Using Second Measurement of De Ritis Ratio to Improve Mortality Prediction in Adult Trauma Patients in Intensive Care Unit
Abstract
:1. Introduction
2. Materials and Methods
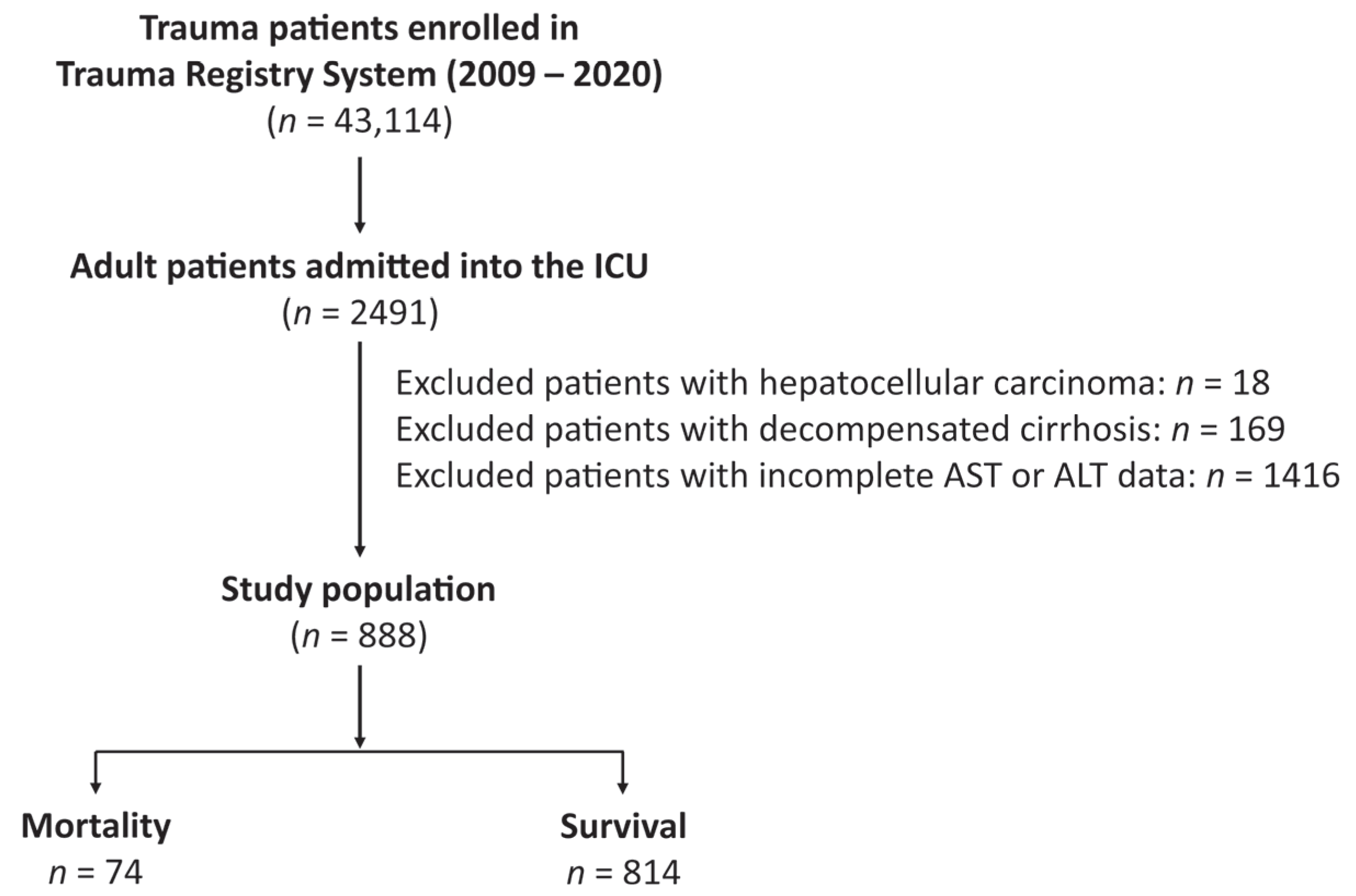
2.1. Study Population and Data Collection
2.2. Statistical Analyses
3. Results
3.1. The Patient and Injury Characteristics of the Death and Survival Patients
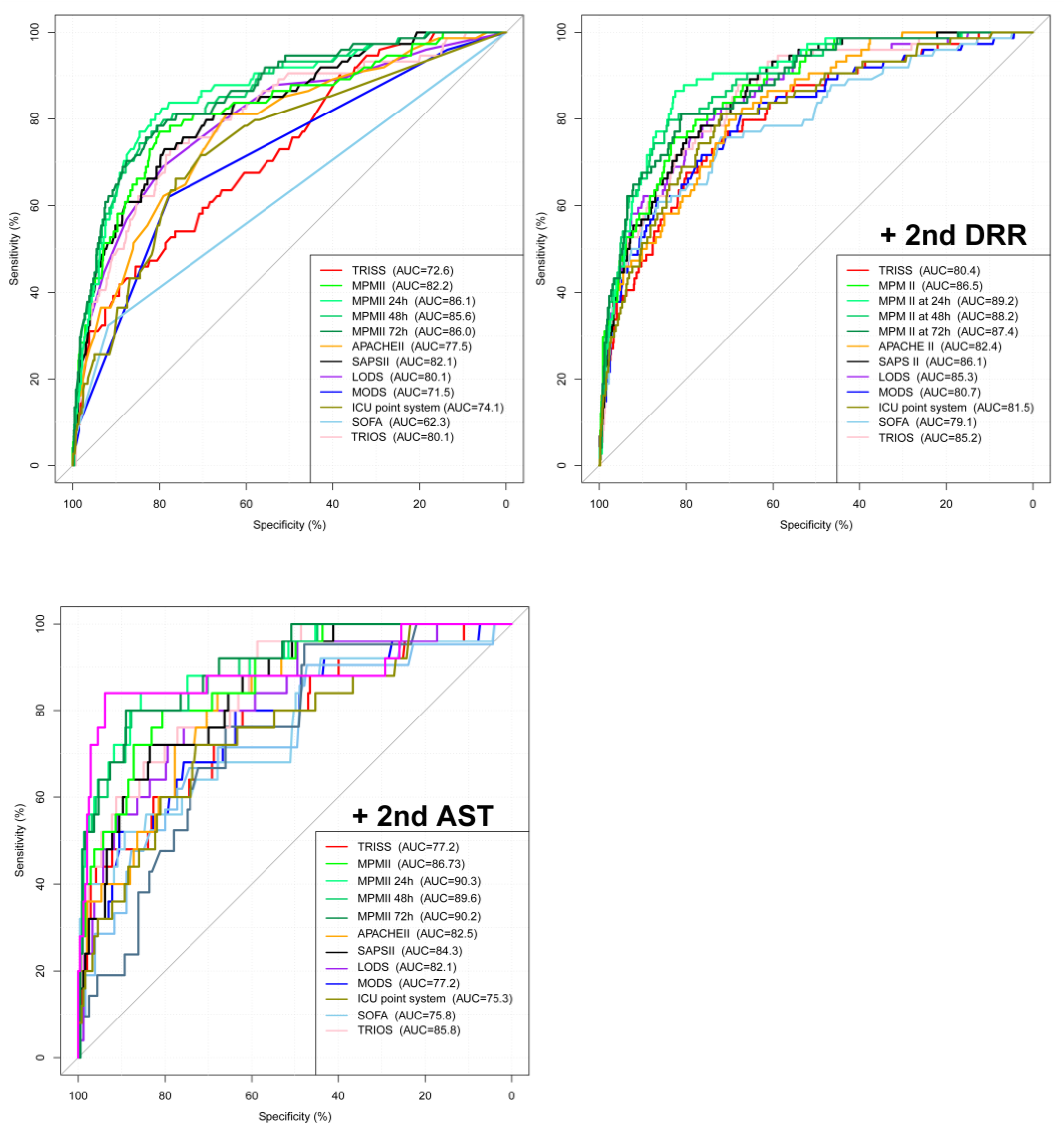
3.2. Analysis of the Plotted ROC Curve
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mo, Q.; Liu, Y.; Zhou, Z.; Li, R.; Gong, W.; Xiang, B.; Tang, W.; Yu, H. Prognostic Value of Aspartate Transaminase/Alanine Transaminase Ratio in Patients with Hepatitis B Virus-Related Hepatocellular Carcinoma Undergoing Hepatectomy. Front. Oncol. 2022, 12, 876900. [Google Scholar] [CrossRef] [PubMed]
- Darstein, F.; Häuser, F.; Straub, B.K.; Wenzel, J.J.; Conradi, R.; Mittler, J.; Lang, H.; Galle, P.R.; Zimmermann, T. Hepatitis E virus genotype 3 is a common finding in liver-transplanted patients undergoing liver biopsy for elevated liver enzymes with a low De Ritis ratio and suspected acute rejection: A real-world cohort. Clin. Transpl. 2018, 32, e13411. [Google Scholar] [CrossRef] [PubMed]
- Botros, M.; Sikaris, K.A. The de ritis ratio: The test of time. Clin. Biochem. Rev. 2013, 34, 117–130. [Google Scholar] [PubMed]
- Sookoian, S.; Pirola, C.J. Liver enzymes, metabolomics and genome-wide association studies: From systems biology to the personalized medicine. World J. Gastroenterol. 2015, 21, 711–725. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Alanine and aspartate aminotransferase and glutamine-cycling pathway: Their roles in pathogenesis of metabolic syndrome. World J. Gastroenterol. 2012, 18, 3775–3781. [Google Scholar] [CrossRef] [PubMed]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Quhal, F.; Abufaraj, M.; Janisch, F.; Mori, K.; Lysenko, I.; Mostafaei, H.; D’Andrea, D.; Mathieu, R.; Enikeev, D.V.; Fajkovic, H.; et al. The significance of De Ritis ratio in patients with radiation-recurrent prostate cancer undergoing salvage radical prostatectomy. Arab J. Urol. 2020, 18, 213–218. [Google Scholar] [CrossRef]
- Li, J.; Cao, D.; Peng, L.; Meng, C.; Xia, Z.; Li, Y.; Wei, Q. Potential Clinical Value of Pretreatment De Ritis Ratio as a Prognostic Biomarker for Renal Cell Carcinoma. Front. Oncol. 2021, 11, 780906. [Google Scholar] [CrossRef] [PubMed]
- Fukui-Kawaura, S.; Kawahara, T.; Araki, Y.; Nishimura, R.; Uemura, K.; Namura, K.; Mizuno, N.; Yao, M.; Uemura, H.; Ikeda, I. A higher De Ritis ratio (AST/ALT) is a risk factor for progression in high-risk non-muscle invasive bladder cancer. Oncotarget 2021, 12, 917–922. [Google Scholar] [CrossRef]
