Transition to Fast Whole-Body SPECT/CT Bone Imaging: An Assessment of Image Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
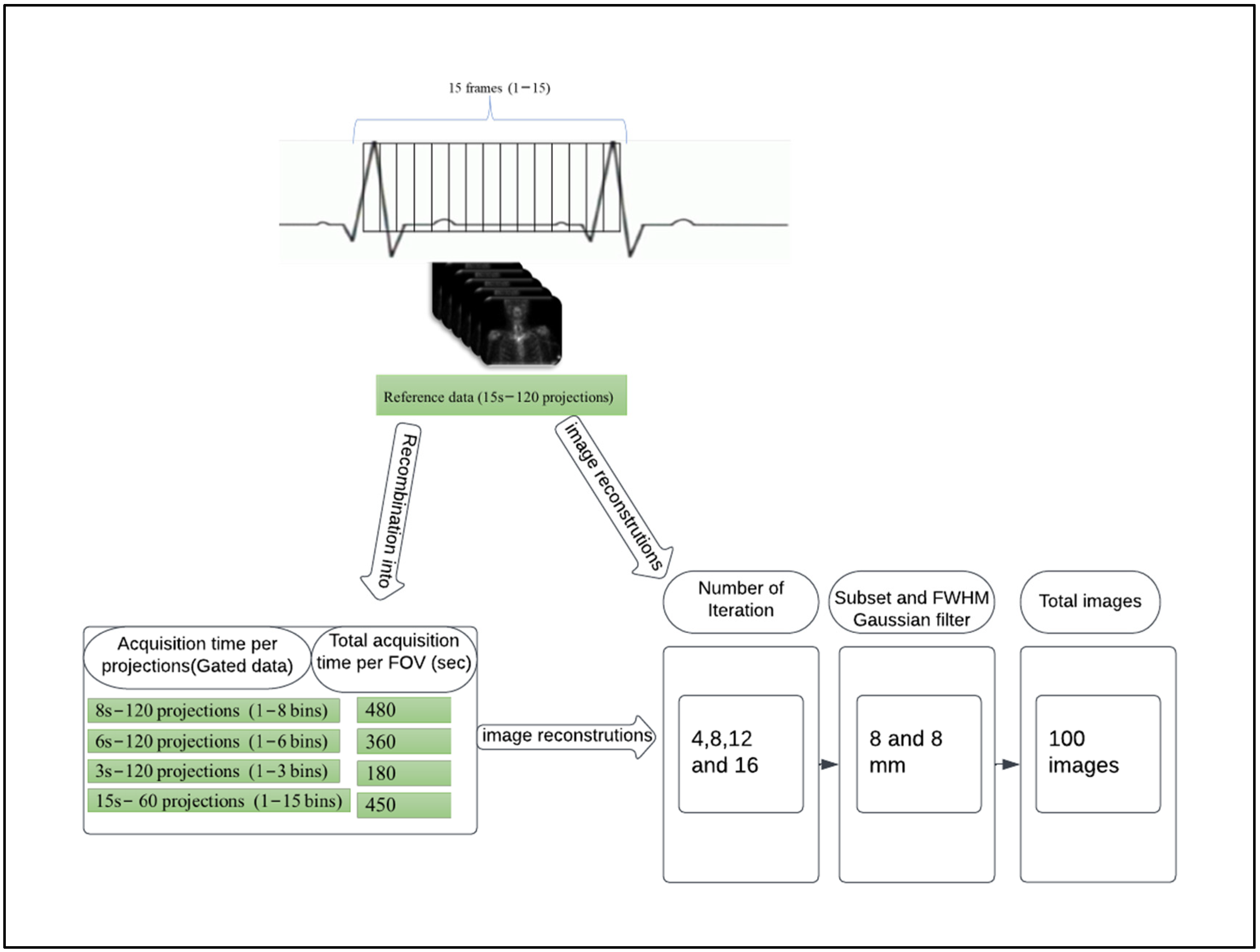
