Fluorescence Melting Curve Analysis for Concurrent Genotyping of Three Tag SNPs in FUT3
Abstract
:1. Introduction
2. Materials and Methods
2.1. DNA Samples
2.2. Probe-Based FMCA for Concurrent Genotyping of c.59T>G, c.314C>T, c.484G>A of FUT3
3. Results
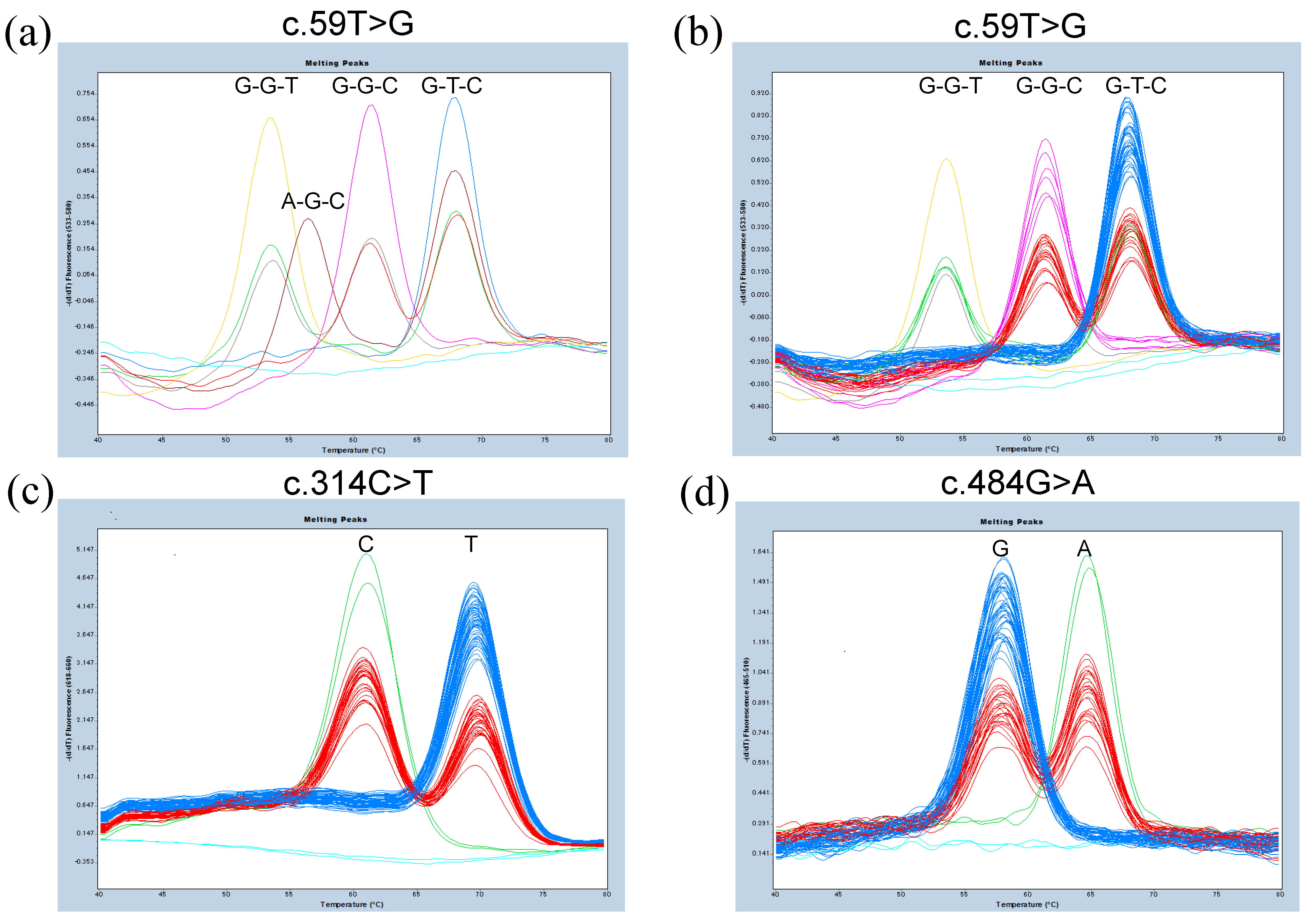
3.1. Probe-Based FMCA for Genotyping of c.59T>G, c.314C>T, and c.484G>A of FUT3
3.2. Validation of Probe-Based FMCA for Genotyping of Three SNPs
3.3. Inferred Le Phenotypes from Combinations of Three SNPs Genotypes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daniels, G. ABO, H, and Lewis Systems. In Human Blood Groups, 3rd ed.; Daniels, G., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 11–95. [Google Scholar]
- Crookston, M.C. Blood group antigens acquired from the plasma. Prog. Clin. Biol. Res. 1980, 43, 99–114. [Google Scholar] [PubMed]
- Henry, S.; Mollicone, R.; Lowe, J.B.; Samuelsson, B.; Larson, G. A second nonsecretor allele of the blood group alpha(1,2)fucosyl-transferase gene (FUT2). Vox Sang. 1996, 70, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Good, A.H.; Yau, O.; Lamontagne, L.R.; Oriol, R. Serological and chemical specificities of twelve monoclonal anti-Lea and anti-Leb antibodies. Vox Sang. 1992, 62, 180–189. [Google Scholar] [CrossRef]
- Koda, Y.; Soejima, M.; Kimura, H. The polymorphisms of fucosyltransferases. Leg. Med. (Tokyo) 2001, 3, 2–14. [Google Scholar] [CrossRef]
- Soejima, M.; Koda, Y. Molecular mechanisms of Lewis antigen expression. Leg. Med. (Tokyo) 2005, 7, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Elmgren, A.; Borjeson, C.; Svensson, L.; Rydberg, L.; Larson, G. DNA sequencing and screening for point mutations in the human Lewis (FUT3) gene enables molecular genotyping of the human Lewis blood group system. Vox Sang. 1996, 70, 97–103. [Google Scholar] [CrossRef]
- Orntoft, T.F.; Vestergaard, E.M.; Holmes, E.; Jakobsen, J.S.; Grunnet, N.; Mortensen, M.; Johnson, P.; Bross, P.; Gregersen, N.; Skorstengaard, K.; et al. Influence of Lewis alpha1-3/4-L-fucosyltransferase (FUT3) gene mutations on enzyme activity, erythrocyte phenotyping, and circulating tumor marker sialyl-Lewis a levels. J. Biol. Chem. 1996, 271, 32260–32268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, H.; Koda, Y.; Soejima, M.; Kimura, H. Significance of each of three missense mutations, G484A, G667A, and G808A, present in an inactive allele of the human Lewis gene (FUT3) for alpha(1,3/1,4)fucosyltransferase inactivation. Glycoconj. J. 1998, 15, 961–967. [Google Scholar] [CrossRef]
- Pang, H.; Liu, Y.; Koda, Y.; Soejima, M.; Jia, J.; Schlaphoff, T.; Du Toit, E.D.; Kimura, H. Five novel missense mutations of the Lewis gene (FUT3) in African (Xhosa) and Caucasian populations in South Africa. Hum. Genet. 1998, 102, 675–680. [Google Scholar] [CrossRef]