- Batur, A.F.; Aydogan, M.F.; Kilic, O.; Korez, M.K.; Gul, M.; Kaynar, M.; Goktas, S.; Akand, M. Comparison of De Ritis Ratio and other systemic inflammatory parameters for the prediction of prognosis of patients with transitional cell bladder cancer. Int. J. Clin. Pract. 2021, 75, e13743. [Google Scholar] [CrossRef]
- Uleri, A.; Hurle, R.; Contieri, R.; Diana, P.; Buffi, N.; Lazzeri, M.; Saita, A.; Casale, P.; Guazzoni, G.; Lughezzani, G. Combination of AST to ALT and neutrophils to lymphocytes ratios as predictors of locally advanced disease in patients with bladder cancer subjected to radical cystectomy: Results from a single-institutional series. Urologia 2022, 89, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Knittelfelder, O.; Delago, D.; Jakse, G.; Reinisch, S.; Partl, R.; Stranzl-Lawatsch, H.; Renner, W.; Langsenlehner, T. The AST/ALT (De Ritis) Ratio Predicts Survival in Patients with Oral and Oropharyngeal Cancer. Diagnostics 2020, 10, 973. [Google Scholar] [CrossRef] [PubMed]
- Ghahari, M.; Salari, A.; Ghafoori Yazdi, M.; Nowroozi, A.; Fotovat, A.; Momeni, S.A.; Nowroozi, M.R.; Amini, E. Association Between Preoperative De Ritis (AST/ALT) Ratio and Oncological Outcomes Following Radical Cystectomy in Patients with Urothelial Bladder Cancer. Clin. Genitourin Cancer 2022, 20, e89–e93. [Google Scholar] [CrossRef] [PubMed]
- Pilarczyk, K.; Carstens, H.; Heckmann, J.; Canbay, A.; Koch, A.; Pizanis, N.; Jakob, H.; Kamler, M. The aspartate transaminase/alanine transaminase (DeRitis) ratio predicts mid-term mortality and renal and respiratory dysfunction after left ventricular assist device implantation. Eur. J. Cardiothorac Surg. 2017, 52, 781–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.Y.; Yu, J.; Hong, J.H.; Lim, B.; Kim, Y.; Hwang, J.H.; Kim, Y.K. Elevated De Ritis Ratio as a Predictor for Acute Kidney Injury after Radical Retropubic Prostatectomy. J. Pers. Med. 2021, 11, 836. [Google Scholar] [CrossRef] [PubMed]
- He, H.M.; He, C.; Zhang, S.C.; You, Z.B.; Lin, X.Q.; Luo, M.Q.; Lin, M.Q.; Guo, Y.S.; Zheng, W.P.; Lin, K.Y. Predictive value of aspartate aminotransferase-to-alanine aminotransferase ratio for contrast-associated acute kidney injury in patients undergoing elective percutaneous coronary intervention. J. Cardiol. 2022, 79, 618–625. [Google Scholar] [CrossRef]
- Steininger, M.; Winter, M.P.; Reiberger, T.; Koller, L.; El-Hamid, F.; Forster, S.; Schnaubelt, S.; Hengstenberg, C.; Distelmaier, K.; Goliasch, G.; et al. De-Ritis Ratio Improves Long-Term Risk Prediction after Acute Myocardial Infarction. J. Clin. Med. 2018, 7, 474. [Google Scholar] [CrossRef] [Green Version]