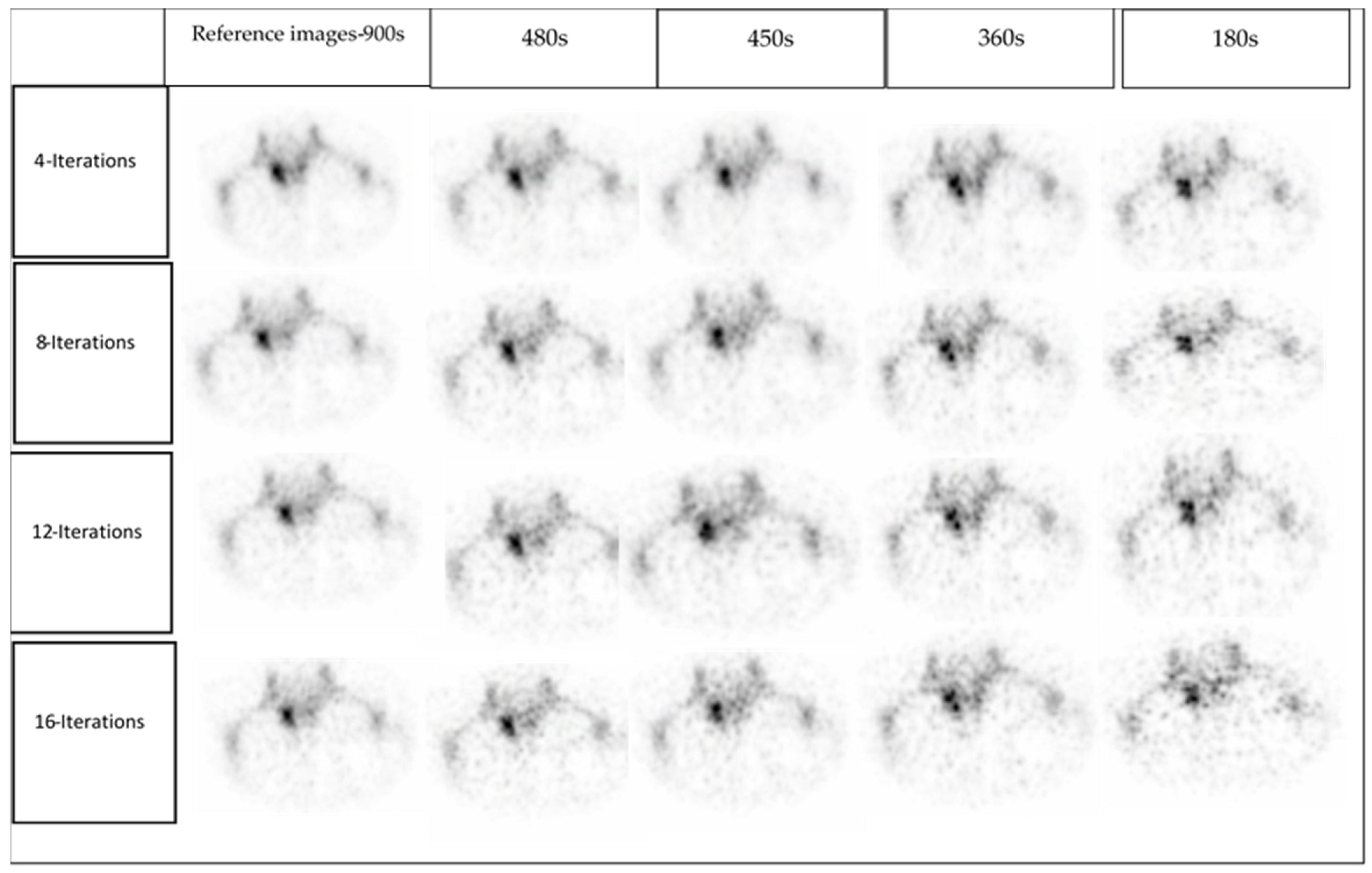
2.2. Data Acquisition and Image Gating
2.3. Image Reconstruction
- (1)
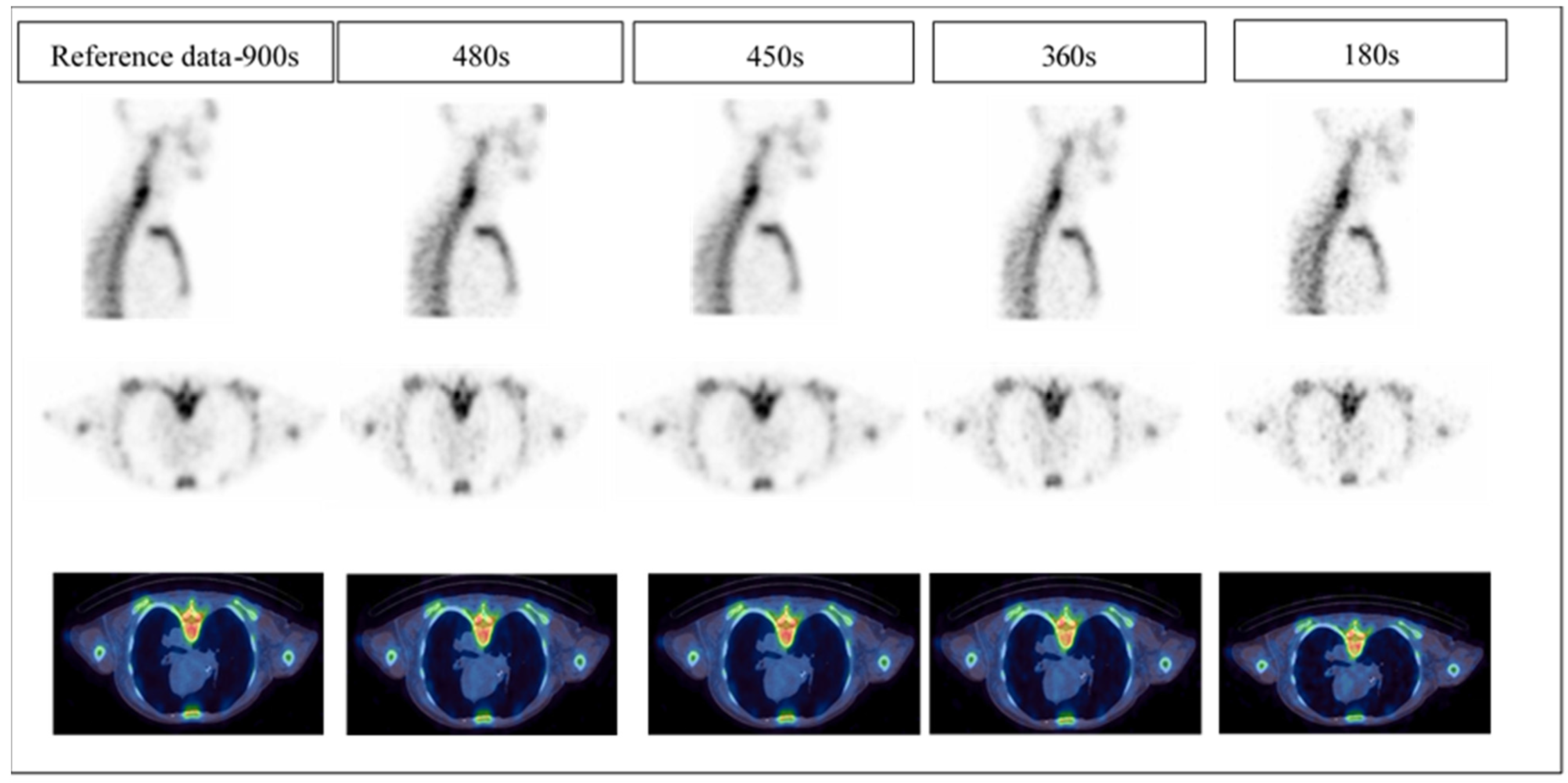
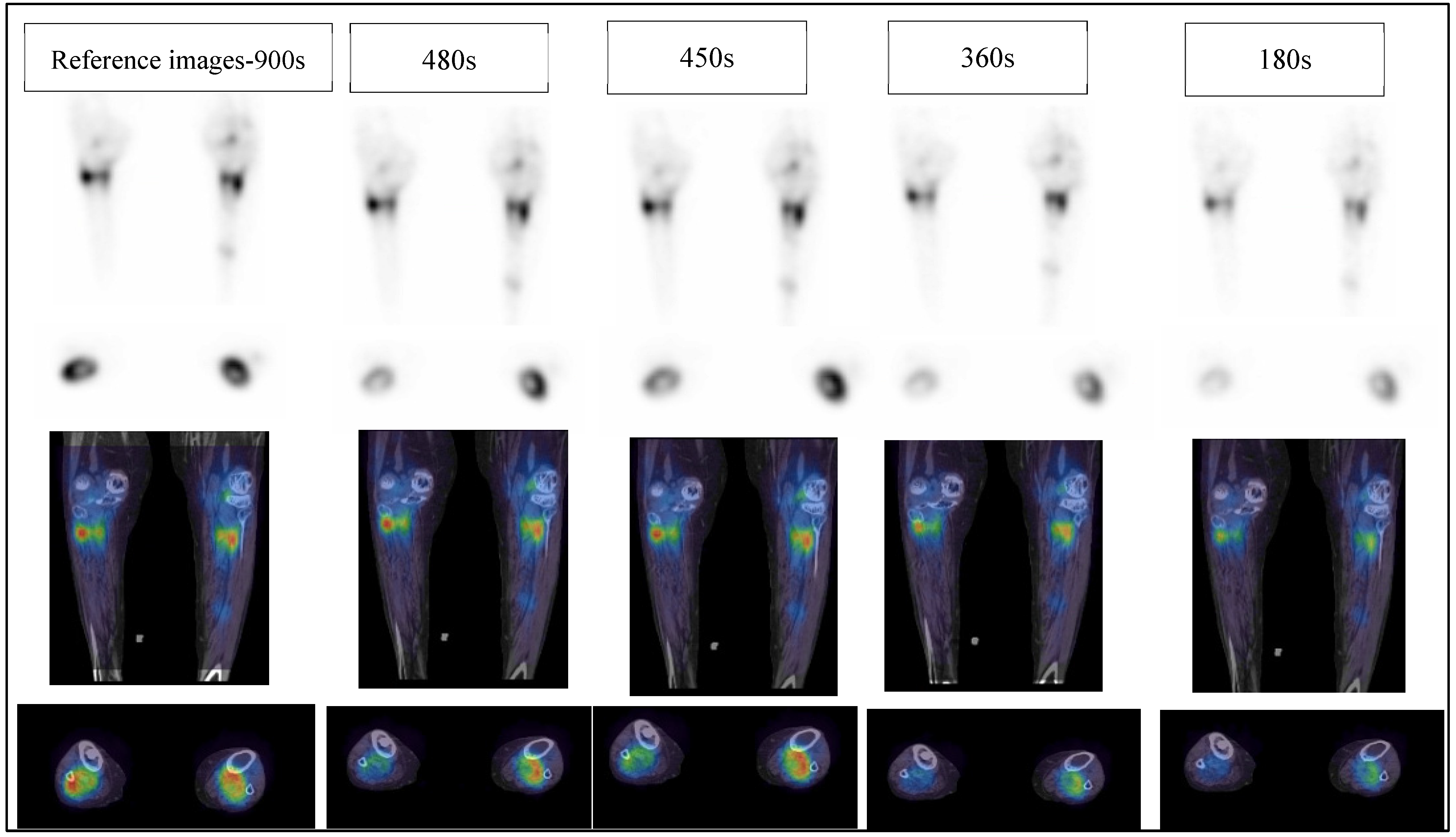
- Reference: full acquisition time (15 s per projection) reconstructed with a total of 120 views (60 stops) resulted in a total acquisition time of 900 s.
- (2)
- Reduced acquisition of 8, 6, and 3 s per projection angle with a total of 120 views (60 stops) resulted in a total acquisition time of 480, 360 and 180 s, respectively.
