Diagnostic and Therapeutic Challenges of Malignant Pleural Mesothelioma
Abstract
1. Introduction
2. Genetics and Risk Factors of Malignant Pleural Mesothelioma
2.1. Asbestos-Related Mesothelioma
2.2. Non Asbestos-Related Mesothelioma
2.3. Mesothelioma and BAP-1 Hereditary Cancer Predisposition Syndrome
2.4. The Role of NF2 and CDKN2A in Malignant Mesothelioma
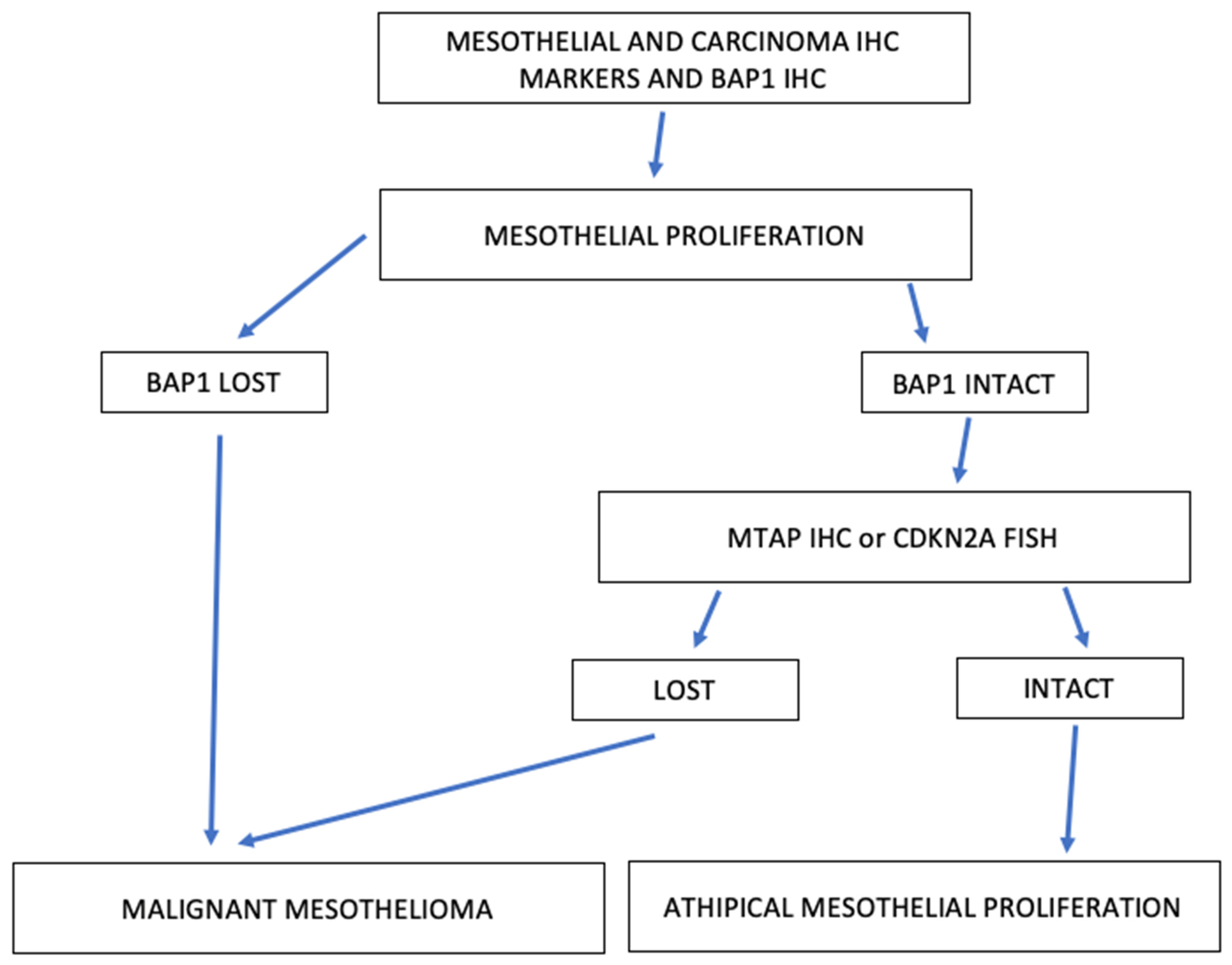
3. Mesothelial Hyperplasia and Mesothelioma In Situ
3.1. Typical and Atypical Mesothelial Hyperplasia
- -
- A reactive proliferation on the surface or at the edge of an organized effusion;
- -
- A pleural surface entrapped in an inflammatory reaction.
