The Capacity of Artificial Intelligence in COVID-19 Response: A Review in Context of COVID-19 Screening and Diagnosis
Abstract
:1. Introduction
2. Methodology
3. AI for COVID-19 Diagnosis
3.1. Areas of Successful Application
3.1.1. AI in Screening and Testing
3.1.2. AI in COVID-19 Detection and Diagnosis
Medical Images for AI COVID-19 Diagnosis
CT and X-ray Medical Images for COVID-19 Diagnosis
Machine Learning (ML) and Deep Learning (DL) for COVID-19 Diagnosis
4. Result: COVID-19 Status
5. Conclusions and Future Recommendation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Laddha, S.; Mnasri, S.; Alghamdi, M.; Kumar, V.; Kaur, M.; Alrashidi, M.; Almuhaimeed, A.; Alshehri, A.; Alrowaily, M.A.; Alkhazi, I. COVID-19 Diagnosis and Classification Using Radiological Imaging and Deep Learning Techniques: A Comparative Study. Diagnostics 2022, 12, 1880, PMCID:PMC9406661. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.W.; Yuan, S.; Kok, K.H.; To, K.K.W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.Y.; Poon, R.W.S.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Wang, Z.; He, Z.; Li, Y.; Wu, Y.; Wang, H.; Liu, Y.; Hao, F.; Tian, H. A follow-up study shows that recovered patients with re-positive PCR test in Wuhan may not be infectious. BMC Med. 2021, 19, 77. [Google Scholar] [CrossRef] [PubMed]
- Roy, S. Physicians’ Dilemma of False-Positive RT-PCR for COVID-19: A Case Report. SN Compr. Clin. Med. 2021, 3, 255–258. [Google Scholar] [CrossRef]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: A report of 1,014 cases. Radiology 2020, 2020, 200642. [Google Scholar]
- Ellahham, S. Artificial intelligence in the diagnosis and management of COVID-19: A narrative review. J. Med. Artif. Intell. 2021, 4, 4. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, H.; Xie, J.; Lin, M.; Ying, L.; Pang, P.; Ji, W. Sensitivity of chest CT for COVID-19: Comparison to RT-PCR. Radiology 2020, 2020, 200432. [Google Scholar] [CrossRef]
- Ozturk, T.; Talo, M.; Yildirim, E.A.; Baloglu, U.B.; Yildirim, O.; Acharya, U.R. Automated detection of COVID-19 cases using deep neural networks with X-ray images. Comput. Biol. Med. 2020, 2020, 103792. [Google Scholar] [CrossRef]
- Schwartz, D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: Maternal coronavirus infections and pregnancy outcomes. Arch. Pathol. Lab. Med. 2020, 144, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Ellahham, S.; Ellahham, N. Use of Artificial Intelligence for Improving Patient Flow and Healthcare Delivery. J. Comput. Sci. Syst. Biol. 2019, 12, 80–85. [Google Scholar]
- Abdulkareem, M.; Petersen, S.E. The Promise of AI in Detection, Diagnosis, and Epidemiology for Combating COVID-19: Beyond the Hype. Front. Artif. Intell. 2021, 4, 652669. [Google Scholar] [CrossRef] [PubMed]
- Rajawat, N.; Hada, B.S.; Meghawat, M.; Lalwani, S.; Kumar, R. C-COVIDNet: A CNN Model for COVID-19 Detection Using Image Processing. Arab. J. Sci. Eng. 2022, 47, 10811–10822. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Javaid, M.; Khan, I.H.; Haleem, A. Artificial Intelligence (AI) applications for COVID-19 pandemic. Diabetes Metab. Syndr. 2020, 14, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Aich, S.; Tripathy, S.; Kim, H.-C. Artificial Intelligence Is Reshaping Healthcare amid COVID-19: A Review in the Context of Diagnosis & Prognosis. Diagnostics 2021, 11, 1604. [Google Scholar]
- Ozsahin, I.; Sekeroglu, B.; Musa, M.S.; Mustapha, M.T.; Uzun Ozsahin, D. Review on Diagnosis of COVID-19 from Chest CT Images Using Artificial Intelligence. Comput. Math. Methods Med. 2020, 2020, 9756518. [Google Scholar] [CrossRef] [PubMed]
- Ramdani, H.; Allali, N.; Chat, L.; El Haddad, S. Covid-19 imaging: A narrative review. Ann. Med. Surg. 2021, 69, 102489. [Google Scholar] [CrossRef]
- Kumar, A.; Mahapatra, R.P. Detection and diagnosis of COVID-19 infection in lungs images using deep learning techniques. Int. J. Imaging Syst. Technol. 2022, 32, 462–475. [Google Scholar] [CrossRef]
- Tsikala Vafea, M.; Atalla, E.; Georgakas, J.; Shehadeh, F.; Mylona, E.K.; Kalligeros, M.; Mylonakis, E. Emerging Technologies for Use in the Study, Diagnosis, and Treatment of Patients with COVID-19. Cell. Mol. Bioeng. 2020, 13, 249–257. [Google Scholar] [CrossRef]