- Moller, M.; Joud, M.; Storry, J.R.; Olsson, M.L. Erythrogene: A database for in-depth analysis of the extensive variation in 36 blood group systems in the 1000 Genomes Project. Blood Adv. 2016, 1, 240–249. [Google Scholar] [CrossRef]
- Nishihara, S.; Hiraga, T.; Ikehara, Y.; Iwasaki, H.; Kudo, T.; Yazawa, S.; Morozumi, K.; Suda, Y.; Narimatsu, H. Molecular behavior of mutant Lewis enzymes in vivo. Glycobiology 1999, 9, 373–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soejima, M.; Munkhtulga, L.; Iwamoto, S.; Koda, Y. Genetic variation of FUT3 in Ghanaians, Caucasians, and Mongolians. Transfusion 2009, 49, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Soejima, M.; Koda, Y. Estimation of Lewis-negative alleles by high-resolution melting analysis of three tag SNPs of FUT3. Vox Sang. 2022, 117, 282–287. [Google Scholar] [CrossRef] [PubMed]
- El Housni, H.; Heimann, P.; Parma, J.; Vassart, G. Single-nucleotide polymorphism genotyping by melting analysis of dual-labeled probes: Examples using factor V Leiden and prothrombin 20210A mutations. Clin. Chem. 2003, 49, 1669–1672. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Liu, Z.; Liao, Y.; Chen, X.; Zhang, Y.; Li, Q. Multiplex fluorescence melting curve analysis for mutation detection with dual-labeled, self-quenched probes. PLoS ONE 2011, 6, e19206. [Google Scholar] [CrossRef]
- Weston, B.W.; Nair, R.P.; Larsen, R.D.; Lowe, J.B. Isolation of a novel human alpha (1,3)fucosyltransferase gene and molecular comparison to the human Lewis blood group alpha (1,3/1,4)fucosyltransferase gene. Syntenic, homologous, nonallelic genes encoding enzymes with distinct acceptor substrate specificities. J. Biol. Chem. 1992, 267, 4152–4160. [Google Scholar]
- Weston, B.W.; Smith, P.L.; Kelly, R.J.; Lowe, J.B. Molecular cloning of a fourth member of a human alpha (1,3)fucosyltransferase gene family. Multiple homologous sequences that determine expression of the Lewis x, sialyl Lewis x, and difucosyl sialyl Lewis x epitopes. J. Biol. Chem. 1992, 267, 24575–24584. [Google Scholar] [CrossRef]
- Soejima, M.; Koda, Y. Rapid genotyping of 508G>A (rs3745635) and 1067T>A (rs3894326) of FUT3 by a duplex Eprobe-mediated melting curve analysis. Vox Sang. 2022, 117, 741–745. [Google Scholar] [CrossRef]
- Boren, T.; Falk, P.; Roth, K.A.; Larson, G.; Normark, S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 1993, 262, 1892–1895. [Google Scholar] [CrossRef]
- Cakir, B.; Heiss, G.; Pankow, J.S.; Salomaa, V.; Sharrett, A.R.; Couper, D.; Weston, B.W. Association of the Lewis genotype with cardiovascular risk factors and subclinical carotid atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) study. J. Intern. Med. 2004, 255, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.; Zhang, D.; Zheng, S.; Guo, M.; Lin, X.; Jiang, Y. Association of ulcerative colitis with FUT2 and FUT3 polymorphisms in patients from Southeast China. PLoS ONE 2016, 11, e0146557. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Han, R.; Chen, M.; Liu, R.; Wu, M.; Zhang, X.; Ma, Y.; Yuan, Y.; Wang, R.; Shuai, Z.; et al. Associations between fucosyltransferase 3 gene polymorphisms and ankylosing spondylitis: A case-control study of an east Chinese population. PLoS ONE 2020, 15, e0237219. [Google Scholar] [CrossRef] [PubMed]