3.2. Mesothelioma In Situ
4. Management
4.1. Surgery
4.2. Radiotherapy
4.3. Medical Treatment
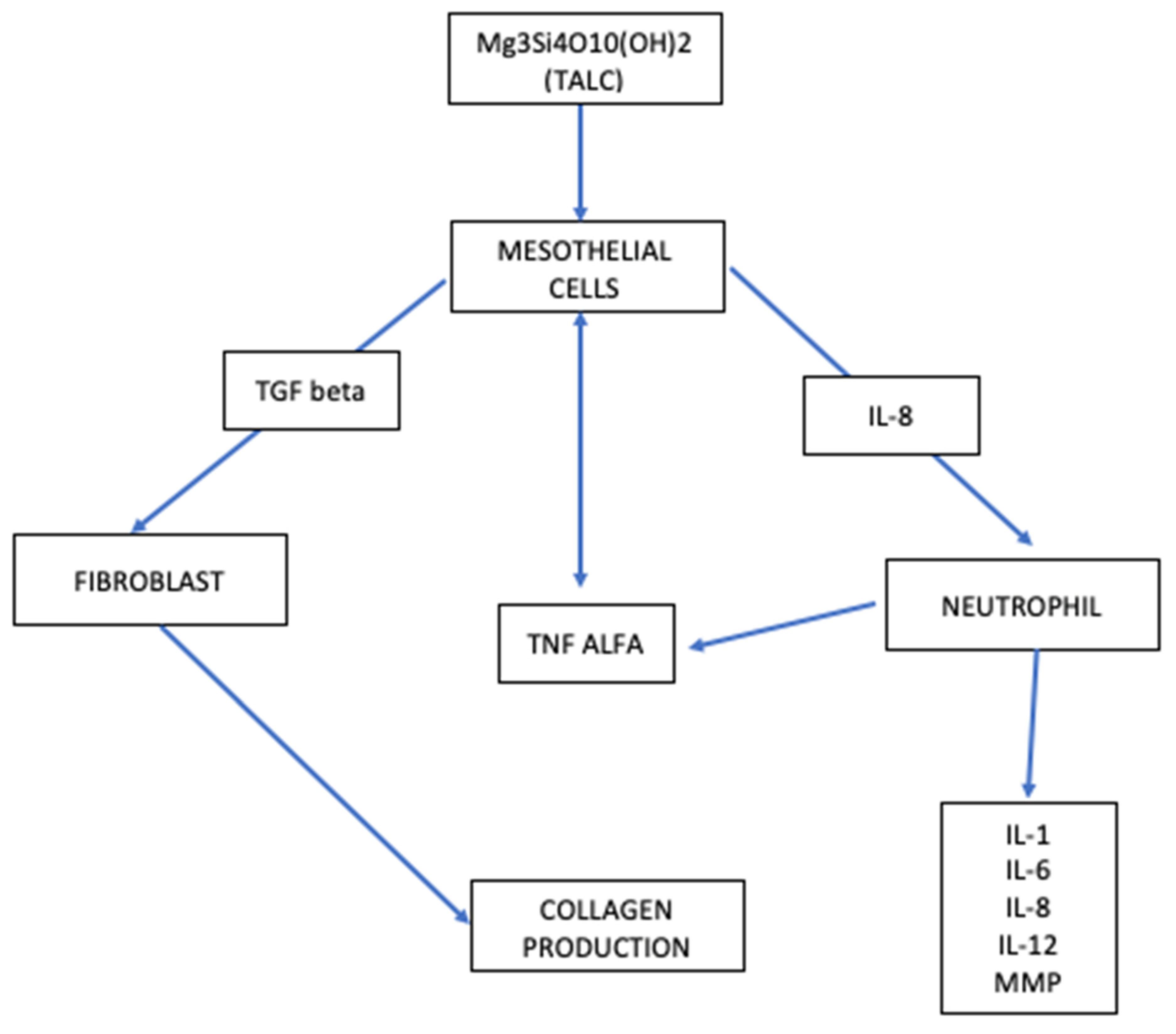
5. Talc: The Potential Link between Mesothelial Hyperplasia and Mesothelioma In Situ
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nakas, A.; Martin-Ucar, A.E.; Edwards, J.G.; Waller, D.A. Localised malignant pleural mesothelioma: A separate clinical entity requiring aggressive local surgery. Eur. J. Cardio-Thoracic Surg. 2008, 33, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Ribak, J.; Lilis, R.; Suzuki, Y.; Penner, L.; Selikoff, I.J. Malignant mesothelioma in a cohort of asbestos insulation workers: Clinical presentation, diagnosis, and causes of death. Occup. Environ. Med. 1988, 45, 182–187. [Google Scholar] [CrossRef] [PubMed]
- SEER Database. Available online: https://seer.cancer.gov (accessed on 20 June 2022).