- Lalmuanawma, S.; Hussain, J.; Chhakchhuak, L. Applications of machine learning and artificial intelligence for Covid-19 (SARS-CoV-2) pandemic: A review. Chaos Solitons Fractals 2020, 139, 110059. [Google Scholar] [CrossRef]
- Suri, J.S.; Puvvula, A.; Majhail, M.; Biswas, M.; Jamthikar, A.D.; Saba, L.; Faa, G.; Singh, I.M.; Oberleitner, R.; Turk, M.; et al. Integration of cardiovascular risk assessment with COVID-19 using artificial intelligence. Rev. Cardiovasc. Med. 2020, 21, 541–560. [Google Scholar] [CrossRef]
- Arora, N.; Banerjee, A.K.; Narasu, M.L. The role of artificial intelligence in tackling COVID-19. Future Virol. 2020, 15, 717–724. [Google Scholar] [CrossRef]
- El-Rashidy, N.; Abdelrazik, S.; Abuhmed, T.; Amer, E.; Ali, F.; Hu, J.W.; El-Sappagh, S. Comprehensive Survey of Using Machine Learning in the COVID-19 Pandemic. Diagnostics 2021, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.; Kamrowski, C.; Langlois, J.; Rozario, P.; Dircks, I.; Grottodden, K.; Martinez, M.; Tee, W.Z.; Sargeant, K.; LaFleur, C.; et al. A Comprehensive Review of Machine Learning Used to Combat COVID-19. Diagnostics 2022, 12, 1853. [Google Scholar] [CrossRef] [PubMed]
- Helwan, A.; Ma’aitah, M.K.; Hamdan, H.; Ozsahin, D.U.; Tuncyurek, O. Radiologists versus deep convolutional neural networks: A Comparative Study for diagnosing COVID-19. Comput. Math. Methods Med. 2021, 2021, 5527271. [Google Scholar] [CrossRef] [PubMed]
- Mulrenan, C.; Rhode, K.; Fischer, B.M. A Literature Review on the Use of Artificial Intelligence for the Diagnosis of COVID-19 on CT and Chest X-ray. Diagnostics 2022, 12, 869. [Google Scholar] [CrossRef]
- Johnson, K.W.; Shameer, K.; Glicksberg, B.S.; Readhead, B.; Sengupta, P.P.; Björkegren, J.L.; Kovacic, J.C.; Dudley, J.T. Enabling precision cardiology through multiscale biology and systems medicine. JACC Basic Transl. Sci. 2017, 2, 311–327. [Google Scholar] [CrossRef]
- Long, J.B.; Ehrenfeld, J.M. The Role of Augmented Intelligence (AI) in Detecting and Preventing the Spread of Novel Coronavirus. J. Med. Syst. 2020, 44, 59. [Google Scholar] [CrossRef] [Green Version]
- Sayan, M.; Sarigul Yildirim, F.; Sanlidag, T.; Uzun, B.; Uzun Ozsahin, D.; Ozsahin, I. Capacity evaluation of diagnostic tests for covid-19 using multicriteria decision-making techniques. Comput. Math. Methods Med. 2020, 2020, 1560250. [Google Scholar] [CrossRef]
- Yildirim, M.; Eroğlu, O.; Eroğlu, Y.; Çinar, A.; Cengil, E. COVID-19 Detection on Chest X-ray Images with the Proposed Model Using Artificial Intelligence and Classifiers. New Gener. Comput. 2022, 15 pages. [Google Scholar] [CrossRef]
- Shen, D.; Wu, G.; Suk, H.-I. Deep learning in medical image analysis. Annu. Rev. Biomed. Eng. 2017, 19, 221–248. [Google Scholar] [CrossRef] [Green Version]
- Wynants, L.; Van Calster, B.; Collins, G.S.; Riley, R.D.; Heinze, G.; Schuit, E.; Bonten, M.M.J.; Dahly, D.L.; Damen, J.A.; Debray, T.P.A.; et al. Prediction models for diagnosis and prognosis of COVID-19 infection: Systematic review and critical appraisal. BMJ 2020, 369, m1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandyopadhyay, S.K.; Dutta, S. Machine learning approach for confirmation of COVID-19 cases: Positive, negative, death and release. medRxiv 2020. [Google Scholar] [CrossRef]
- Goh, K.J.; Kalimuddin, S.; Chan, K.S. Rapid progression to acute respiratory distress syndrome: Review of current understanding of critical illness from coronavirus disease 2019 (COVID-19) infection. Ann. Acad. Med. Singap. 2020, 49, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Pourhomayoun, M.; Shakibi, M. Predicting mortality risk in patients with COVID-19 using artificial intelligence to help medical decision-making. medRxiv 2020, 20, 100178. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Qin, L.; Xu, Z.; Yin, Y.; Wang, X.; Kong, B.; Bai, J.; Lu, Y.; Fang, Z.; Song, Q.; et al. Using Artificial Intelligence to Detect COVID-19 and Community-acquired Pneumonia Based on Pulmonary CT: Evaluation of the Diagnostic Accuracy. Radiology 2020, 296, E65–E71. [Google Scholar] [CrossRef]
- Wang, S.; Zha, Y.; Li, W.; Wu, Q.; Li, X.; Niu, M.; Wang, M.; Qiu, X.; Li, H.; Yu, H.; et al. A Fully Automatic Deep Learning System for COVID-19 Diagnostic and Prognostic Analysis. Eur. Respir. J. 2020, 56, 2000775. [Google Scholar] [CrossRef]