- Matzhold, E.M.; Berghold, A.; Bemelmans, M.K.B.; Banfi, C.; Stelzl, E.; Kessler, H.H.; Steinmetz, I.; Krause, R.; Wurzer, H.; Schlenke, P.; et al. Lewis and ABO histo-blood types and the secretor status of patients hospitalized with COVID-19 implicate a role for ABO antibodies in susceptibility to infection with SARS-CoV-2. Transfusion 2021, 61, 2736–2745. [Google Scholar] [CrossRef] [PubMed]
- Klove-Mogensen, K.; Steffensen, R.; Masmas, T.N.; Glenthoj, A.; Haunstrup, T.M.; Ratcliffe, P.; Hoglund, P.; Hasle, H.; Nielsen, K.R. ABO, secretor, and Lewis carbohydrate histo-blood groups are associated with autoimmune neutropenia of early childhood in Danish patients. Transfusion 2022, 62, 1636–1642. [Google Scholar] [CrossRef]
- Grahn, A.; Elmgren, A.; Aberg, L.; Svensson, L.; Jansson, P.A.; Lonnroth, P.; Larson, G. Determination of Lewis FUT3 gene mutations by PCR using sequence-specific primers enables efficient genotyping of clinical samples. Hum. Mutat. 2001, 18, 358–359. [Google Scholar] [CrossRef]
- Cakir, B.; Pankow, J.S.; Salomaa, V.; Couper, D.; Morris, T.L.; Brantley, K.R.; Hiller, K.M.; Heiss, G.; Weston, B.W. Distribution of Lewis (FUT3) genotype and allele: Frequencies in a biethnic United States population. Ann. Hematol. 2002, 81, 558–565. [Google Scholar] [CrossRef]
- Djousse, L.; Karamohamed, S.; Herbert, A.G.; D’Agostino, R.B.; Cupples, L.A.; Ellison, R.C. Fucosyltransferase 3 polymorphism and atherothrombotic disease in the Framingham Offspring Study. Am. Heart J. 2007, 153, 636–639. [Google Scholar] [CrossRef] [Green Version]
- Hanami, T.; Delobel, D.; Kanamori, H.; Tanaka, Y.; Kimura, Y.; Nakasone, A.; Soma, T.; Hayashizaki, Y.; Usui, K.; Harbers, M. Eprobe mediated real-time PCR monitoring and melting curve analysis. PLoS ONE 2013, 8, e70942. [Google Scholar] [CrossRef] [Green Version]
- Wittwer, C.T.; Reed, G.H.; Gundry, C.N.; Vandersteen, J.G.; Pryor, R.J. High-resolution genotyping by amplicon melting analysis using LCGreen. Clin. Chem. 2003, 49, 853–860. [Google Scholar] [CrossRef] [Green Version]
- Soejima, M.; Koda, Y. Simultaneous genotyping of three major Se enzyme inactivating SNPs of FUT2 based on a triplex probe-based fluorescence melting-curve analysis. Clin. Chim. Acta 2022, 530, 50–54. [Google Scholar] [CrossRef]

| Primer Sequences | Position of FUT3 | Differences with FUT5 | Differences with FUT6 | Amplicon Length |
|---|---|---|---|---|
| Detection of c.59T>G | ||||
| forward primer: 5′-CCTCTCTCCTCTCTTCCCAGA-3′ | −32 to −12 bp | 1 | 2 | 179 bp |
| reverse primer: 5′-ACTGGGAGCCCTAGGGGAT-3′ | 129 to 147 bp | 6 | 10 | |