- Jasiewicz, M.; Siedlaczek, M.; Kasprzak, M.; Gorog, D.A.; Jilma, B.; Siller-Matula, J.; Obońska, K.; Dobosiewicz, R.; Pstrągowski, K.; Kubica, J. Elevated serum transaminases in patients with acute coronary syndromes: Do we need a revision of exclusion criteria for clinical trials? Cardiol. J. 2021; ahead of print. [Google Scholar] [CrossRef]
- Djakpo, D.K.; Wang, Z.Q.; Shrestha, M. The significance of transaminase ratio (AST/ALT) in acute myocardial infarction. Arch Med. Sci. Atheroscler Dis. 2020, 5, e279–e283. [Google Scholar] [CrossRef]
- Zhao, P.Y.; Yao, R.Q.; Ren, C.; Li, S.Y.; Li, Y.X.; Zhu, S.Y.; Yao, Y.M.; Du, X.H. De Ritis Ratio as a Significant Prognostic Factor in Patients with Sepsis: A Retrospective Analysis. J. Surg. Res. 2021, 264, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Arru, F.; De Vito, A.; Sassu, A.; Valdes, G.; Scano, V.; Zinellu, E.; Perra, R.; Madeddu, G.; Carru, C.; et al. The De Ritis ratio as prognostic biomarker of in-hospital mortality in COVID-19 patients. Eur. J. Clin. Investig. 2021, 51, e13427. [Google Scholar] [CrossRef]
- Pranata, R.; Huang, I.; Lim, M.A.; Yonas, E.; Vania, R.; Lukito, A.A.; Nasution, S.A.; Siswanto, B.B.; Kuswardhani, R.A.T. Elevated De Ritis Ratio Is Associated with Poor Prognosis in COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 676581. [Google Scholar] [CrossRef] [PubMed]
- Guzey-Aras, Y.; Yazar, H.; Acar, T.; Kayacan, Y.; Acar, B.A.; Boncuk, S.; Eryilmaz, H.A. The Role of De Ritis Ratio as a Clinical Prognostic Parameter in COVID 19 Patients. Clin. Lab 2021, 67, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Yashashwini, A.; Vedavathi, R. The Study of De Ritis (Ast/ Alt) Ratio in Comparision with Other Parameters for Predicting Poor Prognosis in Covid 19 Patients. J. Assoc. Physicians India 2022, 70, 11–12. [Google Scholar]
- Bulgarelli, L.; Deliberato, R.O.; Johnson, A.E.W. Prediction on critically ill patients: The role of “big data”. J. Crit. Care 2020, 60, 64–68. [Google Scholar] [CrossRef]
- Fu, L.H.; Schwartz, J.; Moy, A.; Knaplund, C.; Kang, M.J.; Schnock, K.O.; Garcia, J.P.; Jia, H.; Dykes, P.C.; Cato, K.; et al. Development and validation of early warning score system: A systematic literature review. J. Biomed. Inf. 2020, 105, 103410. [Google Scholar] [CrossRef] [PubMed]
