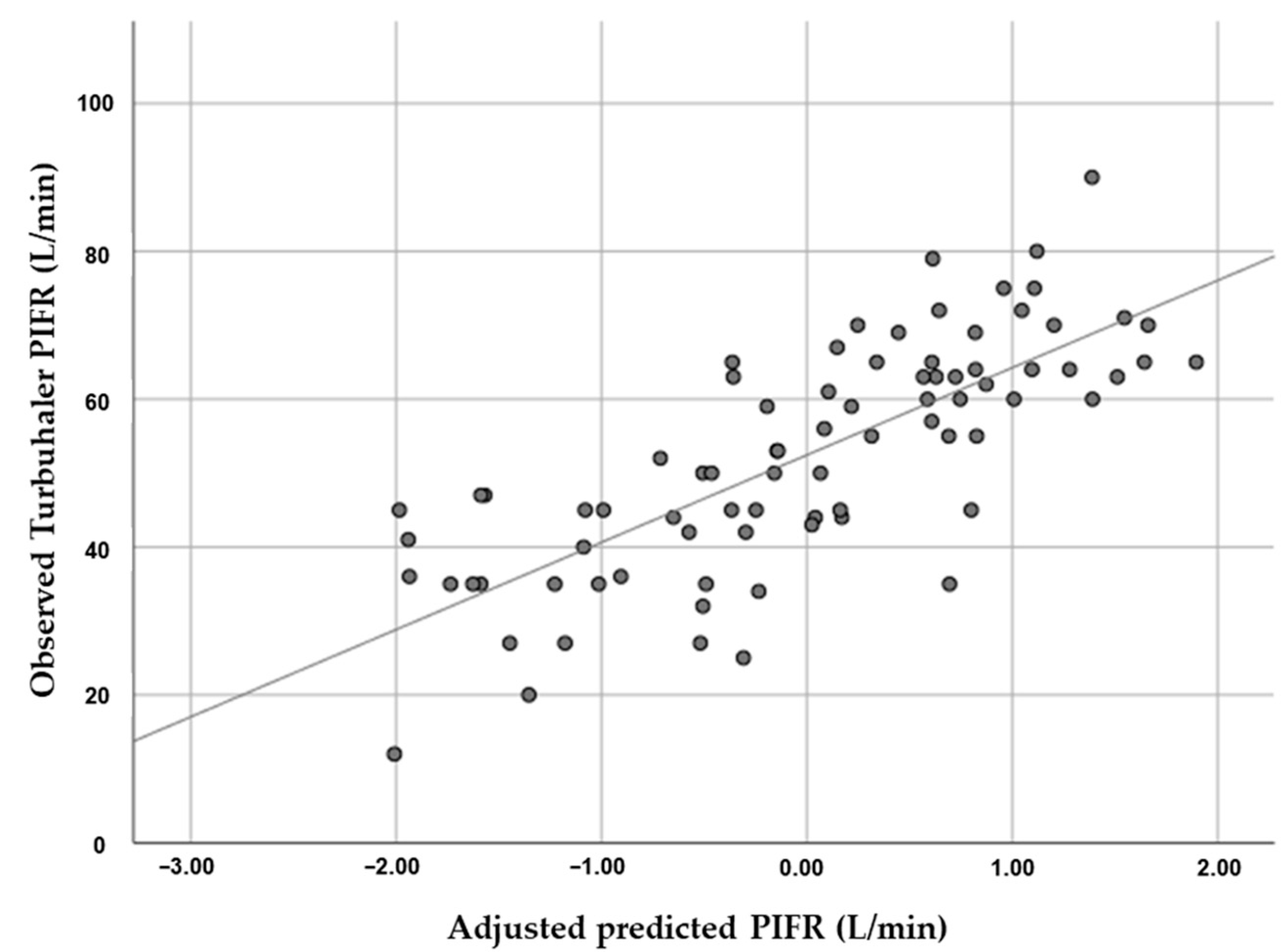
3.3. Association between HGS and PIFR
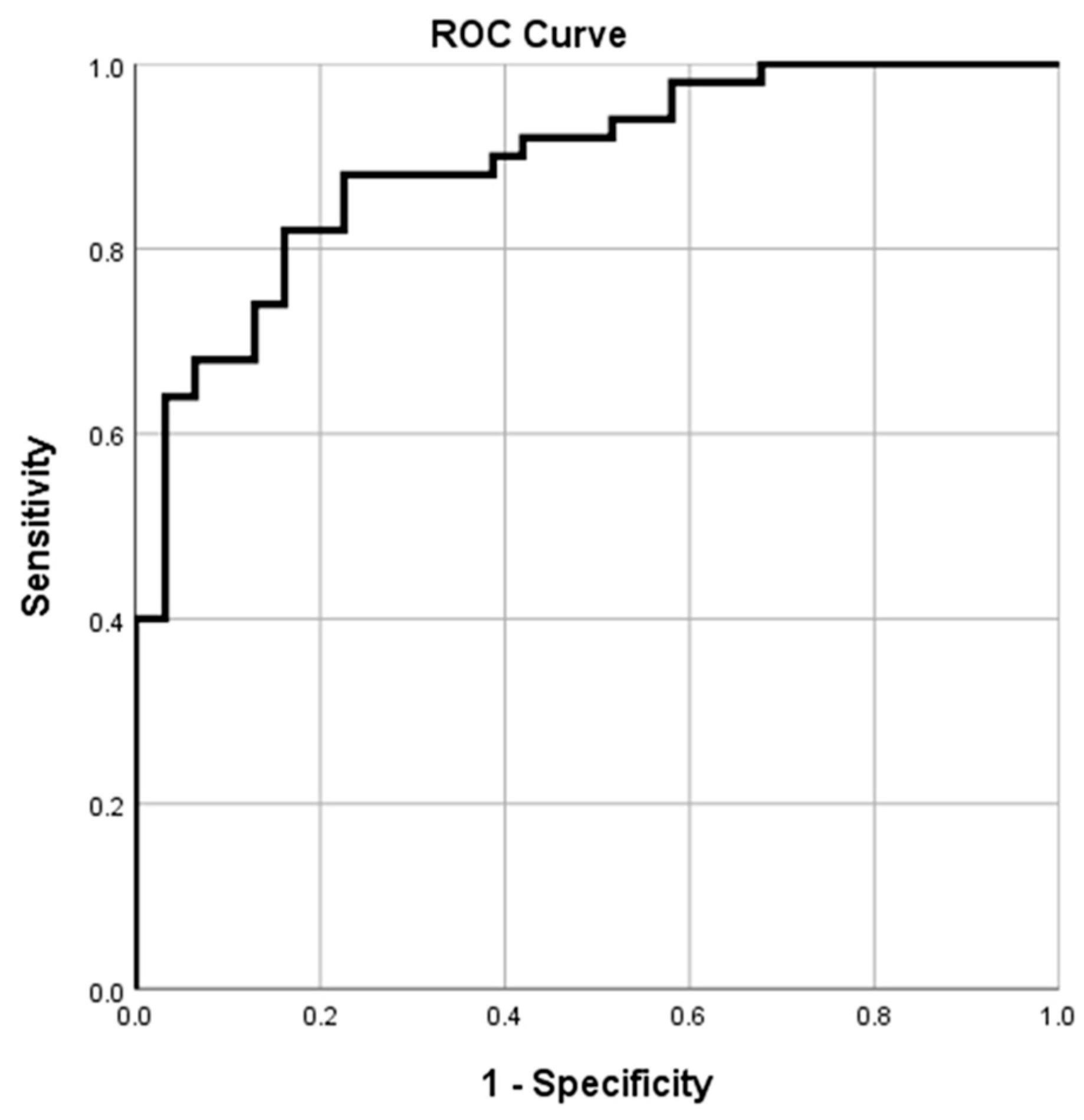
3.4. HGS Cutoff Value for Predicting Optimal Accuhaler and Turbuhaler PIFR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2022 Report). 2022. Available online: https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf (accessed on 14 October 2022).