- Alaa, A.M.; Bolton, T.; Di Angelantonio, E.; Rudd, J.H.F.; van der Schaar, M. Cardiovascular disease risk prediction using automated machine learning: A prospective study of 423,604 UK Biobank participants. PLoS ONE 2019, 14, e0213653. [Google Scholar] [CrossRef] [Green Version]
- Chieregato, M.; Frangiamore, F.; Morassi, M.; Baresi, C.; Nici, S.; Bassetti, C.; Bnà, C.; Galelli, M. A hybrid machine learning/deep learning COVID-19 severity predictive model from CT images and clinical data. Sci. Rep. 2022, 12, 4329. [Google Scholar] [CrossRef]
- Castiglioni, I.; Ippolito, D.; Interlenghi, M.; Monti, C.B.; Salvatore, C.; Schiaffino, S.; Polidori, A.; Gandola, D.; Messa, C.; Sardanelli, F. Artificial intelligence applied on chest X-ray can aid in the diagnosis of COVID-19 infection: A first experience from Lombardy, Italy. medRxiv 2020. [Google Scholar]
- Bogoch, I.I.; Watts, A.; Thomas-Bachli, A.; Huber, C.; Kraemer, M.U.G.; Khan, K. Pneumonia of unknown aetiology in Wuhan, China: Potential for international spread via commercial air travel. J. Travel. Med. 2020, 27, taaa008. [Google Scholar] [CrossRef]
- Bénézit, F.; Le Turnier, P.; Declerck, C.; Paillé, C.; Revest, M.; Dubée, V.; Tattevin, P.; Arvieux, C.; Baldeyrou, M.; Chapplain, J.M.; et al. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect. Dis. 2020, 20, 1014–1015. [Google Scholar] [CrossRef]
- Mei, X.; Lee, H.C.; Diao, K.Y.; Huang, M.; Lin, B.; Liu, C.; Xie, Z.; Ma, Y.; Robson, P.M.; Chung, M.; et al. Artificial intelligence-enabled rapid diagnosis of patients with COVID-19. Nat. Med. 2020, 26, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Mauger, C.; Gilbert, K.; Lee, A.M.; Sanghvi, M.M.; Aung, N.; Fung, K.; Carapella, V.; Piechnik, S.K.; Neubauer, S.; Petersen, S.E.; et al. Right ventricular shape and function: Cardiovascular magnetic resonance reference morphology and biventricular risk factor morphometrics in UK Biobank. J. Cardiovasc. Magn. Reson. 2019, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.X.; Wang, R.; Xiong, Z.; Hsieh, B.; Chang, K.; Halsey, K.; Tran, T.M.L.; Choi, J.W.; Wang, D.C.; Shi, L.B.; et al. Artificial Intelligence Augmentation of Radiologist Performance in Distinguishing COVID-19 from Pneumonia of Other Origin at Chest CT. Radiology 2020, 296, E156–E165. [Google Scholar] [CrossRef]
- Shi, F.; Wang, J.; Shi, J.; Wu, Z.; Wang, Q.; Tang, Z.; He, K.; Shi, Y.; Shen, D. Review of Artificial Intelligence Techniques in Imaging Data Acquisition, Segmentation, and Diagnosis for COVID-19. IEEE Rev. Biomed. Eng. 2021, 14, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Zhao, W.; Xie, X.; Zhong, Z.; Shi, F.; Ma, T.; Liu, J.; Shen, D. Severity assessment of COVID-19 using CT image features and laboratory indices. Phys. Med. Biol. 2021, 66, 035015. [Google Scholar] [CrossRef]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time course of lung changes on chest CT during recovery from 2019 COVID-19 pneumonia. Radiology 2020, 2020, 200370. [Google Scholar]
- Darapaneni, N.; Ranjane, S.; Satya, U.S.P.; Prashanth, D.; Reddy, M.H.; Paduri, A.R.; Adhi, A.K.; Madabhushanam, V. COVID 19 Severity of Pneumonia Analysis Using Chest X Rays. In Proceedings of the 2020 IEEE 15th International Conference on Industrial and Information Systems (ICIIS), Rupnagar, India, 26–28 November 2020; pp. 381–386. [Google Scholar]
- Bullock, J.; Pham, K.H.; Lam, C.S.N.; Luengo-Oroz, M. Mapping the landscape of artificial intelligence applications against COVID-19. J. Artif. Intell. Res. 2020, 69, 807–845. [Google Scholar] [CrossRef]
- Chung, M.; Bernheim, A.; Mei, X.; Zhang, N.; Huang, M.; Zeng, X.; Cui, J.; Xu, W.; Yang, Y.; Fayad, Z.A.; et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology 2020, 295, 202–207. [Google Scholar] [CrossRef] [Green Version]
- Kanne, J.P. Chest CT Findings in 2019 Novel Coronavirus (2019-nCoV) Infections from Wuhan, China: Key Points for the Radiologist. Radiology 2020, 295, 16–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, A.S. COVID-Net. 2020. Available online: https://alexswong.github.io/COVID-Net/ (accessed on 14 October 2022).
