Effect of Dexamethasone on the Incidence and Outcome of COVID-19 Associated Pulmonary Aspergillosis (CAPA) in Critically Ill Patients during First- and Second Pandemic Wave—A Single Center Experience
Abstract
1. Introduction
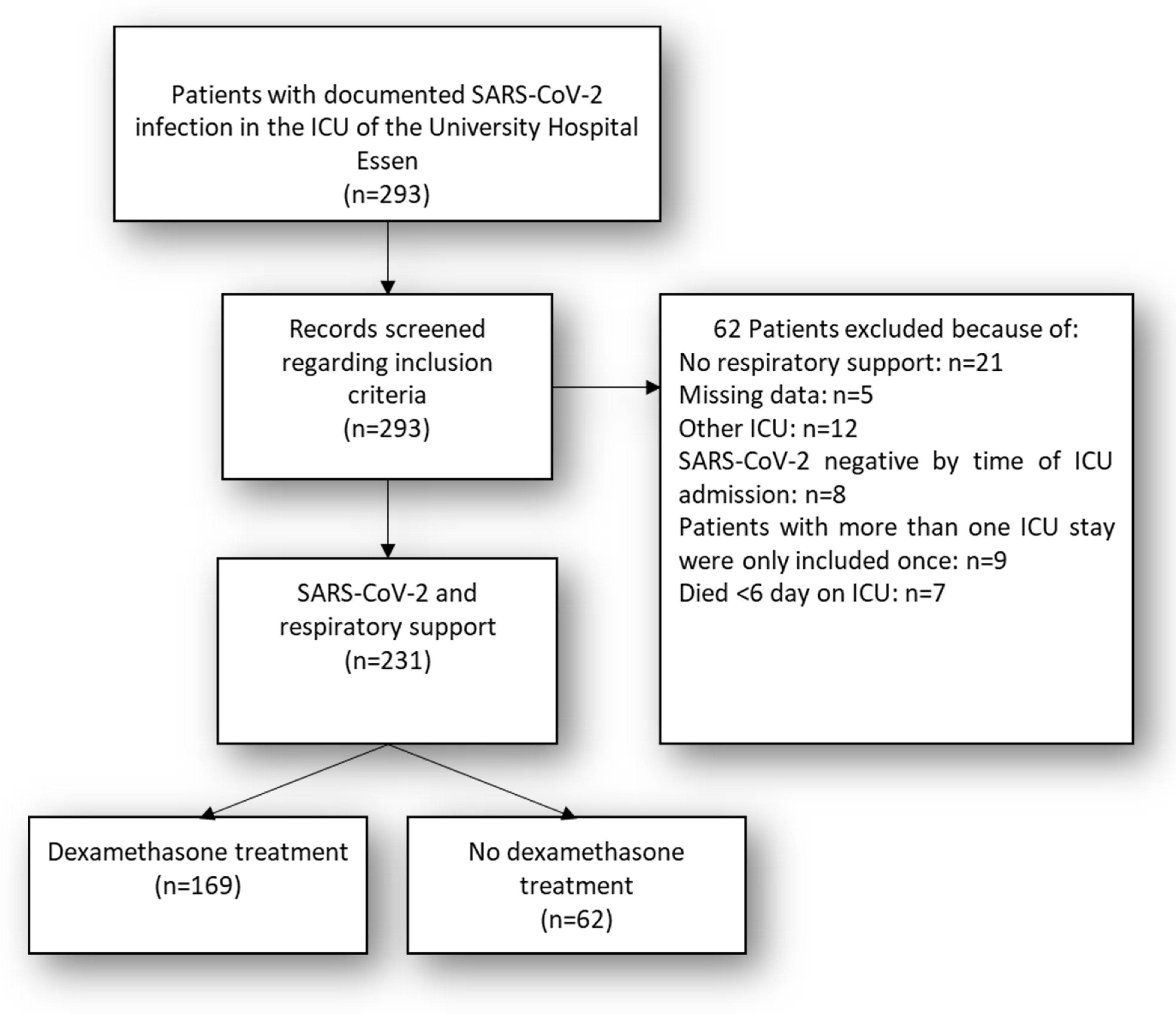
2. Materials and Methods
2.1. Treatment protocols
2.2. Definitions
2.2.1. CAPA
2.2.2. Waves
2.2.3. ARDS
2.3. Sequential Organ Failure Assessment without Glasgow Coma Scale (SOFA Non-GCS)
2.4. Outcome
2.5. Mycological Studies
- ▪
- Blood cultures
- ▪
- Serum
- o
- Galactomannan (GM): twice weekly
- ▪
- BALF
- o
- Gram stain and immediate microscopy
- o
- Cultures
- o
- PCR (multiplex for common causes of pneumonia)
- o
- PCR (Mycobacterium tuberculosis, Pneumocystis jirovecii, Legionella pneumophila)
- o
- PCR [Aspergillus spp. and azole-resistance markers TR34 and TR46.: Aspergillus fumigatus (A. fumigatus), A. terreus, A. flavus]
- o
- PCR (viral panel, including Cytomegalovirus (CMV), Herpes-simplex virus [HSV-1 and HSV-2], Influenza-A, Influenza-B, Respiratory syncytial virus [RSV] and SARS-CoV-2)
- o
- Galactomannan (GM)
- ▪
- Urine
- o
- Culture
- o
- Antigen testing for L. pneumophila and Streptococcus pneumoniae
- ▪
- Nasal and rectal swab
- o
- Screening for Methicillin-resistant Staphylococcus aureus (MRSA), Vancomycin-resistant Enterococcus faecalis/faecium (VRE) and resistant gram-negative pathogens
2.6. Pharmacological Studies and Therapeutic Drug Monitoring (TDM)
3. Statistics
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Kluge, S.; Janssens, U.; Welte, T.; Weber-Carstens, S.; Schälte, G.; Spinner, C.D.; Malin, J.J.; Gastmeier, P.; Langer, F.; Wepler, M.; et al. S3-Leitlinie—Empfehlungen zur stationären Therapie von Patienten mit COVID-19. AWMF Online 2022. [Google Scholar]
- Karagiannidis, C.; Windisch, W.; McAuley, D.F.; Welte, T.; Busse, R. Major differences in ICU admissions during the first and second COVID-19 wave in Germany. Lancet Respir. Med. 2021, 9, e47–e48. [Google Scholar] [CrossRef]
- Koehler, P.; Bassetti, M.; Kochanek, M.; Shimabukuro-Vornhagen, A.; Cornely, O.A. Intensive care management of influenza-associated pulmonary aspergillosis. Clin. Microbiol. Infect. 2019, 25, 1501–1509. [Google Scholar] [CrossRef]
- Schauwvlieghe, A.F.A.D.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van de Veerdonk, F.L.; Gommers, D.; et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- White, P.L.; Dhillon, R.; Cordey, A.; Hughes, H.; Faggian, F.; Soni, S.; Pandey, M.; Whitaker, H.; May, A.; Morgan, M.; et al. A National Strategy to Diagnose Coronavirus Disease 2019-Associated Invasive Fungal Disease in the Intensive Care Unit. Clin. Infect. Dis. 2021, 73, e1634–e1644. [Google Scholar] [CrossRef]