- (3)
- Full acquisition duration (15 s per projection) with a total of 60 projections (30 stops) resulted in a total acquisition time of 450 s.
2.4. Clinical Image Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Clinical Image Quality Assessment
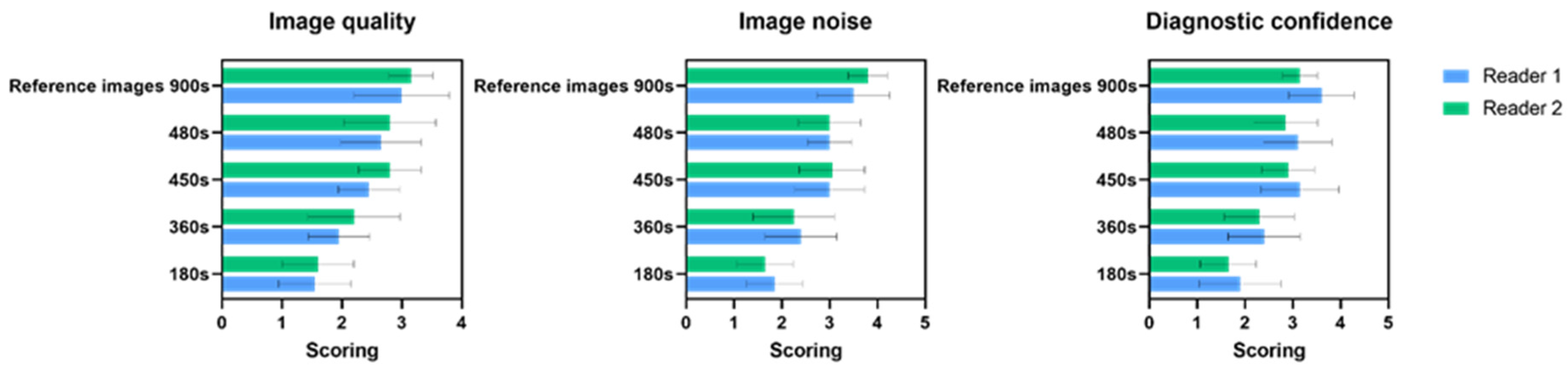
3.1.1. Image Quality
3.1.2. Image Noise
3.1.3. Diagnostics Confidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Helyar, V.; Mohan, H.K.; Barwick, T.; Livieratos, L.; Gnanasegaran, G.; Clarke, S.E.; Fogelman, I. The added value of multislice SPECT/CT in patients with equivocal bony metastasis from carcinoma of the prostate. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Palmedo, H.; Marx, C.; Ebert, A.; Kreft, B.; Ko, Y.; Türler, A.; Vorreuther, R.; Göhring, U.; Schild, H.; Gerhardt, T. Whole-body SPECT/CT for bone scintigraphy: Diagnostic value and effect on patient management in oncological patients. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Ritt, P.; Sanders, J.; Kuwert, T. SPECT/CT technology. Clin. Transl. Imaging 2014, 2, 445–457. [Google Scholar] [CrossRef]
- Stansfield, E.C.; Sheehy, N.; Zurakowski, D.; Vija, A.H.; Fahey, F.H.; Treves, S.T. Pediatric 99mTc-MDP bone SPECT with ordered subset expectation maximization iterative reconstruction with isotropic 3D resolution recovery. Radiology 2010, 257, 793–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grootjans, W.; Meeuwis, A.P.; Slump, C.H.; de Geus-Oei, L.-F.; Gotthardt, M.; Visser, E.P. Performance of 3DOSEM and MAP algorithms for reconstructing low count SPECT acquisitions. Z. Med. Phys. 2016, 26, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Ritt, P.; Kuwert, T. Quantitative SPECT/CT—Technique and Clinical Applications. In Molecular Imaging in Oncology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 565–590. [Google Scholar]
- Even-Sapir, E.; Metser, U.; Mishani, E.; Lievshitz, G.; Lerman, H.; Leibovitch, I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F- Fluoride PET/CT. J. Nucl. Med. 2006, 47, 287–297. [Google Scholar] [PubMed]
- Fleury, V.; Ferrer, L.; Colombié, M.; Rusu, D.; Le Thiec, M.; Kraeber-Bodéré, F.; Campion, L.; Rousseau, C. Advantages of systematic trunk SPECT/CT to planar bone scan (PBS) in more than 300 patients with breast or prostate cancer. Oncotarget 2018, 9, 31744. [Google Scholar] [CrossRef] [Green Version]
- Alqahtani, M.; Fulton, R.; Constable, C.; Willowson, K.; Kench, P. Diagnostic performance of whole-body SPECT/CT in bone metastasis detection using 99mTc-labelled diphosphate: A systematic review and meta-analysis. Clin. Radiol. 2020, 75, 961.e11–961.e24. [Google Scholar] [CrossRef]