- Testa, J.R.; Cheung, M.; Pei, J.; Below, J.E.; Tan, Y.; Sementino, E.; Cox, N.J.; Dogan, A.U.; Pass, H.I.; Trusa, S.; et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat. Genet. 2012, 43, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- WHO. Classification of Tumors, 5th Edition-Cap-Tumor of Pleura e Pericardium; WHO: Geneva, Switzerland, 2021; pp. 200–201.
- Meyerhoff, R.R.; Yang, C.-F.J.; Speicher, P.J.; Gulack, B.C.; Hartwig, M.G.; D’Amico, T.A.; Harpole, D.H.; Berry, M.F. Impact of mesothelioma histologic subtype on outcomes in the Surveillance, Epidemiology, and End Results database. J. Surg. Res. 2015, 196, 23–32. [Google Scholar] [CrossRef]
- Yang, H.; Testa, J.R.; Carbone, M. Mesothelioma Epidemiology, Carcinogenesis, and Pathogenesis. Curr. Treat. Options Oncol. 2008, 9, 147–157. [Google Scholar] [CrossRef]
- de Assis, L.; Isoldi, M.C. The function, mechanisms, and role of the genes PTEN and TP53 and the effects of asbestos in the development of malignant mesothelioma: A review focused on the genes’ molecular mechanisms. Tumor Biol. 2013, 35, 889–901. [Google Scholar] [CrossRef]
- Wagner, J.C.; Sleggs, C.A.; Marchand, P. Diffuse Pleural Mesothelioma and Asbestos Exposure in the North Western Cape Province. Occup. Environ. Med. 1960, 17, 260–271. [Google Scholar] [CrossRef]
- Magnani, C.; Fubini, B.; Mirabelli, D.; Bertazzi, P.A.; Bianchi, C.; Chellini, E.; Gennaro, V.; Marinaccio, A.; Menegozzo, M.; Merler, E.; et al. Pleural mesothelioma: Epidemiological and public health issues. Report from the Second Italian Consensus Conference on Pleural Mesotheli-oma. Med. Lav. 2013, 104, 191–202. [Google Scholar]
- McDonald, A.D.; McDonald, J. Mesothelioma after crocidolite exposure during gas mask manufacture. Environ. Res. 1978, 17, 340–346. [Google Scholar] [CrossRef]
- Tan, C.; Treasure, T. Mesothelioma: Time to take stock. J. R. Soc. Med. 2005, 98, 455–458. [Google Scholar] [CrossRef]
- LaDou, J. The asbestos cancer epidemic. Environ. Health Perspect. 2004, 112, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Boutin, C.; Dumortier, P.; Rey, F.; Viallat, J.R.; De Vuyst, P. Black spots concentrate oncogenic asbestos fibers in the parietal pleura. Thoracoscopic and mineralogic study. Am. J. Respir. Crit. Care Med. 1996, 153, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Jaurand, M.C.; Kaplan, H.; Thiollet, J.; Pinchon, M.C.; Bernaudin, J.F.; Bignon, J. Phagocytosis of chrysotile fibers by pleural mesothelial cells in culture. Am. J. Pathol. 1979, 94, 529–538. [Google Scholar]
- Jaurand, M.-C.; Fleury-Feith, J. Pathogenesis of malignant pleural mesothelioma. Respirology 2005, 10, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ernst, J.D.; Broaddus, V.C. Phagocytosis of Crocidolite Asbestos Induces Oxidative Stress, DNA Damage, and Apoptosis in Mesothelial Cells. Am. J. Respir. Cell Mol. Biol. 2000, 23, 371–378. [Google Scholar] [CrossRef]
- Yegles, M.; Saint-Etienne, L.; Renier, A.; Janson, X.; Jaurand, M.-C. Induction of Metaphase and Anaphase/Telophase Abnormalities by Asbestos Fibers in Rat Pleural Mesothelial Cells In Vitro. Am. J. Respir. Cell Mol. Biol. 1993, 9, 186–191. [Google Scholar] [CrossRef]