- Keuning, B.E.; Kaufmann, T.; Wiersema, R.; Granholm, A.; Pettilä, V.; Møller, M.H.; Christiansen, C.F.; Castela Forte, J.; Snieder, H.; Keus, F.; et al. Mortality prediction models in the adult critically ill: A scoping review. Acta Anaesthesiol. Scand 2020, 64, 424–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinuff, T.; Adhikari, N.K.; Cook, D.J.; Schünemann, H.J.; Griffith, L.E.; Rocker, G.; Walter, S.D. Mortality predictions in the intensive care unit: Comparing physicians with scoring systems. Crit. Care Med. 2006, 34, 878–885. [Google Scholar] [CrossRef]
- Timsit, J.F.; Fosse, J.P.; Troché, G.; De Lassence, A.; Alberti, C.; Garrouste-Orgeas, M.; Azoulay, E.; Chevret, S.; Moine, P.; Cohen, Y. Accuracy of a composite score using daily SAPS II and LOD scores for predicting hospital mortality in ICU patients hospitalized for more than 72 h. Intensive Care Med. 2001, 27, 1012–1021. [Google Scholar] [CrossRef]
- Breslow, M.J.; Badawi, O. Severity scoring in the critically ill: Part 1--interpretation and accuracy of outcome prediction scoring systems. Chest 2012, 141, 245–252. [Google Scholar] [CrossRef]
- Lemeshow, S.; Le Gall, J.R. Modeling the severity of illness of ICU patients. A systems update. JAMA 1994, 272, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, J.R. The use of severity scores in the intensive care unit. Intensive Care Med. 2005, 31, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. Jama 1993, 270, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Lemeshow, S.; Klar, J.; Teres, D.; Avrunin, J.S.; Gehlbach, S.H.; Rapoport, J.; Rué, M. Mortality probability models for patients in the intensive care unit for 48 or 72 h: A prospective, multicenter study. Crit. Care Med. 1994, 22, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Sekulic, A.D.; Trpkovic, S.V.; Pavlovic, A.P.; Marinkovic, O.M.; Ilic, A.N. Scoring Systems in Assessing Survival of Critically Ill ICU Patients. Med. Sci. Monit 2015, 21, 2621–2629. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.C.; Chou, S.E.; Liu, H.T.; Hsieh, T.M.; Su, W.T.; Chien, P.C.; Hsieh, C.H. Performance of Prognostic Scoring Systems in Trauma Patients in the Intensive Care Unit of a Trauma Center. Int. J. Env. Res. Public Health 2020, 17, 7226. [Google Scholar] [CrossRef]
- Le Gall, J.R.; Klar, J.; Lemeshow, S.; Saulnier, F.; Alberti, C.; Artigas, A.; Teres, D. The Logistic Organ Dysfunction system. A new way to assess organ dysfunction in the intensive care unit. ICU Scoring Group. JAMA 1996, 276, 802–810. [Google Scholar] [CrossRef]
- Marshall, J.C.; Cook, D.J.; Christou, N.V.; Bernard, G.R.; Sprung, C.L.; Sibbald, W.J. Multiple organ dysfunction score: A reliable descriptor of a complex clinical outcome. Crit. Care Med. 1995, 23, 1638–1652. [Google Scholar] [CrossRef]