- Israel, O.; Pellet, O.; Biassoni, L.; De Palma, D.; Estrada-Lobato, E.; Gnanasegaran, G.; Kuwert, T.; la Fougère, C.; Mariani, G.; Massalha, S. Two decades of SPECT/CT–the coming of age of a technology: An updated review of literature evidence. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1990–2012. [Google Scholar] [CrossRef] [Green Version]
- Abikhzer, G.; Gourevich, K.; Kagna, O.; Israel, O.; Frenkel, A.; Keidar, Z. Whole-body bone SPECT in breast cancer patients: The future bone scan protocol? Nucl. Med. Commun. 2016, 37, 247–253. [Google Scholar] [CrossRef]
- Herholz, K.; Teipel, S.; Hellwig, S.; Langner, S.; Rijntjes, M.; Klöppel, S.; Weiller, C.; Meyer, P.T. Functional and Molecular Neuroimaging. In Bradley and Daroff’s Neurology in Clinical Practice; Jankovic, J.M.D., Mazziotta, J.C.M.D.P., Pomeroy, S.L.M.D.P., Newman, N.J.M.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 576–600.e510. [Google Scholar]
- Seo, Y.; Mari, C.; Hasegawa, B.H. Technological development and advances in single-photon emission computed tomography/computed tomography. In Seminars in Nuclear Medicine; WB Saunders: Philadelphia, PA, USA, 2008; pp. 177–198. [Google Scholar]
- Alqahtani, M.M.; Willowson, K.P.; Constable, C.; Fulton, R.; Kench, P.L. Optimization of 99mTc whole-body SPECT/CT image quality: A phantom study. J. Appl. Clin. Med. Phys. 2022, 23, e13528. [Google Scholar] [CrossRef]
- Salkica, N.; Begic, A.; Zubovic, S.; Ceric, S.; Basic, A.; Sehic, A.; Julardzija, F.; Tinjak, E. Impact of Reduced Acquisition Time on Bone Single-photon Emission Computed Tomography Images in Oncology Patients. Acta Inform. Med. 2022, 30, 36. [Google Scholar] [CrossRef]
- Bailey, D.L.; Schembri, G.P.; Harris, B.E.; Bailey, E.A.; Cooper, R.A.; Roach, P.J. Generation of planar images from lung ventilation/perfusion SPECT. Ann. Nucl. Med. 2008, 22, 437–445. [Google Scholar] [CrossRef]
- Kyrtatos, P.G.; Navalkissoor, S.; Burniston, M.; Wagner, T. Planar images reprojected from SPECT V/Q data perform similarly to traditional planar V/Q scans in the diagnosis of pulmonary embolism. Nucl. Med. Commun. 2013, 34, 445–450. [Google Scholar] [CrossRef]
- Zeintl, J.; Vija, A.H.; Yahil, A.; Hornegger, J.; Kuwert, T. Quantitative accuracy of clinical 99mTc SPECT/CT using ordered-subset expectation maximization with 3-dimensional resolution recovery, attenuation, and scatter correction. J. Nucl. Med. 2010, 51, 921–928. [Google Scholar] [CrossRef] [Green Version]
- Bailey, D.L.; Kalemis, A. Externally triggered gating of nuclear medicine acquisitions: A useful method for partitioning data. Phys. Med. Biol. 2005, 50, N55. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Hu, P.-C.; Wu, R.-Z.; Gu, Y.-S.; Chen, S.-G.; Yu, H.-J.; Wang, X.-Q.; Song, J.; Shi, H.-C. The image quality, lesion detectability, and acquisition time of 18F-FDG total-body PET/CT in oncological patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2507–2515. [Google Scholar] [CrossRef]