- Jamshidi, M.B.; Lalbakhsh, A.; Talla, J.; Peroutka, Z.; Hadjilooei, F.; Lalbakhsh, P.; Jamshidi, M.; La Spada, L.; Mirmozafari, M.; Dehghani, M.; et al. Artificial Intelligence and COVID-19: Deep Learning Approaches for Diagnosis and Treatment. IEEE Access 2020, 8, 109581–109595. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, K.; Zhang, Z.; Li, K.; Yu, P.S. A survey on applications of artificial intelligence in fighting against COVID-19. arXiv 2020, arXiv:200702202. [Google Scholar] [CrossRef]
- Al-Waisy, A.S.; Al-Fahdawi, S.; Mohammed, M.A.; Abdulkareem, K.H.; Mostafa, S.A.; Maashi, M.S.; Arif, M.; Garcia-Zapirain, B. Covid-chexnet: Hybrid deep learning framework for identifying covid-19 virus in chest x-rays images. Soft Comput. 2020. [Google Scholar] [CrossRef]
- Horry, M.J.; Chakraborty, S.; Paul, M.; Ulhaq, A.; Pradhan, B.; Saha, M.; Shukla, N. X-ray Image Based COVID-19 Detection Using Pre-trained Deep Learning Models. engrXiv 2020. [Google Scholar]
- Lawton, S.; Viriri, S. Detection of COVID-19 from CT Lung Scans Using Transfer Learning. Comput. Intell. Neurosci. 2021, 2021, 5527923. [Google Scholar] [CrossRef]
- Gilanie, G.; Bajwa, U.I.; Waraich, M.M.; Asghar, M.; Kousar, R.; Kashif, A.; Aslam, R.S.; Qasim, M.M.; Rafique, H. Coronavirus (COVID-19) detection from chest radiology images using convolutional neural networks. Biomed. Signal Process. Control 2021, 66, 102490. [Google Scholar] [CrossRef]
- Sajun, A.R.; Zualkernan, I.; Sankalpa, D. Investigating the Performance of FixMatch for COVID-19 Detection in Chest X-rays. Appl. Sci. 2022, 12, 4694. [Google Scholar] [CrossRef]
- Xue, Y.; Onzo, B.M.; Mansour, R.F.; Su, S. Deep Convolutional Neural Network Approach for COVID-19 Detection. Comput. Syst. Sci. Eng. 2022, 42, 201–211. [Google Scholar] [CrossRef]
- Musha, A.; Al Mamun, A.; Tahabilder, A.; Hossen, M.J.; Hossen, B.; Jahan, B.; Ranjbari, S. A deep learning approach for COVID-19 and pneumonia detection from chest X-ray images. Int. J. Electr. Comput. Eng. (IJECE) 2022, 12, 3655. [Google Scholar] [CrossRef]