- Shukla, A.; Gulumian, M.; Hei, T.K.; Kamp, D.; Rahman, Q.; Mossman, B.T. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Free Radic. Biol. Med. 2003, 34, 1117–1129. [Google Scholar] [CrossRef]
- Yang, H.; Bocchetta, M.; Kroczynska, B.; Elmishad, A.G.; Chen, Y.; Liu, Z.; Bubici, C.; Mossman, B.T.; Pass, H.I.; Testa, J.R.; et al. TNF-α inhibits asbestos-induced cytotoxicity via a NF-κB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 10397–10402. [Google Scholar] [CrossRef]
- Bueno, R.; Stawiski, E.W.; Goldstein, L.D.; Durinck, S.; De Rienzo, A.; Modrusan, Z.; Gnad, F.; Nguyen, T.T.; Jaiswal, B.S.; Chirieac, L.R.; et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat. Genet. 2016, 48, 407–416. [Google Scholar] [CrossRef]
- Li, X.; Brownlee, N.A.; Sporn, T.A.; Mahar, A.; Roggli, V.L. Malignant (Diffuse) Mesothelioma in Patients With Hematologic Malignancies: A Clinicopathologic Study of 45 Cases. Arch. Pathol. Lab. Med. 2015, 139, 1129–1136. [Google Scholar] [CrossRef]
- Schubauer-Berigan, M.K.; Daniels, R.D.; Bertke, S.J.; Tseng, C.-Y.; Richardson, D.B. Cancer Mortality through 2005 among a Pooled Cohort of U.S. Nuclear Workers Exposed to External Ionizing Radiation. Radiat. Res. 2015, 183, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Futakuchi, M.; Shimizu, H.; Alexander, D.B.; Yanagihara, K.; Fukamachi, K.; Suzui, M.; Kanno, J.; Hirose, A.; Ogata, A.; et al. Multi-walled carbon nanotubes translocate into the pleural cavity and induce visceral mesothelial proliferation in rats. Cancer Sci. 2012, 103, 2045–2050. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, S.; Kasai, T.; Umeda, Y.; Ohnishi, M.; Sasaki, T.; Matsumoto, M. Carcinogenicity of multi-walled carbon nanotubes: Challenging issue on hazard assessment. J. Occup. Health 2018, 60, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Kodama, Y.; Hoshi, S.; Minami, M.; Kiso, M.; Takezawa, T.; Arai, T.; To, Y.; Teshima, S.; Suzuki, N. Malignant mesothelioma associated with chronic em-pyema with elevation of serum CYFRA19: A case report. Biosci. Trends 2008, 2, 250–254. [Google Scholar]
- Roviaro, G.C.; Sartori, F.; Calabrò, F.; Varoli, F. The association of pleural mesothelioma and tuberculosis. Am. Rev. Respir. Dis. 1982, 126, 569–571. [Google Scholar] [CrossRef]
- Attanoos, R.L.; Churg, A.; Galateau-Salle, F.; Gibbs, A.R.; Roggli, V.L. Malignant Mesothelioma and Its Non-Asbestos Causes. Arch. Pathol. Lab. Med. 2018, 142, 753–760. [Google Scholar] [CrossRef]
- Dostert, C.; Pétrilli, V.; Van Bruggen, R.; Steele, C.; Mossman, B.T.; Tschopp, J. Innate Immune Activation Through Nalp3 Inflammasome Sensing of Asbestos and Silica. Science 2008, 320, 674–677. [Google Scholar] [CrossRef]
- International Agency for Cancer Research (IARC). IARC Monographs–Malaria and Some Polyomaviruses (SV40, BK, JC, and Mekel Cell Viruses), Volume 104. Reviews of Human Car-cinogens. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer, World Health Organization: Geneva, Switzerland, 2014.