- Thientunyakit, T.; Taerakul, T.; Chaudakshetrin, P.; Ubolnuch, K.; Sirithongjak, K. A comparison of the diagnostic performance of half-time SPECT and multiplanar pelvic bone scan in patients with significant bladder artifacts. Nucl. Med. Commun. 2013, 34, 233–239. [Google Scholar] [CrossRef]
- Borges-Neto, S.; Pagnanelli, R.A.; Shaw, L.K.; Honeycutt, E.; Shwartz, S.C.; Adams, G.L.; Coleman, R.E. Clinical results of a novel wide beam reconstruction method for shortening scan time of Tc-99m cardiac SPECT perfusion studies. J. Nucl. Cardiol. 2007, 14, 555–565. [Google Scholar] [CrossRef]
- Aldridge, M.D.; Waddington, W.W.; Dickson, J.C.; Prakash, V.; Ell, P.J.; Bomanji, J.B. Clinical evaluation of reducing acquisition time on single-photon emission computed tomography image quality using proprietary resolution recovery software. Nucl. Med. Commun. 2013, 34, 1116–1123. [Google Scholar] [CrossRef]
- Kapsoritakis, N.; Stathaki, M.; Bourogianni, O.; Tsaroucha, A.; Papadaki, E.; Simos, P.; Koukouraki, S. Clinical impact of targeted single-photon emission computed tomography/computed tomography (SPECT/CT) bone scintigraphy on the assessment of bone metastasis in cancer patients. Nucl. Med. Commun. 2021, 42, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
| Patient | Gender | Age (Years) | Tracer | Injected Dose (MBq) | Diagnosis |
|---|---|---|---|---|---|
| 1 | F | 44 | 99mTc HDP | 841 | Cervical spine spondylodiscitis |
| 2 | M | 55 | 99mTc HDP | 840 | Degenerative changes with mild moderate Osteoplastic activity in cervical spine. |
| 3 | F | 76 | 99mTc HDP | 881 | Multiple fractures in both tibiae and right fibular head |
| 4 | M | 89 | 99mTc HDP | 892 | Osteoplastic activity in the left Lumber 5/sacrum 1 with degenerative change in the hip joints. |
| 5 | M | 60 | 99mTc HDP | 864 | Osteoplastic activity with degenerative changes |
| Image Quality | Image Noise | Diagnostic Confidence | |
|---|---|---|---|
| 1 | Unacceptable | Excessive noise | Not confident |
| 2 | Acceptable | Moderately increased | Slightly confident |
| 3 | Good | Average noise | Fairly confident |
| 4 | Excellent | Low image noise | Highly confident |
| Iterations Number | Reference Images-900 s | 480 s | 450 s | 360 s | 180 s | All | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | p Values (1) | Mean ± SD | p Values (1) | Mean ± SD | p Values (1) | Mean ± SD | p Values (1) | p Values (2) | |
| 4 | 3.50 ± 0.52 | 3.10 ± 0.73 | 0.79 | 2.80 ± 0.63 | 0.097 | 2.30 ± 0.82 | 0.011 * | 1.70 ± 82 | <0.001 * | <0.001 * |
| 8 | 3.20 ± 0.63 | 2.90 ± 0.73 | 0.86 | 2.60 ± 0.51 | 0.18 | 2.00 ± 0.47 | 0.001 * | 1.50 ± 0.52 | <0.001 * | <0.001 * |