- Canario, D.A.H.; Fromke, E.; Patetta, M.A.; Eltilib, M.T.; Reyes-Gonzalez, J.P.; Rodriguez, G.C.; Cornejo, V.A.F.; Dunckner, S.; Stewart, J.K. Using artificial intelligence to risk stratify COVID-19 patients based on chest X-ray findings. Intell.-Based Med. 2022, 6, 100049. [Google Scholar] [CrossRef]
- El-Dahshan, E.S.A.; Bassiouni, M.M.; Hagag, A.; Chakrabortty, R.K.; Loh, H.; Acharya, U.R. RESCOVIDTCNnet: A residual neural network-based framework for COVID-19 detection using TCN and EWT with chest X-ray images. Expert Syst. Appl. 2022, 204, 117410. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.; Darwish, A.; Hassanien, A.E. Classification of COVID19 X-ray Images Based on Transfer Learning InceptionV3 Deep Learning Model. In Digital Transformation and Emerging Technologies for Fighting COVID-19 Pandemic: Innovative Approaches; Studies in Systems, Decision and Control; Springer: Cham, Switzerland, 2021; Volume 322, pp. 111–119. [Google Scholar]
- Khan, I.U.; Aslam, N.; Anwar, T.; Alsaif, H.S.; Chrouf, S.M.B.; Alzahrani, N.A.; Alamoudi, F.A.; Kamaleldin, M.M.A.; Awary, K.B. Using a Deep Learning Model to Explore the Impact of Clinical Data on COVID-19 Diagnosis Using Chest X-ray. Sensors 2022, 22, 669. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, G.; Chang, V.; Kant Singh, K.; Shankar, A. Adopt: Automatic deep learning and optimization-based approach for detection of novel coronavirus COVID-19 disease using X-ray images. J. Biomol. Struct. Dyn. 2022, 40, 5836–5847. [Google Scholar] [CrossRef]
- Ieracitano, C.; Mammone, N.; Versaci, M.; Varone, G.; Ali, A.R.; Armentano, A.; Calabrese, G.; Ferrarelli, A.; Turano, L.; Tebala, C.; et al. A Fuzzy-enhanced Deep Learning Approach for Early Detection of COVID-19 Pneumonia from Portable Chest X-ray Images. Neurocomputing 2022, 481, 202–215. [Google Scholar] [CrossRef]