- Salpeter, S.R. Bronchodilators in COPD: Impact of beta-agonists and anticholinergics on severe exacerbations and mortality. Int. Chronic Obstr. Pulm. Dis. 2007, 2, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Samarghandi, A.; Ioachimescu, O.C.; Qayyum, R. Association between peak inspiratory flow rate and hand grip muscle strength in hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease. PLoS ONE 2020, 15, e0227737. [Google Scholar] [CrossRef] [PubMed]
- Kawamatawong, T.; Khiawwan, S.; Pornsuriyasak, P. Peak inspiratory flow rate measurement by using In-Check DIAL for the different inhaler devices in elderly with obstructive airway diseases. J. Asthma Allergy 2017, 10, 17–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Association for Respiratory Care. Aerosol consensus statement—1991. Respir. Care 1991, 36, 916–921. [Google Scholar]
- Al-Showair, R.A.; Tarsin, W.Y.; Assi, K.H.; Pearson, S.B.; Chrystyn, H. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? Respir. Med. 2007, 101, 2395–2401. [Google Scholar] [CrossRef] [Green Version]
- Newman, S.P. Drug delivery to the lungs from dry powder inhalers. Curr. Opin. Pulm. Med. 2003, 9 (Suppl. 1), S17–S20. [Google Scholar] [CrossRef]
- Ghosh, S.; Ohar, J.A.; Drummond, M.B. Peak Inspiratory Flow Rate in Chronic Obstructive Pulmonary Disease: Implications for Dry Powder Inhalers. J. Aerosol. Med. Pulm. Drug Deliv. 2017, 30, 381–387. [Google Scholar] [CrossRef]
- Sharma, G.; Mahler, D.A.; Mayorga, V.M.; Deering, K.L.; Harshaw, O.; Ganapathy, V. Prevalence of Low Peak Inspiratory Flow Rate at Discharge in Patients Hospitalized for COPD Exacerbation. Chronic Obstr. Pulm Dis. 2017, 4, 217–224. [Google Scholar] [CrossRef]
- Ghosh, S.; Pleasants, R.A.; Ohar, J.A.; Donohue, J.F.; Drummond, M.B. Prevalence and factors associated with suboptimal peak inspiratory flow rates in COPD. Int. J. Chron Obstruct Pulmon Dis. 2019, 14, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Harb, H.S.; Laz, N.I.; Rabea, H.; Abdelrahim, M.E. Prevalence and predictors of suboptimal peak inspiratory flow rate in COPD patients. Eur. J. Pharm. Sci. 2020, 147, 105298. [Google Scholar] [CrossRef]
- Bhatt, N.Y.; Wood, K.L. What defines abnormal lung function in older adults with chronic obstructive pulmonary disease? Drugs Aging 2008, 25, 717–728. [Google Scholar] [CrossRef]
- Black, L.F.; Hyatt, R.E. Maximal respiratory pressures: Normal values and relationship to age and sex. Am. Rev. Respir. Dis. 1969, 99, 696–702. [Google Scholar]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Burtin, C.; Ter Riet, G.; Puhan, M.A.; Waschki, B.; Garcia-Aymerich, J.; Pinto-Plata, V.; Celli, B.; Watz, H.; Spruit, M.A. Handgrip weakness and mortality risk in COPD: A multicentre analysis. Thorax 2016, 71, 86–87. [Google Scholar] [CrossRef] [Green Version]