- Vassar, M.J.; Wilkerson, C.L.; Duran, P.J.; Perry, C.A.; Holcroft, J.W. Comparison of APACHE II, TRISS, and a proposed 24-h ICU point system for prediction of outcome in ICU trauma patients. J. Trauma 1992, 32, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; de Mendonça, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M.; Sprung, C.L.; Colardyn, F.; Blecher, S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, C.H.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Differences between the sexes in motorcycle-related injuries and fatalities at a Taiwanese level I trauma center. Biomed. J. 2017, 40, 113–120. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Liu, H.T.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Motorcycle-related hospitalizations of the elderly. Biomed. J. 2017, 40, 121–128. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Chen, Y.C.; Hsu, S.Y.; Hsieh, H.Y.; Chien, P.C. Defining polytrauma by abbreviated injury scale >/= 3 for a least two body regions is insufficient in terms of short-term outcome: A cross-sectional study at a level I trauma center. Biomed. J. 2018, 41, 321–327. [Google Scholar] [CrossRef]
- Crismale, J.F.; Friedman, S.L. Acute Liver Injury and Decompensated Cirrhosis. Med. Clin. N. Am. 2020, 104, 647–662. [Google Scholar] [CrossRef]
- Elf, S.E.; Chen, J. Targeting glucose metabolism in patients with cancer. Cancer 2014, 120, 774–780. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.Y.; Hsu, C.Y.; Chiou, H.Y.; Lee, H.A.; Hsu, L.M.; Chang, P.Y.; Kurniawan, A.L.; Chao, J.C. Association between Dietary Patterns and Serum Hepatic Enzyme Levels in Adults with Dyslipidemia and Impaired Fasting Plasma Glucose. Nutrients 2021, 13, 987. [Google Scholar] [CrossRef]
- Colomba, J.; Netedu, S.R.; Lehoux-Dubois, C.; Coriati, A.; Boudreau, V.; Tremblay, F.; Cusi, K.; Rabasa-Lhoret, R.; Leey, J.A. Hepatic enzyme ALT as a marker of glucose abnormality in men with cystic fibrosis. PLoS ONE 2019, 14, e0219855. [Google Scholar] [CrossRef] [Green Version]
- Zoppini, G.; Cacciatori, V.; Negri, C.; Stoico, V.; Lippi, G.; Targher, G.; Bonora, E. The aspartate aminotransferase-to-alanine aminotransferase ratio predicts all-cause and cardiovascular mortality in patients with type 2 diabetes. Medicine 2016, 95, e4821. [Google Scholar] [CrossRef]
- Sripradha, R.; Sridhar, M.G.; Agrawal, A. Can protein carbonyl/glutathione ratio be used as a potential biomarker to assess oxidative stress in alcoholic hepatitis? Indian J. Med. Sci. 2010, 64, 476–483. [Google Scholar] [PubMed]