- Nayak, S.R.; Nayak, D.R.; Sinha, U.; Arora, V.; Pachori, R.B. Application of deep learning techniques for detection of COVID-19 cases using chest X-ray images: A comprehensive study. Biomed. Signal Process. Control 2020, 64, 102365. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Kobayashi, D.; Nishioka, E.; Matsuo, H.; Urase, Y.; Onoue, K.; Ishikura, R.; Kitamura, Y.; Sakai, E.; Tomita, M.; et al. Deep learning model for the automatic classification of COVID-19 pneumonia, non-COVID-19 pneumonia, and the healthy: A multi-center retrospective study. Sci. Rep. 2022, 12, 8214. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, K.; Koundal, D. A novel fusion based convolutional neural network approach for classification of COVID-19 from chest X-ray images. Biomed. Signal Process. Control 2022, 77, 103778. [Google Scholar] [CrossRef]
- Duda, R.O.; Hart, P.E.; Education, I.C. Machine Learning Artificial Intelligence, Data Scienc. In Pattern Classification and Scene Analysis; Wiley: New York, NY, USA, 1973; Volume 3. [Google Scholar]
- Pesapane, F.; Codari, M.; Sardanelli, F. Artificial intelligence in medical imaging: Threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur. Radiol. Exp. 2018, 2, 35. [Google Scholar] [CrossRef] [Green Version]
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural. Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef] [Green Version]
- Panwar, H.; Gupta, P.; Siddiqui, M.K.; Morales-Menendez, R.; Singh, V. Application of deep learning for fast detection of COVID-19 in X-rays using nCOVnet. Chaos Solitons Fractals 2020, 138, 109944. [Google Scholar] [CrossRef]
- Gozes, O.; Frid-Adar, M.; Greenspan, H.; Browning, P.D.; Zhang, H.; Ji, W.; Bernheim, A.; Siegel, E. Rapid AI development cycle for the coronavirus (COVID-19) pandemic: Initial results for automated detection & Patient Monitoring Using Deep Learning CT image analysis. arXiv 2020, arXiv:2003.05037p. [Google Scholar]