- Puhan, M.A.; Siebeling, L.; Zoller, M.; Muggensturm, P.; ter Riet, G. Simple functional performance tests and mortality in COPD. Eur. Respir. J. 2013, 42, 956–963. [Google Scholar] [CrossRef] [Green Version]
- Mahler, D.A.; Wells, C.K. Evaluation of clinical methods for rating dyspnea. Chest 1988, 93, 580–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Kline Leidy, N. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef] [Green Version]
- Manuyakorn, W.; Direkwattanachai, C.; Benjaponpitak, S.; Kamchaisatian, W.; Sasisakulporn, C.; Teawsomboonkit, W. Sensitivity of Turbutester and Accuhaler tester in asthmatic children and adolescents. Pediatr. Int. 2010, 52, 118–125. [Google Scholar] [CrossRef]
- Chrystyn, H. Is inhalation rate important for a dry powder inhaler? Using the In-Check Dial to identify these rates. Respir. Med. 2003, 97, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Tsuburai, T.; Komase, Y.; Tsuruoka, H.; Oyama, B.; Muraoka, H.; Hida, N.; Kobayashi, T.; Matsushima, S. The relationship between peak inspiratory flow and hand grip strength measurement in men with mild chronic obstructive pulmonary disease. BMC Pulm. Med. 2022, 22, 65. [Google Scholar] [CrossRef]
- van der Palen, J. Peak inspiratory flow through diskus and turbuhaler, measured by means of a peak inspiratory flow meter (In-Check DIAL). Respir. Med. 2003, 97, 285–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saiphoklang, N.; Chomchoey, C. Eosinophilia and parasitic infestations in patients with chronic obstructive pulmonary disease. Sci. Rep. 2020, 10, 12490. [Google Scholar] [CrossRef] [PubMed]
- Frohnhofen, H.; Hagen, O. Handgrip strength measurement as a predictor for successful dry powder inhaler treatment: Application in older individuals with COPD. Z Gerontol. Geriatr. 2011, 44, 245–249. [Google Scholar] [CrossRef]
- Saiphoklang, N.; Tepwimonpetkun, C. Interest of hand grip strength to predict outcome in mechanically ventilated patients. Heart Lung 2020, 49, 637–640. [Google Scholar] [CrossRef]
- Strandkvist, V.J.; Backman, H.; Röding, J.; Stridsman, C.; Lindberg, A. Hand grip strength is associated with forced expiratory volume in 1 second among subjects with COPD: Report from a population-based cohort study. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2527. [Google Scholar] [CrossRef]

| Characteristic | N = 81 |