- Mitaka, H.; Kuno, T.; Takagi, H.; Patrawalla, P. Incidence and mortality of COVID-19-associated pulmonary aspergillosis: A systematic review and meta-analysis. Mycoses 2021, 64, 993–1001. [Google Scholar] [CrossRef]
- Chong, W.H.; Saha, B.K.; Neu, K.P. Comparing the clinical characteristics and outcomes of COVID-19-associate pulmonary aspergillosis (CAPA): A systematic review and meta-analysis. Infection 2022, 50, 43–56. [Google Scholar] [CrossRef]
- Kariyawasam, R.M.; Dingle, T.C.; Kula, B.E.; Vandermeer, B.; Sligl, W.I.; Schwartz, I.S. Defining COVID-19-associated pulmonary aspergillosis: Systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 920–927. [Google Scholar] [CrossRef]
- Prattes, J.; Wauters, J.; Giacobbe, D.R.; Salmanton-Garcia, J.; Maertens, J.; Bourgeois, M.; Reynders, M.; Rutsaert, L.; Van Regenmortel, N.; Lormans, P.; et al. Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients-a multinational observational study by the European Confederation of Medical Mycology. Clin. Microbiol. Infect. 2022, 28, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Prattes, J.; Wauters, J.; Giacobbe, D.R.; Lagrou, K.; Hoenigl, M. on behalf of the ECMM-CAPA Study Group. Diagnosis and treatment of COVID-19 associated pulmonary apergillosis in critically ill patients: Results from a European confederation of medical mycology registry. Intensive Care Med. 2021, 47, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Brüggemann, R.J.M.; Azoulay, E.; Bassetti, M.; Blot, S.; Buil, J.B.; Calandra, T.; Chiller, T.; Clancy, C.J.; Cornely, O.A.; et al. Taskforce report on the diagnosis and clinical management of COVID-19 associated pulmonary aspergillosis. Intensive Care Med. 2021, 47, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Salmanton-García, J.; Sprute, R.; Stemler, J.; Bartoletti, M.; Dupont, D.; Valerio, M.; García-Vidal, C.; Falces-Romero, I.; Machado, M.; de la Villa, S.; et al. COVID-19-Associated Pulmonary Aspergillosis, March-August 2020. Emerg. Infect. Dis. 2021, 27, 1077–1086. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Geng, Y.; Li, K.; Wu, H.; Chen, J.; Chai, X.; Li, S.; Zheng, D. Fine-Grained Assessment of COVID-19 Severity Based on Clinico-Radiological Data Using Machine Learning. Int. J. Environ. Res. Public Health 2022, 19, 10665. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef]