- Darji, P.A.; Nayak, N.R.; Ganavdiya, S.; Batra, N.; Guhathakurta, R. Feature extraction with capsule network for the COVID-19 disease prediction though X-ray images. Mater. Today Proc. 2022, 56, 3556–3560. [Google Scholar] [CrossRef] [PubMed]
- López, V.; Čukić, M. A dynamical model of SARS-CoV-2 based on people flow networks. Saf. Sci. 2021, 134, 105034. [Google Scholar] [CrossRef] [PubMed]
- Sakib, S.; Tazrin, T.; Fouda, M.M.; Fadlullah, Z.M.; Guizani, M. DL-CRC: Deep Learning-Based Chest Radiograph Classification for COVID-19 Detection: A Novel Approach. IEEE Access 2020, 8, 171575–171589. [Google Scholar] [CrossRef] [PubMed]
- Vaid, S.; Kalantar, R.; Bhandari, M. Deep learning COVID-19 detection bias: Accuracy through artificial intelligence. Int. Orthop. 2020, 44, 1539–1542. [Google Scholar] [CrossRef]
- Ting, D.S.W.; Carin, L.; Dzau, V.; Wong, T.Y. Digital technology and COVID-19. Nat. Med. 2020, 26, 459–461. [Google Scholar] [CrossRef]
- Han, Z.; Wei, B.; Hong, Y.; Li, T.; Cong, J.; Zhu, X.; Wei, H.; Zhang, W. Accurate screening of COVID-19 using attention-based deep 3D multiple instance learning. IEEE Trans. Med. Imaging 2020, 39, 2584–2594. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Liu, C.; Sun, Q.; Peng, W. Differentiating novel coronavirus pneumonia from general pneumonia based on machine learning. Biomed. Eng. Online 2020, 19, 66. [Google Scholar] [CrossRef]
- Sakagianni, A.; Feretzakis, G.; Kalles, D.; Koufopoulou, C.; Kaldis, V. Setting up an easy-to-use machine learning pipeline for medical decision support: A case study for COVID-19 diagnosis based on deep learning with CT scans. Stud. Health Technol. Inform. 2020, 272, 13–16. [Google Scholar]