- Louie, B.H.; Kurzrock, R. BAP1: Not just a BRCA1-associated protein. Cancer Treat. Rev. 2020, 90, 102091. [Google Scholar] [CrossRef]
- Ismail, I.H.; Davidson, R.; Gagné, J.-P.; Xu, Z.Z.; Poirier, G.G.; Hendzel, M.J. Germline Mutations in BAP1 Impair Its Function in DNA Double-Strand Break Repair. Cancer Res. 2014, 74, 4282–4294. [Google Scholar] [CrossRef]
- Yu, H.; Pak, H.; Hammond-Martel, I.; Ghram, M.; Rodrigue, A.; Daou, S.; Barbour, H.; Corbeil, L.; Hébert, J.; Drobetsky, E.; et al. Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proc. Natl. Acad. Sci. USA 2013, 111, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Machida, Y.J.; Machida, Y.; Vashisht, A.A.; Wohlschlegel, J.A.; Dutta, A. The Deubiquitinating Enzyme BAP1 Regulates Cell Growth via Interaction with HCF-1. J. Biol. Chem. 2009, 284, 34179–34188. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Jia, R.; Zhang, L.; Xu, S.; Wu, Q.; Song, X.; Zhang, H.; Ge, S.; Xu, X.L.; Fan, X. BAP1 regulates cell cycle progression through E2F1 target genes and mediates transcriptional silencing via H2A monoubiquitination in uveal melanoma cells. Int. J. Biochem. Cell Biol. 2015, 60, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Mashtalir, N.; Daou, S.; Hammond-Martel, I.; Ross, J.; Sui, G.; Hart, G.W.; Rauscher, F.J.; Drobetsky, E.; Milot, E.; et al. The Ubiquitin Carboxyl Hydrolase BAP1 Forms a Ternary Complex with YY1 and HCF-1 and Is a Critical Regulator of Gene Expression. Mol. Cell. Biol. 2010, 30, 5071–5085. [Google Scholar] [CrossRef] [PubMed]
- Bononi, A.; Giorgi, C.; Patergnani, S.; Larson, D.; Verbruggen, K.; Tanji, M.; Pellegrini, L.; Signorato, V.; Olivetto, F.; Pastorino, S.; et al. BAP1 regulates IP3R3-mediated Ca2+ flux to mitochondria suppressing cell transformation. Nature 2017, 546, 549–553. [Google Scholar] [CrossRef]
- Quetel, L.; Meiller, C.; Assié, J.; Blum, Y.; Imbeaud, S.; Montagne, F.; Tranchant, R.; De Wolf, J.; Caruso, S.; Copin, M.; et al. Genetic alterations of malignant pleural mesothelioma: Association with tumor heterogeneity and overall survival. Mol. Oncol. 2020, 14, 1207–1223. [Google Scholar] [CrossRef]
- Ohar, J.A.; Cheung, M.; Talarchek, J.; Howard, S.E.; Howard, T.D.; Hesdorffer, M.; Peng, H.; Rauscher, F.J.; Testa, J.R. Germline BAP1 Mutational Landscape of Asbestos-Exposed Malignant Mesothelioma Patients with Family History of Cancer. Cancer Res. 2016, 76, 206–215. [Google Scholar] [CrossRef]
- Haugh, A.M.; Njauw, C.-N.; Bubley, J.A.; Verzì, A.E.; Zhang, B.; Kudalkar, E.; Vandenboom, T.; Walton, K.; Swick, B.; Kumar, R.; et al. Genotypic and Phenotypic Features of BAP1 Cancer Syndrome: A Report of 8 New Families and Review of Cases in the Literatur. JAMA Dermatol. 2017, 153, 999–1006. [Google Scholar] [CrossRef]
- Baumann, F.; Flores, E.; Napolitano, A.; Kanodia, S.; Taioli, E.; Pass, H.; Yang, H.; Carbone, M. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis 2014, 36, 76–81. [Google Scholar] [CrossRef]
- Xu, J.; Kadariya, Y.; Cheung, M.; Pei, J.; Talarchek, J.; Sementino, E.; Tan, Y.; Menges, C.W.; Cai, K.Q.; Litwin, S.; et al. Germline Mutation of Bap1 Accelerates Development of Asbestos-Induced Malignant Mesothelioma. Cancer Res. 2014, 74, 4388–4397. [Google Scholar] [CrossRef]
- Kadariya, Y.; Cheung, M.; Xu, J.; Pei, J.; Sementino, E.; Menges, C.W.; Cai, K.Q.; Rauscher, F.J.; Klein-Szanto, A.J.; Testa, J.R. Bap1 Is a Bona Fide Tumor Suppressor: Genetic Evidence from Mouse Models Carrying Heterozygous Germline Bap1 Mutations. Cancer Res. 2016, 76, 2836–2844. [Google Scholar] [CrossRef] [PubMed]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.O.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent Mutation of BAP1 in Metastasizing Uveal Melanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef] [PubMed]