| 59T-probe: 5′-HEX-TCTGGCCGCACTGCTATTTCAGC-BHQ1-3′ | 48 to 70 bp | |||
| Detection of c.314C>T | ||||
| forward primer: 5′-TGTGGCTCTGTCCCGCTGT-3′ | 225 to 243 bp | 3 | 5 | 140 bp |
| reverse primer: 5′-GGAGGCGTGACTTAGGGTTGG-3′ | 344 to 364 bp | 8 | 8 | |
| 314C-probe: 5′-Cy5-ACAGGCAGACACGGTCATCGT-BHQ2-3′ | 303 to 323 bp | |||
| Detection of c.484G>A | ||||
| forward primer: 5′-AGCCACCCCCTAACTGCCA-3′ | 413 to 431bp | 6 | 9 | 158 bp |
| reverse primer: 5′-GGTCTTGGCCGAGAGGTTGA-3′ | 551 to 570 bp | 0 | 0 | |
| 484A-probe: 5′-FAM-CAGCAACTCCGACATCTTCA-BHQ1-3′ | 480 to 499 bp |
| 55-59-61/55-59-61 | Tm (°C) | |
|---|---|---|
| Subject 1 | G-T-C/G-T-C | 68.0 |
| Subject 2 | G-T-C/G-G-C | 68.0/61.5 |
| Subject 3 | G-G-C/G-G-C | 61.5 |
| Subject 4 | G-T-C/G-G-T | 68.5/53.5 |
| Subject 5 | G-G-C/G-G-T | 61.5/53.5 |
| Subject 6 | G-G-T/G-G-T | 53.5 |
| Subject 7 | G-T-C/A-G-C | 68.0/56.5 |
| DNA not available | G-G-C/A-G-C | - |
| DNA not available | A-G-C/A-G-C | - |
| DNA not available | G-G-T/A-G-C | - |
| c.59T>G | c.314C>T | c.484G>A | Lewis Genotype | Lewis Phenotype | Number of Ghanaians | Number of Caucasians |
|---|---|---|---|---|---|---|
| T/T | C/C | G/G | Le/Le | positive | 23 | 51 |
| T/T | C/C | G/A | Le/le | positive | 23 | 0 |
| T/G | C/C | G/A | le/le | negative | 9 | 0 |
| T/T | C/T | G/A | le/le | negative | 7 | 0 |
| T/T | C/C | A/A | le/le | negative | 2 | 0 |
| T/G | C/C | G/G | Le/le | positive | 17 | 8 |
| T/G | C/T | G/G | le/le | negative | 3 | 7 |
| G/G | C/C | G/G | le/le | negative | 14 | 1 |
| T/T | C/T | G/G | Le/le | positive | 8 | 31 |
| T/T | T/T | G/G | le/le | negative | 0 | 2 |
| SNPs | Inferred | Actual | Frequency (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Africa | America | E. Asia | Europe | S. Asia | Global | |||
| 59T-508A | Le | le | 0.23 | 0 | 0.20 | 0 | 0.20 | 0.14 |
| 59T-1067A | Le | le | 0.08 | 0 | 0.60 | 0.10 | 0.30 | 0.22 |
| 202C-314C | Le | le | 0.38 | 0 | 0 | 0 | 0.40 | 0.18 |
| 59G-484A-1067A | le | le | 0 | 0 | 0.10 | 0 | 0 | 0.02 |
| Uncharacterized | Le | ? | 1.60 | 0.99 | 0.60 | 1.10 | 1.32 | 1.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soejima, M.; Koda, Y. Fluorescence Melting Curve Analysis for Concurrent Genotyping of Three Tag SNPs in FUT3. Diagnostics 2022, 12, 3039. https://doi.org/10.3390/diagnostics12123039
Soejima M, Koda Y. Fluorescence Melting Curve Analysis for Concurrent Genotyping of Three Tag SNPs in FUT3. Diagnostics. 2022; 12(12):3039. https://doi.org/10.3390/diagnostics12123039
Chicago/Turabian StyleSoejima, Mikiko, and Yoshiro Koda. 2022. "Fluorescence Melting Curve Analysis for Concurrent Genotyping of Three Tag SNPs in FUT3" Diagnostics 12, no. 12: 3039. https://doi.org/10.3390/diagnostics12123039
APA StyleSoejima, M., & Koda, Y. (2022). Fluorescence Melting Curve Analysis for Concurrent Genotyping of Three Tag SNPs in FUT3. Diagnostics, 12(12), 3039. https://doi.org/10.3390/diagnostics12123039