- Tai, Y.S.; Chen, C.H.; Huang, C.Y.; Tai, H.C.; Wang, S.M.; Pu, Y.S. Diabetes mellitus with poor glycemic control increases bladder cancer recurrence risk in patients with upper urinary tract urothelial carcinoma. Diabetes Metab. Res. Rev. 2015, 31, 307–314. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.E.; Byun, S.S.; Kim, H.H.; Kwak, C.; Hong, S.K. De Ritis ratio (aspartate transaminase/alanine transaminase ratio) as a significant prognostic factor after surgical treatment in patients with clear-cell localized renal cell carcinoma: A propensity score-matched study. BJU Int. 2017, 119, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Rief, P.; Pichler, M.; Raggam, R.; Hafner, F.; Gerger, A.; Eller, P.; Brodmann, M.; Gary, T. The AST/ALT (De-Ritis) ratio: A novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. Medicine 2016, 95, e3843. [Google Scholar] [CrossRef]
- Tan, X.; Xiao, K.; Liu, W.; Chang, S.; Zhang, T.; Tang, H. Prognostic factors of distal cholangiocarcinoma after curative surgery: A series of 84 cases. Hepatogastroenterology 2013, 60, 1892–1895. [Google Scholar] [PubMed]

| Variables | Total | Mortality | p-Value | ||
|---|---|---|---|---|---|
| (n = 888) | No (n = 814) | Yes (n = 74) | |||
| Sex | Female | 289 (32.6%) | 265 (32.6%) | 24 (32.4%) | >0.999 |
| Male | 599 (67.5%) | 549 (67.4%) | 50 (67.6%) | ||
| HTN | No | 597 (67.2%) | 558 (68.6%) | 39 (52.7%) | 0.007 |
| Yes | 291 (32.8%) | 256 (31.5%) | 35 (47.3%) | ||
| CAD | No | 817 (92.0%) | 754 (92.6%) | 63 (85.1%) | 0.040 |
| Yes | 71 (8.0%) | 60 (7.4%) | 11 (14.9%) | ||
| ESRD | No | 868 (97.8%) | 802 (98.5%) | 66 (89.2%) | <0.001 |
| Yes | 20 (2.3%) | 12 (1.5%) | 8 (10.8%) | ||
| CVA | No | 854 (96.2%) | 785 (96.4%) | 69 (93.2%) | 0.194 |
| Yes | 34 (3.8%) | 29 (3.6%) | 5 (6.8%) | ||
| DM | No | 718 (80.9%) | 663 (81.5%) | 55 (74.3%) | 0.163 |
| Yes | 170 (19.1%) | 151 (18.6%) | 19 (25.7%) | ||
| AIS (Head) | 0 | 225 (25.3%) | 214 (26.3%) | 11 (14.9%) | <0.001 |
| 1 | 8 (0.9%) | 8 (1.0%) | 0 (0.0%) | ||
| 2 | 18 (2.0%) | 17 (2.1%) | 1 (1.4%) | ||
| 3 | 76 (8.6%) | 72 (8.9%) | 4 (5.4%) | ||
| 4 | 400 (45.1%) | 376 (46.2%) | 24 (32.4%) | ||
| 5 | 160 (18.0%) | 127 (15.6%) | 33 (44.6%) | ||
| 6 | 1 (0.1%) | 0 (0.0%) | 1 (1.4%) | ||
| AIS (Face) | 0 | 715 (80.5%) | 651 (80.0%) | 64 (86.5%) | 0.357 |
| 1 | 20 (2.3%) | 20 (2.5%) | 0 (0.0%) | ||
| 2 | 146 (16.4%) | 136 (16.7%) | 10 (13.5%) | ||
| 3 | 7 (0.8%) | 7 (0.9%) | 0 (0.0%) | ||
| AIS (Thorax) | 0 | 569 (64.1%) | 524 (64.4%) | 45 (60.8%) | 0.523 |
| 1 | 25 (2.8%) | 25 (3.1%) | 0 (0.0%) | ||
| 2 | 56 (6.3%) | 51 (6.3%) | 5 (6.8%) | ||
| 3 | 134 (15.1%) | 122 (15.0%) | 12 (16.2%) | ||
| 4 | 91 (10.3%) | 81 (10.0%) | 10 (13.5%) | ||
| 5 | 13 (1.5%) | 11 (1.4%) | 2 (2.7%) | ||
| AIS (Abdomen) | 0 | 668 (75.2%) | 605 (74.3%) | 63 (85.1%) | 0.040 |