- Cigognetti, M.; Lonardi, S.; Fisogni, S.; Balzarini, P.; Pellegrini, V.; Tironi, A.; Bercich, L.; Bugatti, M.; Rossi, G.; Murer, B.; et al. BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod. Pathol. 2015, 28, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Minikes, A.M.; Gao, M.; Bian, H.; Li, Y.; Stockwell, B.R.; Chen, Z.-N.; Jiang, X. Intercellular interaction dictates cancer cell ferroptosis via NF2–YAP signalling. Nature 2019, 572, 402–406. [Google Scholar] [CrossRef]
- Bachir, S.; Shah, S.; Shapiro, S.; Koehler, A.; Mahammedi, A.; Samy, R.N.; Zuccarello, M.; Schorry, E.; Sengupta, S. Neurofibromatosis Type 2 (NF2) and the Implications for Vestibular Schwannoma and Meningioma Pathogenesis. Int. J. Mol. Sci. 2021, 22, 690. [Google Scholar] [CrossRef]
- Evans, D.G.R. Neurofibromatosis type 2 (NF2): A clinical and molecular review. Orphanet J. Rare Dis. 2009, 4, 16. [Google Scholar] [CrossRef]
- Bianchi, A.B.; I Mitsunaga, S.; Cheng, J.Q.; Klein, W.M.; Jhanwar, S.C.; Seizinger, B.; Kley, N.; Klein-Szanto, A.J.; Testa, J.R. High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc. Natl. Acad. Sci. USA 1995, 92, 10854–10858. [Google Scholar] [CrossRef]
- Thurneysen, C.; Opitz, I.; Kurtz, S.; Weder, W.; Stahel, R.A.; Felley-Bosco, E. Functional inactivation of NF2/merlin in human mesothelioma. Lung Cancer 2009, 64, 140–147. [Google Scholar] [CrossRef]
- Guo, G.; Chmielecki, J.; Goparaju, C.; Heguy, A.; Dolgalev, I.; Carbone, M.; Seepo, S.; Meyerson, M.; Pass, H.I. Whole-Exome Sequencing Reveals Frequent Genetic Alterations in BAP1, NF2, CDKN2A, and CUL1 in Malignant Pleural Mesothelioma. Cancer Res. 2015, 75, 264–269. [Google Scholar] [CrossRef]
- Kinzler, K.W.; Vogelstein, B. Gatekeepers and caretakers. Nature 1997, 386, 761–763. [Google Scholar] [CrossRef]
- Serrano, M. The Tumor Suppressor Protein p16INK4a. Exp. Cell Res. 1997, 237, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Komata, T.; Kanzawa, T.; Takeuchi, H.; Germano, I.M.; Schreiber, M.; Kondo, Y.; Kondo, S. Antitumour effect of cyclin-dependent kinase inhibitors (p16INK4A, p18INK4C, p19INK4D, p21WAF1/CIP1 and p27KIP1) on malignant glioma cells. Br. J. Cancer 2003, 88, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Poi, M.J.; Tsai, M.-D. Regulatory Mechanisms of Tumor Suppressor P16INK4A and Their Relevance to Cancer. Biochemistry 2011, 50, 5566–5582. [Google Scholar] [CrossRef] [PubMed]
- Hirao, T.; Bueno, R.; Chen, C.-J.; Gordon, G.J.; Heilig, E.; Kelsey, K.T. Alterations of the p16INK4 locus in human malignant mesothelial tumors. Carcinogenesis 2002, 23, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.; Jackson, S.; Jones, J.; Holme, J.; Lyons, J.; Barrett, E.; Taylor, P.; Bishop, P.; Hodgson, C.; Green, M.; et al. Homozygous deletion of CDKN2A in malignant mesothelioma: Diagnostic utility, patient characteristics and survival in a UK mesothelioma centre. Lung Cancer 2020, 150, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Misawa, N.; Akatsuka, S.; Kohyama, N.; Sekido, Y.; Takahashi, T.; Toyokuni, S. Frequent homozygous deletion of Cdkn2a/2b in tremolite-induced malignant mesothelioma in rats. Cancer Sci. 2020, 111, 1180–1192. [Google Scholar] [CrossRef]
- Cagle, P.T.; Churg, A. Differential Diagnosis of Benign and Malignant Mesothelial Proliferations on Pleural Biopsies. Arch. Pathol. Lab. Med. 2005, 129, 1421–1427. [Google Scholar] [CrossRef]
- Savic, I.; Myers, J. Update on Diagnosing and Reporting Malignant Pleural Mesothelioma. Acta Med. Acad. 2021, 50, 197–208. [Google Scholar] [CrossRef]