| 12 | 2.90 ± 0.31 | 2.40 ± 0.69 | 0.18 | 2.60 ± 0.51 | 0.55 | 2.00 ± 0.47 | 0.005 * | 1.50 ± 0.52 | 0.001 * | <0.001 * |
| 16 | 2.70 ± 0.67 | 2.50 ± 0.52 | 0.73 | 2.50 ± 0.52 | 0.73 | 2.00 ± 0.81 | 0.054 | 1.60 ± 0.51 | 0.014 * | 0.004 * |
| Iterations Number | Reference Images-900 s | 480 s | 450 s | 360 s | 180 s | All | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | p Values (1) | Mean ± SD | p Values (1) | Mean ± SD | p Values (1) | Mean ± SD | p Values (1) | p Values (2) | |
| 4 | 4.00 ± 0.00 | 3.30 ± 0.48 | 0. 012 * | 3.10 ± 0.56 | 0.004 * | 2.30 ± 0.82 | <0.001 * | 2.00 ± 0.81 | <0.001 * | <0.001 * |
| 8 | 3.60 ± 0.69 | 3.10 ± 0.56 | 0.429 | 3.15 ± 0.73 | 0.542 | 2.40 ± 0.84 | 0.021 * | 1.60 ± 0.51 | <0.001 * | <0.001 * |
| 12 | 3.60 ± 0.69 | 2.90 ± 0.56 | 0.131 | 3.10 ± 0.87 | 0.638 | 2.40 ± 0.69 | 0.021 * | 1.70 ± 0.48 | 0.002 * | <0.001 * |
| 16 | 3.40 ± 0.69 | 2.70 ± 0.48 | 0.143 | 2.80 ± 0.63 | 0.320 | 2.20 ± 0.91 | 0.051 | 1.70 ± 0.48 | 0.002 * | <0.001 * |
| Iterations Number | Reference Images-900 s | 480 s | 450 s | 360 s | 180 s | All | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | p Values (1) | Mean ± SD | p Values (1) | Mean ± SD | p Values (1) | Mean ± SD | p Values (1) | p Values (2) | |
| 4 | 3.70 ± 0.48 | 3.10 ± 0.56 | 0.125 | 3.20 ± 0.78 | 0.458 | 2.50 ± 0.70 | 0.003 * | 1.90 ± 0.87 | <0.001 * | <0.001 * |
| 8 | 3.30 ± 0.67 | 3.10 ± 0.73 | 0.968 | 3.00 ± 0.47 | 0.777 | 2.50 ± 0.70 | 0.115 | 1.60 ± 0.51 | <0.001 * | <0.001 * |
| 12 | 3.40 ± 0.51 | 2.90 ± 0.87 | 0.546 | 3.00 ± 0.81 | 0.690 | 2.30 ± 0.67 | 0.006 * | 1.80 ± 0.91 | 0.002 * | <0.001 * |
| 16 | 3.10 ± 0.56 | 2.80 ± 0.63 | 0.798 | 2.90 ± 0.73 | 0.959 | 2.10 ± 0.87 | 0.082 | 1.80 ± 0.63 | 0.006 * | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, M.; Willowson, K.; Fulton, R.; Constable, C.; Kench, P. Transition to Fast Whole-Body SPECT/CT Bone Imaging: An Assessment of Image Quality. Diagnostics 2022, 12, 2938. https://doi.org/10.3390/diagnostics12122938
Alqahtani M, Willowson K, Fulton R, Constable C, Kench P. Transition to Fast Whole-Body SPECT/CT Bone Imaging: An Assessment of Image Quality. Diagnostics. 2022; 12(12):2938. https://doi.org/10.3390/diagnostics12122938
Chicago/Turabian StyleAlqahtani, Mansour, Kathy Willowson, Roger Fulton, Chris Constable, and Peter Kench. 2022. "Transition to Fast Whole-Body SPECT/CT Bone Imaging: An Assessment of Image Quality" Diagnostics 12, no. 12: 2938. https://doi.org/10.3390/diagnostics12122938
APA StyleAlqahtani, M., Willowson, K., Fulton, R., Constable, C., & Kench, P. (2022). Transition to Fast Whole-Body SPECT/CT Bone Imaging: An Assessment of Image Quality. Diagnostics, 12(12), 2938. https://doi.org/10.3390/diagnostics12122938