| 2 | 77 (8.7%) | 75 (9.2%) | 2 (2.7%) | ||
| 3 | 80 (9.0%) | 78 (9.6%) | 2 (2.7%) | ||
| 4 | 43 (4.8%) | 37 (4.6%) | 6 (8.1%) | ||
| 5 | 20 (2.3%) | 19 (2.3%) | 1 (1.4%) | ||
| AIS (Extremity) | 0 | 523 (58.9%) | 472 (58.0%) | 51 (68.9%) | 0.528 |
| 1 | 5 (0.6%) | 5 (0.6%) | 0 (0.0%) | ||
| 2 | 214 (24.1%) | 202 (24.8%) | 12 (16.2%) | ||
| 3 | 129 (14.5%) | 119 (14.6%) | 10 (13.5%) | ||
| 4 | 16 (1.8%) | 15 (1.8%) | 1 (1.4%) | ||
| 5 | 1 (0.1%) | 1 (0.1%) | 0 (0.0%) | ||
| AIS (External) | 0 | 848 (95.5%) | 779 (95.7%) | 69 (93.2%) | 0.667 |
| 1 | 28 (3.2%) | 25 (3.1%) | 3 (4.1%) | ||
| 2 | 7 (0.8%) | 6 (0.7%) | 1 (1.4%) | ||
| 3 | 1 (0.1%) | 1 (0.1%) | 0 (0.0%) | ||
| 4 | 1 (0.1%) | 1 (0.1%) | 0 (0.0%) | ||
| 5 | 3 (0.3%) | 2 (0.3%) | 1 (1.4%) | ||
| Variables | Total | Mortality | p-Value | |
|---|---|---|---|---|
| (n = 888) | No (n = 814) | Yes (n = 74) | ||
| Age (years) | 59 [40, 70] | 58 [39, 69] | 71 [58, 78] | <0.001 |
| BMI | 23.9 [21.4, 27.1] | 23.9 [21.4, 27.1] | 23.8 [21.4, 28.0] | 0.861 |
| Temperature (°C) | 36.5 [36.1, 37.0] | 36.5 [36.1, 37.0] | 36.2 [36.0, 36.7] | 0.001 |
| HR (beats/min) | 92 [80, 106] | 92 [80, 106] | 98 [86, 112] | 0.030 |
| SBP (mmHg) | 140 [122, 156] | 139 [123, 156] | 141 [116, 158] | 0.675 |
| RR (times/min) | 19 [18, 20] | 19 [18, 20] | 20 [17, 22] | 0.932 |
| GCS | 11 [7, 15] | 11 [8, 15] | 6 [3, 11] | <0.001 |
| ISS | 20 [16, 25] | 20 [16, 25] | 25 [19, 29] | <0.001 |
| Glucose (mg/dL) | 159 [133, 205] | 158 [132, 198] | 197 [157, 250] | <0.001 |
| HCO3 (meq/L) | 21.7 [19.3, 23.6] | 21.8 [19.4, 23.7] | 20.4 [18.5, 22.5] | 0.003 |
| Na (mEq/L) | 138 [136, 140] | 138 [136, 140] | 139 [136, 141] | 0.599 |
| K (mEq/L) | 3.6 [3.2, 3.9] | 3.6 [3.3, 3.9] | 3.7 [3.1, 4.2] | 0.742 |
| RBC (106/μL) | 4.4 [3.9, 4.9] | 4.4 [3.9, 4.9] | 4.4 [3.9, 4.8] | 0.864 |
| WBC (103/μL) | 11.6 [8.4, 15.7] | 11.6 [8.6, 15.5] | 11.4 [7.5, 16.0] | 0.572 |
| Neutrophil (%) | 79.5 [68.1, 85.5] | 79.3 [68.0, 85.5] | 81.1 [69.5, 85.2] | 0.702 |
| Hb (g/dL) | 13.1 [11.5, 14.4] | 13.1 [11.6, 14.4] | 13.0 [10.9, 14.6] | 0.665 |
| Hct (%) | 39.2 [34.8, 42.9] | 39.2 [35.1, 42.9] | 39.7 [33.7, 42.6] | 0.594 |
| Platelets (103/μL) | 215 [169, 266] | 215 [169, 266] | 215 [172, 265] | 0.729 |
| INR | 1.05 [1.01, 1.12] | 1.05 [1.00, 1.12] | 1.09 [1.03, 1.20] | <0.001 |
| BUN (mg/dL) | 15 [11, 19] | 14 [11, 19] | 19 [13, 26] | <0.001 |
| Cr (mg/dL) | 0.96 [0.76, 1.21] | 0.94 [0.75, 1.19] | 1.19 [0.95, 2.06] | <0.001 |
| BUN/Cr | 14.97 [11.39, 19.23] | 15.04 [11.46, 19.25] | 13.69 [10.72, 19.09] | 0.192 |
| Albumin (g/dL) | 3.3 [2.9, 3.7] | 3.4 [2.9, 3.8] | 2.9 [2.5, 3.6] | <0.001 |