- Dacic, S.; Roy, S.; Lyons, M.A.; von der Thusen, J.H.; Galateau-Salle, F.; Churg, A. Whole exome sequencing reveals BAP1 somatic abnormalities in mesothelioma in situ. Lung Cancer 2020, 149, 1–4. [Google Scholar] [CrossRef]
- Simon, F.; Johnen, G.; Krismann, M.; Müller, K.-M. Chromosomal alterations in early stages of malignant mesotheliomas. Virchows Arch. 2005, 447, 762–767. [Google Scholar] [CrossRef]
- Pulford, E.; Henderson, D.W.; Klebe, S. Malignant mesothelioma in situ: Diagnostic and clinical considerations. Pathology 2020, 52, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Churg, A.; Hwang, H.; Tan, L.; Qing, G.; Taher, A.; Tong, A.; Bilawich, A.; Dacic, S. Malignant mesothelioma in situ. Histopathology 2018, 72, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Beasley, M.B.; Galateau-Salle, F.; Dacic, S. Pleural mesothelioma classification update. Virchows Arch. 2021, 478, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.B.; Dacic, S.; Miller, C.; Cheung, S.; Churg, A. Utility of Methylthioadenosine Phosphorylase Compared With BAP1 Immunohistochemistry, and CDKN2A and NF2 Fluorescence In Situ Hybridization in Separating Reactive Mesothelial Proliferations From Epithelioid Malignant Mesotheliomas. Arch. Pathol. Lab. Med. 2018, 142, 1549–1553. [Google Scholar] [CrossRef] [PubMed]
- Churg, A.; Salle, F.G.; Roden, A.C.; Attanoos, R.; Von Der Thusen, J.H.; Tsao, M.; Chang, N.; De Perrot, M.; Dacic, S. Malignant mesothelioma in situ: Morphologic features and clinical outcome. Mod. Pathol. 2019, 33, 297–302. [Google Scholar] [CrossRef]
- Sharif, S.; Zahid, I.; Routledge, T.; Scarci, M. Does positron emission tomography offer prognostic in-formation in malignant pleural mesothelioma? Interact. Cardiovasc. Thorac. Surg. 2011, 12, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Zahid, I.; Sharif, S.; Routledge, T.; Scarci, M. What is the best way to diagnose and stage malignant pleural mesothelioma? Interact. Cardiovasc. Thorac. Surg. 2011, 12, 254–259. [Google Scholar] [CrossRef]
- Bueno, R.; Opitz, I.; IASLC Mesothelioma Taskforce. Surgery in Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2018, 13, 1638–1654. [Google Scholar] [CrossRef]
- Scherpereel, A.; Opitz, I.; Berghmans, T.; Psallidas, I.; Glatzer, M.; Rigau, D.; Astoul, P.; Bölükbas, S.; Boyd, J.; Coolen, J.; et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur. Respir. J. 2020, 55, 1900953. [Google Scholar] [CrossRef]
- Sugarbaker, D.J. Macroscopic complete resection: The goal of primary surgery in multimo-dality therapy for pleural mesothelioma. J. Thorac. Oncol. 2006, 1, 175–176. [Google Scholar] [CrossRef]
- Opitz, I.; Furrer, K. Preoperative Identification of Benefit from Surgery for Malignant Pleural Mesothelioma. Thorac. Surg. Clin. 2020, 30, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Buikhuisen, W.; Hiddinga, B.I.; Baas, P.; van Meerbeeck, J. Second line therapy in malignant pleural mesothelioma: A systematic review. Lung Cancer 2015, 89, 223–231. [Google Scholar] [CrossRef]
- Brims, F.J.H.; Meniawy, T.M.; Duffus, I.; de Fonseka, D.; Segal, A.; Creaney, J.; Maskell, N.; Lake, R.A.; de Klerk, N.; Nowak, A.K. A Novel Clinical Prediction Model for Prognosis in Malignant Pleural Mesothelioma Using Decision Tree Analysis. J. Thorac. Oncol. 2016, 11, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Zalcman, G.; Mazieres, J.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Molinier, O.; Corre, R.; Monnet, I.; Gounant, V.; et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase 3 trial. Lancet 2015, 387, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- LaFave, L.M.; Béguelin, W.; Koche, R.; Teater, M.; Spitzer, B.; Chramiec, A.; Papalexi, E.; Keller, M.D.; Hricik, T.; Konstantinoff, K.; et al. Loss of BAP1 function leads to EZH2-dependent transformation. Nat. Med. 2015, 21, 1344–1349. [Google Scholar] [CrossRef]