|---|---|
| Age, years | 73.3 ± 8.9 |
| Male | 70 (86.4) |
| Body mass index, kg/m2 | 22.5 ± 3.9 |
| Active smoking | 4 (4.9) |
| Smoking, pack-years | 24.9 ± 22.2 |
| Dominant right hand | 72 (88.9) |
| Maximal Accuhaler PIFR, L/min | 63.7 ± 18.9 |
| Maximal Turbuhaler PIFR, L/min | 52.4 ± 15.4 |
| HGS, kg | 29.2 ± 8.0 |
| Comorbidity | |
| Hypertension | 44 (54.3) |
| Diabetes mellitus | 21 (25.9) |
| Coronary artery disease | 14 (17.3) |
| Congestive heart failure | 5 (6.2) |
| Atrial fibrillation | 4 (4.9) |
| Lung cancer | 4 (4.9) |
| Bronchiectasis | 4 (4.9) |
| Depression | 1 (1.2) |
| Vital sign | |
| Systolic blood pressure, mmHg | 128.4 ± 21.5 |
| Diastolic blood pressure, mmHg | 71.3 ± 12.2 |
| Pulse rate, beats/min | 81.4 ± 14.3 |
| Breathing frequency, breaths/min | 17.0 ± 1.9 |
| Oxygen saturation, % | 96.4 ± 4.0 |
| Spirometric data | |
| Post-BD FEV1, L | 1.45 ± 0.60 |
| Post-BD FEV1, % predicted | 65.3 ± 23.7 |
| Post-BD FVC, L | 2.50 ± 0.90 |
| Post-BD FVC, % predicted | 82.7 ± 22.1 |
| Post-BD FEV1/FVC, % | 58.0 ± 11.0 |
| Functional performance | |
| CAT scores | 9.5 ± 6.5 |
| CAT score < 10 | 40 (49.4) |
| CAT score ≥ 10 | 41 (50.6) |
| mMRC scores | 1.5 ± 1.1 |
| mMRC < 2 | 42 (51.9) |
| mMRC ≥ 2 | 39 (48.1) |
| Spirometry grading | |
| 1 | 32 (39.5) |
| 2 | 24 (29.6) |
| 3 | 16 (19.8) |
| 4 | 9 (11.1) |
| GOLD classification | |
| A | 35 (43.2) |
| B | 39 (48.1) |
| C | 0 (0) |
| D | 7 (8.6) |
| Medication | |
| SABD | 25 (30.9) |
| SABD dose, puffs/day | 1.0 ± 1.9 |
| Inhaled LABA | 2 (2.5) |
| LAMA | 43 (53.1) |
| LABA/LAMA | 21 (25.9) |
| ICS/LABA | 36 (44.4) |
| Xanthine | 30 (37.0) |
| Phosphodieterase-4 inhibitor | 4 (4.9) |
| Oral beta-2 agonist | 5 (6.2) |
| Azithromycin | 5 (6.2) |
| Acetylcysteine | 36 (44.4) |
| Variable | Accuhaler | Turbuhaler | ||||
|---|---|---|---|---|---|---|
| Optimal | Suboptimal | p-Value | Optimal | Suboptimal | p-Value | |
| Patients | 50 (61.7) | 31 (38.3) | NA | 33 (40.7) | 48 (59.3) | NA |
| Maximal PIFR, L/min | 75.6 ± 12.3 | 44.7 ± 9.9 | <0.001 | 67.4 ± 6.7 | 42.2 ± 10.5 | <0.001 |
| HGS, kg | 33.2 ± 6.9 | 22.8 ± 4.7 | <0.001 | 35.1 ± 6.3 | 25.3 ± 6.4 | <0.001 |
| Gender | ||||||
| Male | 49 (98.0) | 21 (67.7) | <0.001 | 33 (100) | 37 (77.1) | 0.002 |
| Female | 1 (2.0) | 10 (32.3) | 0 (0) | 11 (22.9) | ||
| Age, years | 70.0 ± 8.8 | 78.7 ± 6.2 | <0.001 | 67.9 ± 8.6 | 77.0 ± 7.2 | <0.001 |
| Height, cm | 165.7 ± 6.1 | 158.6 ± 8.0 | <0.001 | 166.8 ± 5.1 | 160.4 ± 8.1 | <0.001 |
| BMI, kg/m2 | 22.8 ± 4.0 | 22.0 ± 3.7 | 0.372 | 23.2 ± 3.6 | 22.1 ± 4.0 | 0.190 |