- Ahuja, S.; Panigrahi, B.K.; Dey, N.; Rajinikanth, V.; Gandhi, T.K. Deep transfer learning-based automated detection of COVID-19 from lung CT scan slices. Appl. Intell. 2021, 51, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Kumar, V.; Vaishali, M.K. Classification of COVID-19 patients from chest CT images using multi-objective differential evolution-based convolutional neural networks. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Alom, M.Z.; Rahman, M.M.S.; Nasrin, M.S.; Taha, T.M.; Asari, V.K. COVID MTNet: COVID-19 detection with multi-task deep learning approaches. arXiv 2020, arXiv:2004.03747. [Google Scholar]

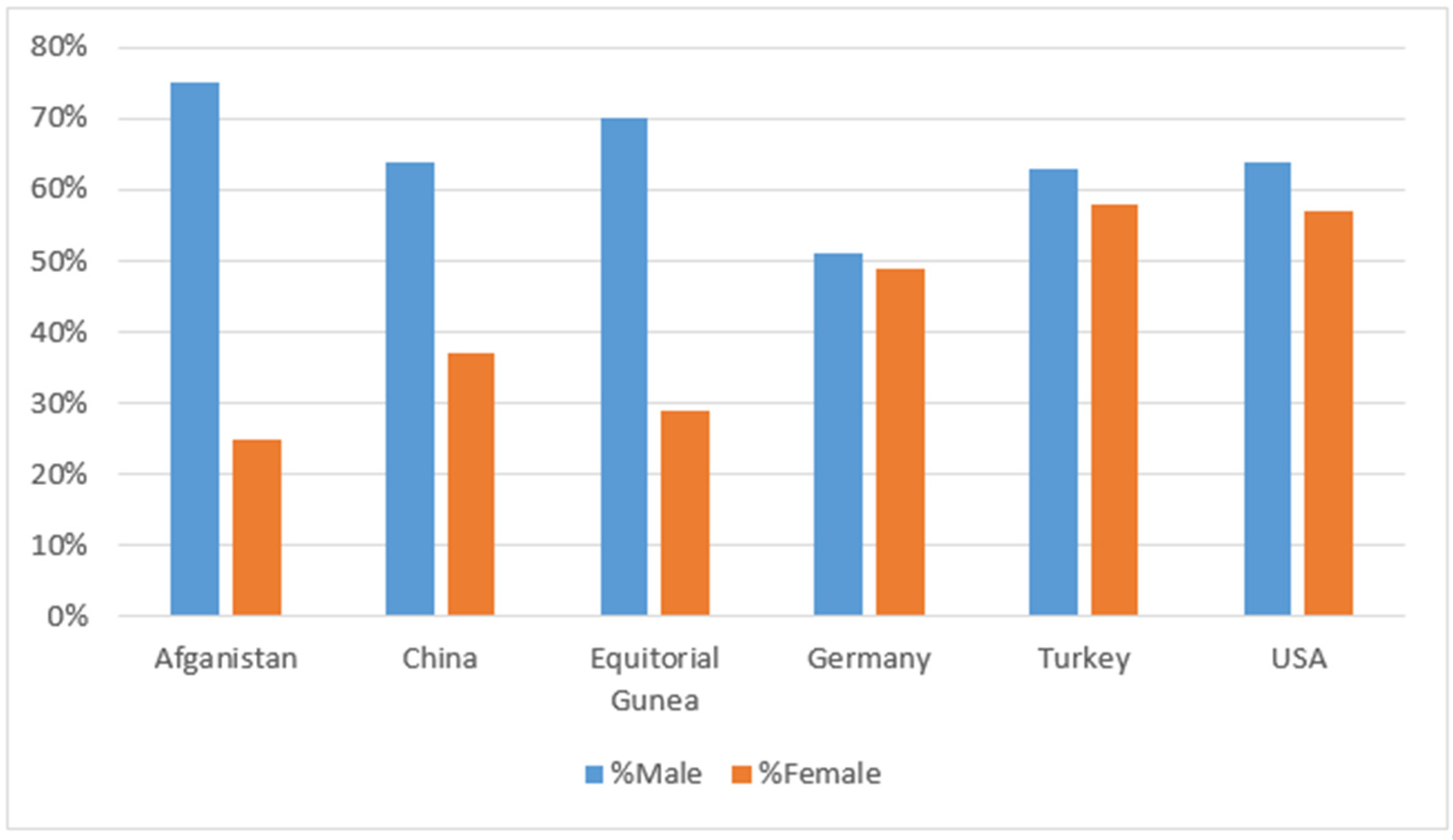
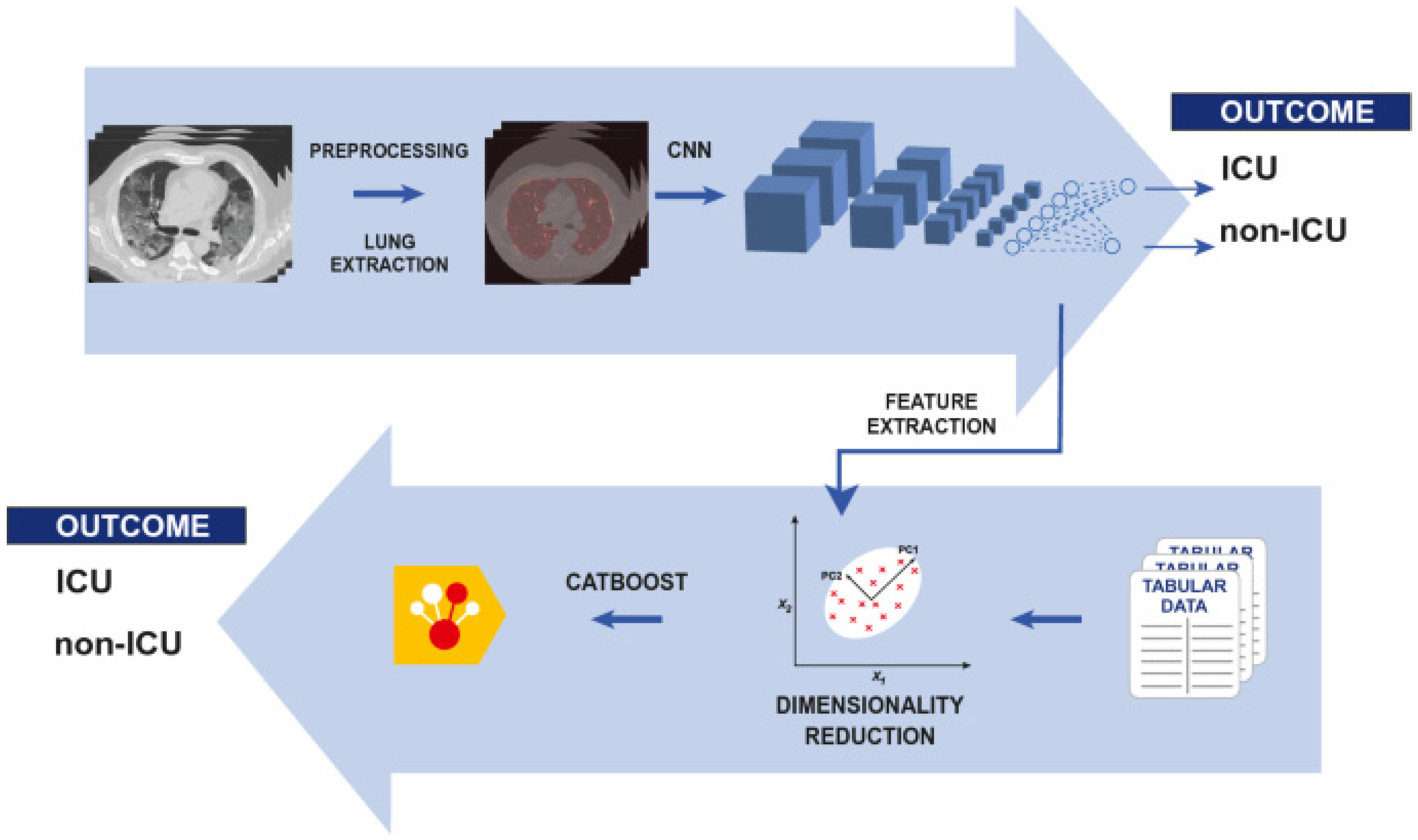
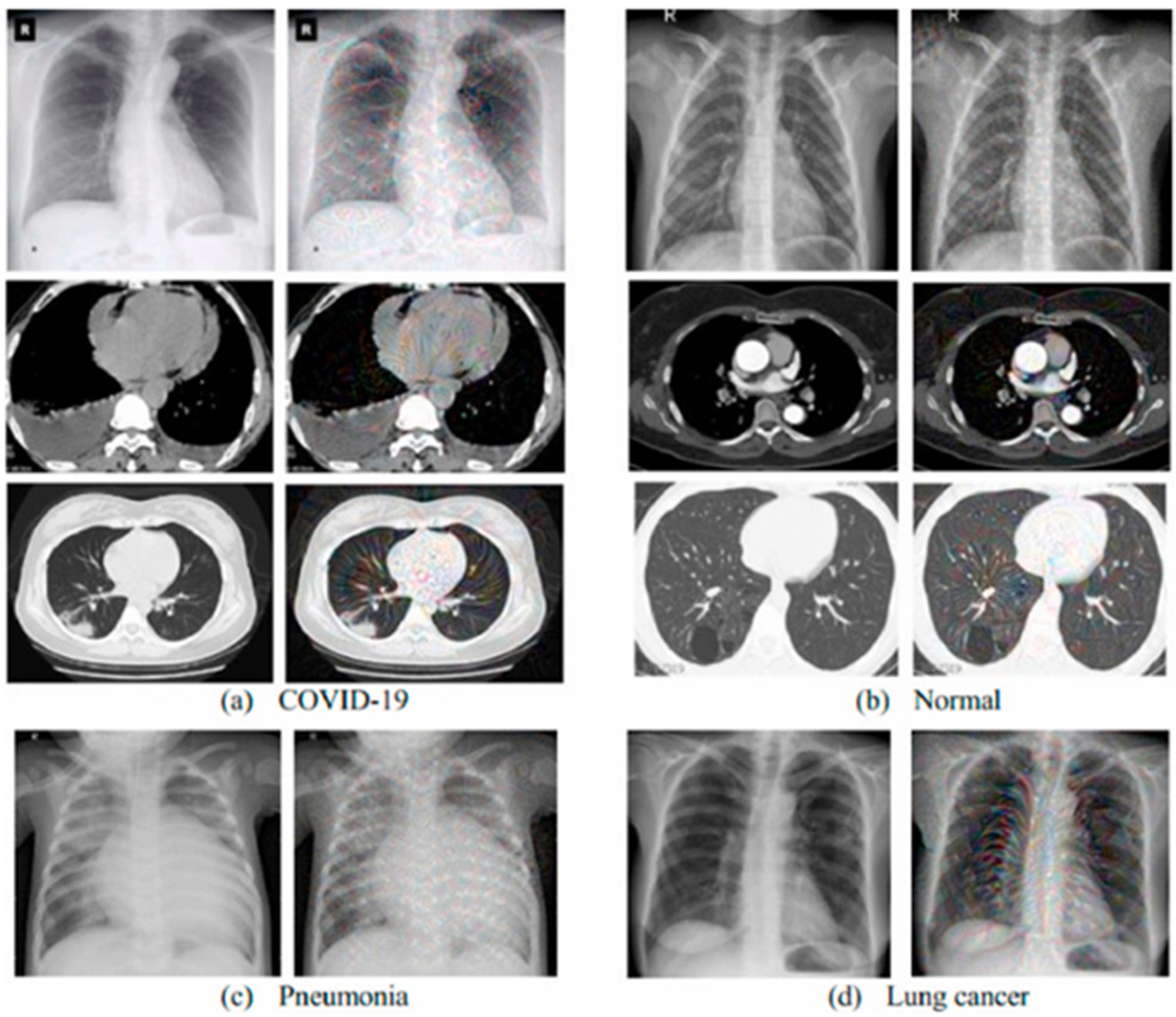

| Year | Reference | Type | Model | Dataset | Accuracy |
|---|---|---|---|---|---|
| 2020 | [42] | Screening | - | image and clinical data | |
| [43] | screening | - | |||
| [50] | Images | RCNN | CXR | - | |
| [57] | Images | VGG19 | CXR | 99.99% | |
| [58] | images | VGG19 | CXR | 83% | |
| [44] | Images | 3D CNN | CT slices | 86.7% | |
| 2021 | |||||
| [79] | Images | Deep learning | CT | 86.30%. | |
| [86] | Images | CNN | CT | 92.21% | |
| 2022 | [18] | Images | CP-CXR | CXR | 100% |
| [62] | Image | Deep learning | CXR | 97.67% | |
| [67] | Image | join-fusion AI system | CXR | 97% | |
| [69] | Image | CovNNet | CXR | 80.9% | |
| [78] | XR-CAPS/UNet | CXR | 93.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozsahin, D.U.; Isa, N.A.; Uzun, B. The Capacity of Artificial Intelligence in COVID-19 Response: A Review in Context of COVID-19 Screening and Diagnosis. Diagnostics 2022, 12, 2943. https://doi.org/10.3390/diagnostics12122943
Ozsahin DU, Isa NA, Uzun B. The Capacity of Artificial Intelligence in COVID-19 Response: A Review in Context of COVID-19 Screening and Diagnosis. Diagnostics. 2022; 12(12):2943. https://doi.org/10.3390/diagnostics12122943
Chicago/Turabian StyleOzsahin, Dilber Uzun, Nuhu Abdulhaqq Isa, and Berna Uzun. 2022. "The Capacity of Artificial Intelligence in COVID-19 Response: A Review in Context of COVID-19 Screening and Diagnosis" Diagnostics 12, no. 12: 2943. https://doi.org/10.3390/diagnostics12122943