- Latgé, J.-P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, 1–75. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti, M.; Pascale, R.; Cricca, M.; Rinaldi, M.; Maccaro, A.; Bussini, L.; Fornaro, G.; Tonetti, T.; Pizzilli, G.; Francalanci, E.; et al. Epidemiology of Invasive Pulmonary Aspergillosis Among Intubated Patients With COVID-19: A Prospective Study. Clin. Infect. Dis. 2021, 73, e3606–e3614. [Google Scholar] [CrossRef]
- Gregoire, E.; Pirotte, B.F.; Moerman, F.; Altdorfer, A.; Gaspard, L.; Firre, E.; Moonen, M.; Fraipont, V.; Ernst, M.; Darcis, G. Incidence and Risk Factors of COVID-19-Associated Pulmonary Aspergillosis in Intensive Care Unit-A Monocentric Retrospective Observational Study. Pathogens 2021, 10, 1370. [Google Scholar] [CrossRef]
- Gangneux, J.-P.; Dannaoui, E.; Fekkar, A.; Luyt, C.-E.; Botterel, F.; De Prost, N.; Tadié, J.-M.; Reizine, F.; Houzé, S.; Timsit, J.F.; et al. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: The French multicentre MYCOVID study. Lancet Respir. Med. 2022, 10, 180–190. [Google Scholar] [CrossRef]
- Ergün, M.; Brüggemann, R.J.M.; Alanio, A.; Dellière, S.; van Arkel, A.; Bentvelsen, R.G.; Rijpstra, T.; van der Sar-van der Brugge, S.; Lagrou, K.; Janssen, N.A.F.; et al. Aspergillus Test Profiles and Mortality in Critically Ill COVID-19 Patients. J. Clin. Microbiol. 2021, 59, e0122921. [Google Scholar] [CrossRef]
- Herbstreit, F.; Overbeck, M.; Berger, M.M.; Skarabis, A.; Brenner, T.; Schmidt, K. Characteristics of Critically Ill Patients with COVID-19 Compared to Patients with Influenza-A Single Center Experience. J. Clin. Med. 2021, 10, 2056. [Google Scholar] [CrossRef]
- RKI. Aktualisierung zur “Retrospektiven Phaseneinteilung der COVID-19-Pandemie in Deutschland”. Epidemiologisches Bulletin, 16 September 2021; p. 13. [Google Scholar]
- ARDS Definition of Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Dubler, S.; Lenz, M.; Zimmermann, S.; Richter, D.C.; Weiss, K.H.; Mehrabi, A.; Mieth, M.; Bruckner, T.; Weigand, M.A.; Brenner, T.; et al. Does vancomycin resistance increase mortality in Enterococcus faecium bacteraemia after orthotopic liver transplantation? A retrospective study. Antimicrob. Resist. Infect. Control 2020, 9, 22. [Google Scholar] [CrossRef]
- BIO-RAD. PlateliaTM Aspergillus EIA 96 TESTS. 2009. Available online: https://commerce.bio-rad.com/webroot/web/pdf/inserts/CDG/en/62796_881045_EN.pdf (accessed on 27 November 2022).