| Active smoking | 3 (6.0) | 1 (3.2) | 1.000 | 2 (6.1) | 2 (4.2) | 1.000 |
| Smoking, pack-years | 27.5 ± 22.4 | 20.7 ± 21.7 | 0.182 | 25.3 ± 18.1 | 24.6 ± 24.9 | 0.892 |
| Dominant hand | ||||||
| Left | 7 (14.0) | 2 (6.5) | 0.471 | 3 (9.1) | 6 (12.5) | 0.731 |
| Right | 43 (86.0) | 29 (93.5) | 30 (90.9) | 42 (87.5) | ||
| Comorbidity | ||||||
| Diabetes mellitus | 12 (24.0) | 9 (29.0) | 0.615 | 8 (24.2) | 13 (27.1) | 0.774 |
| Hypertension | 28 (56.0) | 16 (51.6) | 0.700 | 21 (63.6) | 23 (47.9) | 0.163 |
| Coronary artery disease | 6 (12.0) | 8 (25.8) | 0.110 | 5 (15.2) | 9 (18.8) | 0.674 |
| Congestive heart failure | 3 (6.0) | 2 (6.5) | 1.000 | 2 (6.1) | 3 (6.3) | 1.000 |
| Atrial fibrillation | 2 (4.0) | 2 (6.5) | 0.635 | 2 (6.1) | 2 (4.2) | 1.000 |
| Lung cancer | 1 (2.0) | 3 (9.7) | 0.154 | 1 (3.0) | 3 (6.3) | 0.642 |
| Bronchiectasis | 1 (2.0) | 3 (9.7) | 0.154 | 0 (0.0) | 4 (8.3) | 0.142 |
| Vital sign | ||||||
| SBP, mmHg | 126.9 ± 24.4 | 130.9 ± 15.9 | 0.424 | 130.2 ± 16.0 | 127.3 ± 24.7 | 0.554 |
| DBP, mmHg | 73.0 ± 12.9 | 68.8 ± 10.7 | 0.126 | 74.6 ± 13.0 | 69.1 ± 11.2 | 0.044 |
| Pulse rate, beats/min | 81.3 ± 14.2 | 81.6 ± 14.6 | 0.932 | 80.7 ± 13.2 | 81.9 ± 15.1 | 0.713 |
| Respiratory rate, breaths/min | 16.6±1.8 | 17.6±1.96 | 0.031 | 16.3±1.6 | 17.4±2.0 | 0.007 |
| SpO2, % | 96.6±4.5 | 96.1±3.1 | 0.578 | 96.3±5.3 | 96.5±2.8 | 0.837 |
| Spirometry data | ||||||
| Post-BD FEV1, % | 70.6 ± 20.2 | 56.8 ± 26.6 | 0.010 | 75.2 ± 18.4 | 58.5 ± 24.7 | 0.001 |
| Post-BD FVC, % | 88.5 ± 18.1 | 73.4 ± 24.9 | 0.002 | 93.0 ± 17.9 | 75.7 ± 22.0 | <0.001 |
| Spirometric grade 3 and 4 | 12 (24.0) | 13 (16.0) | 0.089 | 5 (15.2) | 20 (41.7) | 0.011 |
| Functional performance | ||||||
| CAT scores | 7.8 ± 6.3 | 12.2 ± 6.0 | 0.003 | 5.5 ± 5.1 | 12.2 ± 5.9 | <0.001 |
| CAT ≥ 10 | 20 (40.0) | 21 (67.7) | 0.015 | 7 (21.2) | 34 (70.8) | <0.001 |
| mMRC scores | 1.2 ± 1.1 | 2.0 ± 0.9 | 0.001 | 0.9 ± 0.9 | 2.0 ± 1.0 | <0.001 |
| mMRC ≥ 2 | 17 (34.0) | 22 (71.0) | 0.001 | 6 (18.2) | 33 (68.8) | <0.001 |
| GOLD group D | 5 (10.0) | 2 (6.5) | 0.702 | 1 (3.0) | 6 (12.5) | 0.136 |
| Medication | ||||||
| SABD | 11 (22.0) | 14 (45.2) | 0.028 | 5 (15.2) | 20 (41.7) | 0.014 |
| SABD dose, puffs/day | 0.6±1.3 | 1.6±2.6 | 0.043 | 0.4±1.1 | 1.4±2.3 | 0.008 |
| LABA | 1 (2.0) | 1 (3.2) | 1.000 | 1 (3.0) | 1 (2.1) | 1.000 |
| LAMA | 28 (56.0) | 15 (48.4) | 0.505 | 15 (45.5) | 28 (58.3) | 0.254 |
| LABA/LAMA | 10 (20.0) | 11 (35.5) | 0.122 | 10 (30.3) | 11 (22.9) | 0.456 |
| ICS/LABA | 25 (50.0) | 11 (35.5) | 0.201 | 14 (42.4) | 22 (45.8) | 0.762 |
| Theophylline | 10 (20.0) | 5 (16.1) | 0.663 | 6 (18.2) | 9 (18.8) | 0.948 |