| Bilirubin (mg/dL) | 0.8 [0.6, 1.1] | 0.8 [0.6, 1.1] | 0.8 [0.5, 1.4] | 0.826 |
| 1st AST (U/L) | 53 [33, 128] | 53 [33, 128] | 54 [34, 118] | 0.768 |
| 1st ALT (U/L) | 37 [22, 88] | 37 [22, 92] | 29 [19, 61] | 0.050 |
| 2nd AST (U/L) | 40 [26, 66] | 39 [26, 63] | 57 [32, 107] | <0.001 |
| 2nd ALT (U/L) | 34 [20, 62] | 34 [20, 62] | 25 [16, 56] | 0.066 |
| 1st DRR | 1.49 [1.19, 1.88] | 1.46 [1.18, 1.85] | 1.66 [1.41, 2.26] | <0.001 |
| 2nd DRR | 1.22 [0.85, 1.69] | 1.17 [0.83, 1.61] | 1.92 [1.24, 3.28] | <0.001 |
| Variables | Total | Mortality | p-Value | |
|---|---|---|---|---|
| (n = 888) | No (n = 814) | Yes (n = 74) | ||
| TRISS | 0.93 [0.79, 0.97] | 0.93 [0.82, 0.97] | 0.79 [0.27, 0.93] | <0.001 |
| MPM II | 11.9 [7.1, 22.5] | 11.4 [6.9, 19.2] | 44.4 [23.5, 69.9] | <0.001 |
| MPM24 II | 7.8 [4.4, 14.1] | 7.2 [4.1, 12.5] | 29.4 [16.7, 49.9] | <0.001 |
| MPM48 II | 10.2 [5.6, 17.8] | 9.4 [5.2, 15.7] | 34.9 [19.5, 56.4] | <0.001 |
| MPM72 II | 12.1 [6.6, 21.3] | 10.8 [6.1, 18.2] | 40.6 [22.7, 60.2] | <0.001 |
| APACHE II | 13 [8, 18] | 12 [8, 18] | 21 [16, 26] | <0.001 |
| SAPS II | 28 [19, 38] | 27 [18, 37] | 49 [35, 56] | <0.001 |
| LODS | 2 [1, 4] | 2 [1, 4] | 6 [4, 8] | <0.001 |
| MODS | 3 [1, 5] | 2 [1, 4] | 5 [4, 6] | <0.001 |
| 24 h ICU point system | 1 [0, 4] | 1 [0, 3] | 4 [2, 6] | <0.001 |
| SOFA | 3 [1, 5] | 3 [1, 5] | 5 [4, 8] | <0.001 |
| TRIOS | 8.7 [4.3, 17.7] | 7.6 [4.2, 15.0] | 23.6 [14.8, 41.5] | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, W.-T.; Rau, C.-S.; Chou, S.-E.; Tsai, C.-H.; Chien, P.-C.; Hsieh, C.-H. Using Second Measurement of De Ritis Ratio to Improve Mortality Prediction in Adult Trauma Patients in Intensive Care Unit. Diagnostics 2022, 12, 2930. https://doi.org/10.3390/diagnostics12122930
Su W-T, Rau C-S, Chou S-E, Tsai C-H, Chien P-C, Hsieh C-H. Using Second Measurement of De Ritis Ratio to Improve Mortality Prediction in Adult Trauma Patients in Intensive Care Unit. Diagnostics. 2022; 12(12):2930. https://doi.org/10.3390/diagnostics12122930
Chicago/Turabian StyleSu, Wei-Ti, Cheng-Shyuan Rau, Sheng-En Chou, Ching-Hua Tsai, Peng-Chen Chien, and Ching-Hua Hsieh. 2022. "Using Second Measurement of De Ritis Ratio to Improve Mortality Prediction in Adult Trauma Patients in Intensive Care Unit" Diagnostics 12, no. 12: 2930. https://doi.org/10.3390/diagnostics12122930
APA StyleSu, W.-T., Rau, C.-S., Chou, S.-E., Tsai, C.-H., Chien, P.-C., & Hsieh, C.-H. (2022). Using Second Measurement of De Ritis Ratio to Improve Mortality Prediction in Adult Trauma Patients in Intensive Care Unit. Diagnostics, 12(12), 2930. https://doi.org/10.3390/diagnostics12122930