- Kirchhoff, L.; Braun, L.M.; Schmidt, D.; Dittmer, S.; Dedy, J.; Herbstreit, F.; Stauf, R.; Steckel, N.K.; Buer, J.; Rath, P.M.; et al. COVID-19-associated pulmonary aspergillosis in ICU patients in a German reference centre: Phenotypic and molecular characterisation of Aspergillus fumigatus isolates. Mycoses 2022, 65, 458–465. [Google Scholar] [CrossRef]
- Chung, H.; Lee, H.; Han, H.K.; An, H.; Lim, K.S.; Lee, Y.J.; Cho, J.Y.; Yoon, S.H.; Jang, I.J.; Yu, K.S. A pharmacokinetic comparison of two voriconazole formulations and the effect of CYP2C19 polymorphism on their pharmacokinetic profiles. Drug Des. Dev. Ther. 2015, 9, 2609–2616. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, J.; Huelsewede, J.; Buer, J.; Rath, P.-M. Comparison and evaluation of a novel bioassay and high-performance liquid chromatography for the clinical measurement of serum voriconazole concentrations. Mycoses 2011, 54, e421–e428. [Google Scholar] [CrossRef] [PubMed]
- Lahmer, T.; Kriescher, S.; Herner, A.; Rothe, K.; Spinner, C.D.; Schneider, J.; Mayer, U.; Neuenhahn, M.; Hoffmann, D.; Geisler, F.; et al. Invasive pulmonary aspergillosis in critically ill patients with severe COVID-19 pneumonia: Results from the prospective AspCOVID-19 study. PLoS ONE 2021, 16, e0238825. [Google Scholar] [CrossRef]
- Riera, J.; Roncon-Albuquerque, R.; Fuset, M.P.; Alcantara, S.; Blanco-Schweizer, P. on behalf of the ECMOVIBER Study Group. Increased mortality in patients with COVID-19 receiving extracorporeal respiratory support during the second wave of the pandemic. Intens Care Med. 2021, 47, 1490–1493. [Google Scholar] [CrossRef] [PubMed]
- Fekkar, A.; Lampros, A.; Mayaux, J.; Poignon, C.; Demeret, S.; Constantin, J.-M.; Marcelin, A.-G.; Monsel, A.; Luyt, C.-E.; Blaize, M. Occurrence of Invasive Pulmonary Fungal Infections in Patients with Severe COVID-19 Admitted to the ICU. Am. J. Respir. Crit. Care Med. 2021, 203, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Goncer, I.; Thomas, S.; Foden, P.; Richardson, M.D.; Ashworth, A.; Barker, J.; Geraghty, C.G.; Muldoon, E.G.; Felton, T.W. Invasive pulmonary aspergillosis is associated with adverse clinical outcomes in critically ill patients receiving veno-venous extracorporeal membrane oxygenation. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Azoulay, E.; Kullberg, B.-J.; Ruhnke, M.; Shoham, S.; Vazquez, J.; Giacobbe, D.R.; Calandra, T. EORTC/MSGERC Definitions of Invasive Fungal Diseases: Summary of Activities of the Intensive Care Unit Working Group. Clin. Infect. Dis. 2021, 72, S121–S127. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfari, M.; Arastehfar, A.; Davoodi, L.; Charati, J.Y.; Moazeni, M.; Abastabar, M.; Haghani, I.; Mirzakhani, R.; Mayahi, S.; Fang, W.; et al. Pervasive but Neglected: A Perspective on COVID-19-Associated Pulmonary Mold Infections Among Mechanically Ventilated COVID-19 Patients. Front. Med. 2021, 8, 649675. [Google Scholar] [CrossRef]