- De Bondt, C.; Psallidas, I.; Van Schil, P.E.Y.; van Meerbeeck, J.P. Combined modality treatment in mesothelioma: A systemic literature review with treatment recommendations. Transl. Lung Cancer Res. 2018, 7, 562–573. [Google Scholar] [CrossRef]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- A Fennell, D.; King, A.; Mohammed, S.; Greystoke, A.; Anthony, S.; Poile, C.; Nusrat, N.; Scotland, M.; Bhundia, V.; Branson, A.; et al. Abemaciclib in patients with p16ink4A-deficient mesothelioma (MiST2): A single-arm, open-label, phase 2 trial. Lancet Oncol. 2022, 23, 374–381. [Google Scholar] [CrossRef]
- Mierzejewski, M.; Korczynski, P.; Krenke, R.; Janssen, J.P. Chemical pleurodesis–a review of mechanisms involved in pleural space obliteration. Respir. Res. 2019, 20, 247. [Google Scholar] [CrossRef]
- Kaya, S.O.; Bir, F.; Atalay, H.; Onem, G.; Aytekin, F.O.; Saçar, M. Effect of Diclofenac on Experimental Pleurodesis Induced by Tetracycline in Rabbits. J. Investig. Med. 2005, 53, 267–270. [Google Scholar] [CrossRef]
- Calon, A.; Lonardo, E.; Berenguer-Llergo, A.; Espinet, E.; Hernando-Momblona, X.; Iglesias, M.; Sevillano, M.; Palomo-Ponce, S.; Tauriello, D.V.F.; Byrom, D.; et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Genet. 2015, 47, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Calon, A.; Espinet, E.; Palomo-Ponce, S.; Tauriello, D.V.F.; Iglesias, M.; Céspedes, M.V.; Sevillano, M.; Nadal, C.; Jung, P.; Zhang, X.H.-F.; et al. Dependency of Colorectal Cancer on a TGF-β-Driven Program in Stromal Cells for Metastasis Initiation. Cancer Cell 2012, 22, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Bintcliffe, O.J.; Lee, G.Y.; Rahman, N.M.; Maskell, N.A. The management of benign non-infective pleural effusions. Eur. Respir. Rev. 2016, 25, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Karpathiou, G.; Stefanou, D.; Froudarakis, M.E. Pleural neoplastic pathology. Respir. Med. 2015, 109, 931–943. [Google Scholar] [CrossRef]
- Karpathiou, G.; Péoc’H, M.; Sundaralingam, A.; Rahman, N.; Froudarakis, M.E. Inflammation of the Pleural Cavity: A Review on Pathogenesis, Diagnosis and Implications in Tumor Pathophysiology. Cancers 2022, 14, 1415. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moro, J.; Sobrero, S.; Cartia, C.F.; Ceraolo, S.; Rapanà, R.; Vaisitti, F.; Ganio, S.; Mellone, F.; Rudella, S.; Scopis, F.; et al. Diagnostic and Therapeutic Challenges of Malignant Pleural Mesothelioma. Diagnostics 2022, 12, 3009. https://doi.org/10.3390/diagnostics12123009
Moro J, Sobrero S, Cartia CF, Ceraolo S, Rapanà R, Vaisitti F, Ganio S, Mellone F, Rudella S, Scopis F, et al. Diagnostic and Therapeutic Challenges of Malignant Pleural Mesothelioma. Diagnostics. 2022; 12(12):3009. https://doi.org/10.3390/diagnostics12123009
Chicago/Turabian StyleMoro, Jacopo, Simona Sobrero, Carlotta Francesca Cartia, Simona Ceraolo, Roberta Rapanà, Federico Vaisitti, Stefano Ganio, Federica Mellone, Stefano Rudella, Federico Scopis, and et al. 2022. "Diagnostic and Therapeutic Challenges of Malignant Pleural Mesothelioma" Diagnostics 12, no. 12: 3009. https://doi.org/10.3390/diagnostics12123009
APA StyleMoro, J., Sobrero, S., Cartia, C. F., Ceraolo, S., Rapanà, R., Vaisitti, F., Ganio, S., Mellone, F., Rudella, S., Scopis, F., La Paglia, D., Cacciatore, C. C., Ruffini, E., & Leo, F. (2022). Diagnostic and Therapeutic Challenges of Malignant Pleural Mesothelioma. Diagnostics, 12(12), 3009. https://doi.org/10.3390/diagnostics12123009