| Doxophylline | 7 (14.0) | 8 (25.8) | 0.184 | 7 (21.2) | 8 (16.7) | 0.605 |
| Roflumilast | 3 (6.0) | 1 (3.2) | 1.000 | 1 (3.0) | 3 (6.3) | 0.642 |
| Oral beta2 agonist | 3 (6.0) | 2 (6.5) | 1.000 | 2 (6.1) | 3 (6.3) | 1.000 |
| Azithromycin | 2 (4.0) | 3 (9.7) | 0.366 | 0 (0) | 5 (10.4) | 0.076 |
| Acetylcysteine | 18 (36.0) | 18 (58.1) | 0.052 | 11 (33.3) | 25 (52.1) | 0.095 |
| Factor | Correlation Coefficient | p-Value |
|---|---|---|
| Accuhaler PIFR | 0.591 | <0.001 |
| Turbuhaler PIFR | 0.614 | <0.001 |
| Variables | Regression Coefficients | 95% CI of Coefficients | p-Value |
|---|---|---|---|
| Accuhaler | |||
| Intercept | −30.340 | −114.534, 53.853 | 0.475 |
| Hand grip strength, kg | 0.274 | 0.132, 1.170 | 0.015 |
| Age, years | −0.206 | −0.847, −0.025 | 0.038 |
| Height, cm | 0.219 | 0.063, 1.019 | 0.027 |
| Forced vital capacity, % | 1.019 | 0.099, 0.389 | 0.001 |
| Short-acting bronchodilator dose, puffs/day | −0.148 | −3.118, 0.238 | 0.091 |
| Turbuhaler | |||
| Intercept | 56.196 | 20.715, 91.676 | 0.002 |
| Hand grip strength, kg | 0.321 | 0.210, 1.032 | 0.004 |
| Female | −0.196 | −16.718, −0.850 | 0.030 |
| Age, years | −0.224 | −0.717, −0.058 | 0.022 |
| Forced vital capacity, % | 0.304 | 0.096, 0.330 | 0.001 |
| Short-acting bronchodilator dose, puffs/day | −0.147 | −2.508, 0.173 | 0.087 |
| Variable | Cutoff Value | AUC | 95% CI | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | p-Value |
|---|---|---|---|---|---|---|---|---|
| HGS for Accuhaler, kg | 26.75 | 0.892 | 0.824–0.961 | 82.00 | 83.90 | 89.14 | 74.32 | <0.001 |
| HGS for Turbuhaler, kg | 31.90 | 0.862 | 0.779–0.945 | 78.80 | 89.60 | 83.87 | 86.03 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suriyakul, A.; Saiphoklang, N.; Barjaktarevic, I.; Cooper, C.B. Correlation between Hand Grip Strength and Peak Inspiratory Flow Rate in Patients with Stable Chronic Obstructive Pulmonary Disease. Diagnostics 2022, 12, 3050. https://doi.org/10.3390/diagnostics12123050
Suriyakul A, Saiphoklang N, Barjaktarevic I, Cooper CB. Correlation between Hand Grip Strength and Peak Inspiratory Flow Rate in Patients with Stable Chronic Obstructive Pulmonary Disease. Diagnostics. 2022; 12(12):3050. https://doi.org/10.3390/diagnostics12123050
Chicago/Turabian StyleSuriyakul, Apisara, Narongkorn Saiphoklang, Igor Barjaktarevic, and Christopher B. Cooper. 2022. "Correlation between Hand Grip Strength and Peak Inspiratory Flow Rate in Patients with Stable Chronic Obstructive Pulmonary Disease" Diagnostics 12, no. 12: 3050. https://doi.org/10.3390/diagnostics12123050