- Permpalung, N.; Chiang, T.P.; Massie, A.B.; Zhang, S.X.; Avery, R.K.; Nematollahi, S.; Ostrander, D.; Segev, D.L.; Marr, K.A. Coronavirus Disease 2019—Associated Pulmonary Aspergillosis in Mechanically Ventilated Patients. Clin. Infect. Dis. 2022, 74, 83–91. [Google Scholar] [CrossRef]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef]
- Mircescu, M.M.; Lipuma, L.; van Rooijen, N.; Pamer, E.G.; Hohl, T.M. Essential role for neutrophils but not alveolar macrophages at early time points following Aspergillus fumigatus infection. J. Infect. Dis. 2009, 200, 647–656. [Google Scholar] [CrossRef]
- Yang, D.; Chu, H.; Hou, Y.; Chai, Y.; Shuai, H.; Lee, A.C.-Y.; Zhang, X.; Wang, Y.; Hu, B.; Huang, X.; et al. Attenuated Interferon and Proinflammatory Response in SARS-CoV-2—Infected Human Dendritic Cells Is Associated With Viral Antagonism of STAT1 Phosphorylation. J. Infect. Dis. 2020, 222, 734–745. [Google Scholar] [CrossRef]
| DEXA (n = 169) | Non-DEXA (n = 62) | p | |
|---|---|---|---|
| Demographics | |||
| Age [years] # | 61.03 ± 13.82 | 57.11 ± 14.89 | 0.072 |
| Male | 117 (69.2%) | 53 (85.5%) | 0.013 * |
| Co-morbidity | |||
| BMI [kg/m2] # | 28.63 ± 5.58 | 31.27 ± 7.82 | 0.018 * |
| Smoker | 5 (8.1%) | 11 (6.5%) | 0.680 |
| CCI # | 3.37 ± 3.11 | 2.43 ± 2.30 | 0.013 * |
| SAPS II # | 34.10 ± 15.14 | 33.67 ± 14.59 | 0.854 |
| Horowitz Index # | 184.77 ± 131.79 | 139.35 ± 73.27 | 0.001 ** |
| Non-GCS SOFA | 7.65 ± 3.08 | 7.62 ± 3.25 | 0.963 |
| Complications | |||
| Bacteraemia | 33 (53.2%) | 98 (58.0%) | 0.494 |
| Candidemia | 6 (9.7%) | 10 (5.9%) | 0.319 |
| Probable CAPA | 4 (6.5%) | 13 (7.7%) | 0.749 |
| Invasive ventilation | 48 (77.4%) | 113 (66.9%) | 0.122 |
| Kidney replacement therapy | 27 (43.5%) | 67 (39.6%) | 0.593 |
| ECMO | 19 (30.6%) | 53 (31.4%) | 0.917 |
| Outcome | |||
| Length of ICU stay [days] # | 14.16 ± 12.60 | 13.68 ± 12.41 | 0.795 |
| Survivor | 97 (57.4%) | 37 (59.7%) | 0.756 |
| pCAPA (n = 17) | Non-CAPA (n = 214) | p | |
|---|---|---|---|
| Demographics | |||
| Age [years] # | 59.41 ± 14.38 | 58.06 ± 14.73 | 0.716 |
| Male | 14 (82.4%) | 156 (72.9%) | 0.395 |
| Co-morbidity | |||
| BMI [kg/m2] # | 31.45 ± 7.05 | 30.49 ± 7.41 | 0.608 |
| Smoker | 2 (11.76%) | 14 (6.54%) | 0.414 |
| Leucocytes [106/nl] | 14.83 ± 7.34 | 24.24 ± 145.78 | 0.790 |
| Dexamethasone | 13 (76.5%) | 156 (72.9%) | 0.100 |
| CCI # | 2.82 ± 2.24 | 2.67 ± 2.60 | 0.811 |
| SAPS II # | 41.18 ± 9.05 | 33.19 ± 14.91 | 0.036 |
| Horowitz Index # | 141.60 ± 71.38 | 152.36 ± 96.26 | 0.653 |
| Non-GCS SOFA | 8.59 ± 2.58 | 7.52 ± 3.25 | 0.192 |
| Complications | |||
| Bacteraemia | 15 (88.24%) | 113 (52.80%) | 0.018 * |
| Candidemia | 2 (11.76%) | 14 (6.54%) | 0.414 |
| Intubation | 17 (100%) | 144 (67.29%) | 0.005 ** |
| Kidney replacement therapy | 12 (70.59%) | 82 (38.32%) | 0.009 ** |
| ECMO | 9 (52.94%) | 63 (29.44%) | 0.044 * |
| Wave 1 a | 3 (7.69%) | 36 (92.31%) | 0.930 |
| Wave 2 b | 14 (7.29%) | 178 (92.71%) | 0.930 |
| Outcome | |||
| Length of ICU stay [days] # | 22.71 ± 12.34 | 13.10 ± 12.19 | 0.002 ** |
| Survivor | 5 (29.41%) | 129 (60.28%) | 0.013 * |
| N = 155 § | Odds Ratio (95% CI) | p |
|---|---|---|
| Multivariable Analysis | ||
| Age | 1.015 (0.957–1.076) | 0.628 |
| BMI [kg/m2] | 1.024 (0.956–1.098) | 0.497 |
| Smoker | 0.218 (0.307–1.543) | 0.127 |
| CCI | 0.032 (0.767–1.389) | 0.835 |
| SAPS II | 1.055 (0.971–1.147) | 0.203 |
| Non-GCS SOFA | 1.023 (0.813–1.287) | 0.847 |
| Dexamethasone | 0.585 (0.503–6.801) | 0.669 |
| Horowitz Index | 1.003 (0.995–1.010) | 0.463 |
| Dialysis | 1.146 (0.262–5.010) | 0.857 |
| ECMO | 0.847 (0.255–2.811) | 0.787 |
| Time in ICU [days] | 1.048 (1.004–1.095) | 0.033 ** |
| Waves | 0.730 (0.044–12.101) | 0.827 |
| N = 155 § | Odds Ratio (95% CI) | p |
|---|---|---|
| Multivariable Analysis | ||
| Age | 1.038 (0.978–1.103) | 0.218 |
| BMI [kg/m2] | 1.020 (0.947–1.097) | 0.605 |
| Smoker | 1.499 (0.163–13.786) | 0.721 |
| CCI | 1.0933 (0.845–1.414) | 0.497 |
| SAPS II | 1.086 (1.001–1.177) | 0.046 * |
| Non-GCS SOFA | 1.423 (1.19–1.809) | 0.004 ** |
| Dexamethasone | 0.722 (0.067–7.830) | 0.789 |
| probable CAPA | 0.416 (0.063–2.731) | 0.361 |
| Horowitz Index | 0.989 (0.982–0.997) | 0.006 ** |
| Kidney replacement therapy | 0.246 (0.069–0.876) | 0.030 * |
| ECMO | 0.122 (0.036–0.414) | 0.001 ** |
| Length of ICU stay [days] | 0.945 (0.902–0.990) | 0.017 * |
| Waves | 0.898 (0.066–12.282 | 0.936 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubler, S.; Turan, Ö.C.; Schmidt, K.D.; rath, P.-m.; Verhasselt, H.-L.; Maier, S.; Skarabis, A.; Brenner, T.; Herbstreit, F. Effect of Dexamethasone on the Incidence and Outcome of COVID-19 Associated Pulmonary Aspergillosis (CAPA) in Critically Ill Patients during First- and Second Pandemic Wave—A Single Center Experience. Diagnostics 2022, 12, 3049. https://doi.org/10.3390/diagnostics12123049
Dubler S, Turan ÖC, Schmidt KD, rath P-m, Verhasselt H-L, Maier S, Skarabis A, Brenner T, Herbstreit F. Effect of Dexamethasone on the Incidence and Outcome of COVID-19 Associated Pulmonary Aspergillosis (CAPA) in Critically Ill Patients during First- and Second Pandemic Wave—A Single Center Experience. Diagnostics. 2022; 12(12):3049. https://doi.org/10.3390/diagnostics12123049
Chicago/Turabian StyleDubler, Simon, Ömer Can Turan, Karsten Daniel Schmidt, Peter-michael rath, Hedda-Luise Verhasselt, Sandra Maier, Annabell Skarabis, Thorsten Brenner, and Frank Herbstreit. 2022. "Effect of Dexamethasone on the Incidence and Outcome of COVID-19 Associated Pulmonary Aspergillosis (CAPA) in Critically Ill Patients during First- and Second Pandemic Wave—A Single Center Experience" Diagnostics 12, no. 12: 3049. https://doi.org/10.3390/diagnostics12123049
APA StyleDubler, S., Turan, Ö. C., Schmidt, K. D., rath, P.-m., Verhasselt, H.-L., Maier, S., Skarabis, A., Brenner, T., & Herbstreit, F. (2022). Effect of Dexamethasone on the Incidence and Outcome of COVID-19 Associated Pulmonary Aspergillosis (CAPA) in Critically Ill Patients during First- and Second Pandemic Wave—A Single Center Experience. Diagnostics, 12(12), 3049. https://doi.org/10.3390/diagnostics12123049







