Effectiveness of Abdominal Ultrasonography for Improving the Prognosis of Pancreatic Cancer during Medical Checkup: A Single Center Retrospective Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Initial Diagnosis and Follow-Up
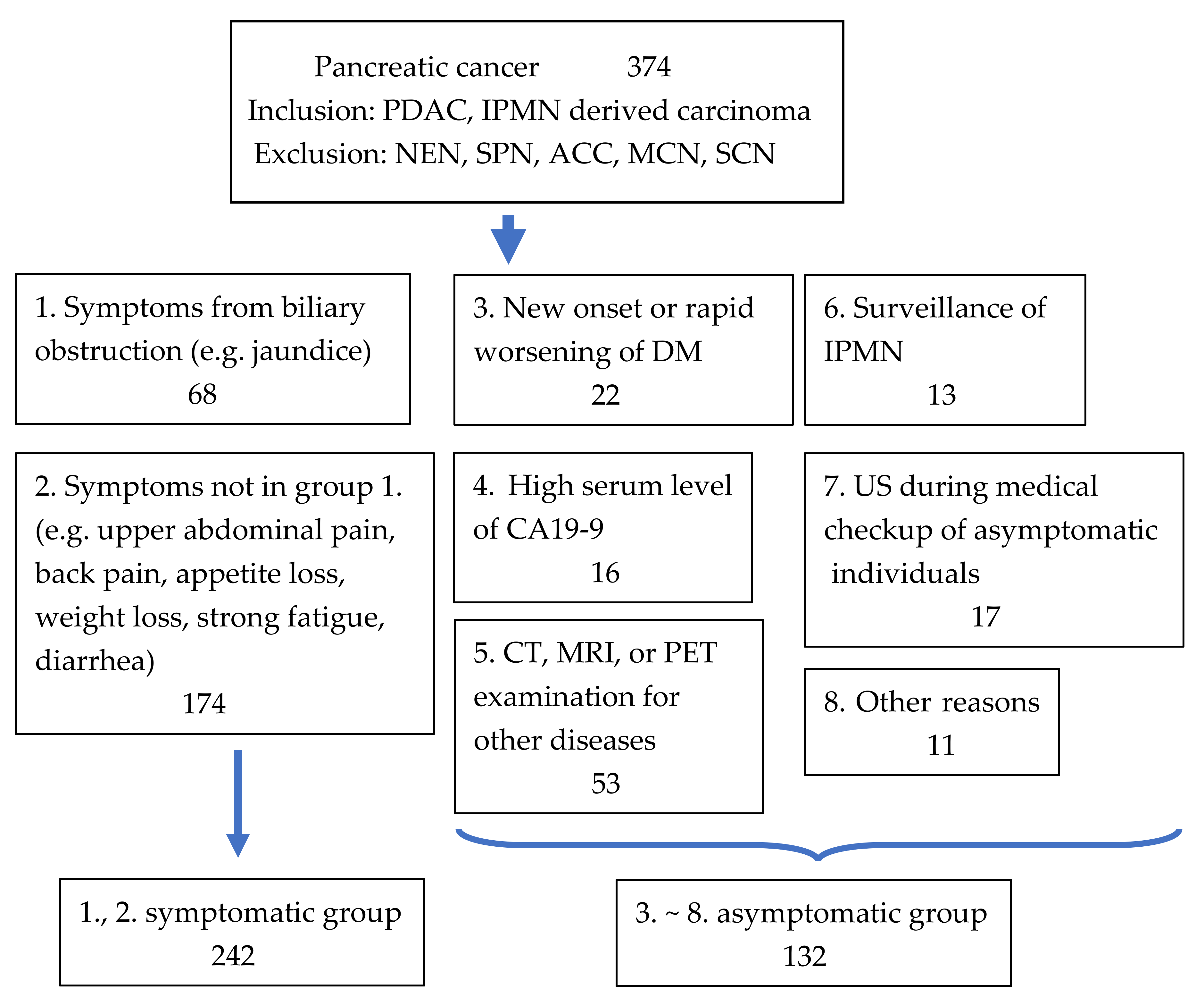
2.3. Grouping of Patients with Pancreatic Cancer According to Their Diagnostic Approach
2.4. Evaluations
2.5. Predictive Factors of Operable Pancreatic Cancers and Long-Term Prognosis in the Symptomatic Group and the Ultrasonography Medical Checkup Group
2.6. Details of Patients Diagnosed through Ultrasonography during Medical Checkup
2.7. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Patient Characteristics in Each Group According to How Pancreatic Cancer Was Diagnosed
3.3. Patients’ Prognosis in Each Group According to How PC Was Diagnosed
3.4. Excision Ratio and Prognosis in the Symptomatic Group Plus US Medical Checkup Group
3.5. Details of Patients Found through Medical Checkup with Abdominal Ultrasonography
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Egawa, S.; Toma, H.; Ohigashi, H.; Okusaka, T.; Nakao, A.; Hatori, T.; Maguchi, H.; Yanagisawa, A.; Tanaka, A. A digest of the Pancreatic Cancer Registry Report 2007. Suizo 2008, 23, 105–123. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early detection of pancreatic cancer: Opportunities and challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanno, S.; Nakano, Y.; Koizumi, K.; Sugiyama, Y.; Nakamura, K.; Sasajima, J.; Nishikawa, T.; Mizukami, Y.; Yanagawa, N.; Fujii, T.; et al. Pancreatic ductal adenocarcinomas in long-term follow-up patients with branch duct intraductal papillary mucinous neoplasms. Pancreas 2010, 39, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, K.; Shiratori, K. Risk factors of pancreatic cancer-family history, past history, and complications. Shokakinaika 2012, 55, 70–73. (In Japanese) [Google Scholar]
- Matsubayashi, H.; Maeda, A.; Kanemoto, H.; Uesaka, K.; Yamazaki, K.; Hironaka, S.; Miyagi, Y.; Ikehara, H.; Ono, H.; Klein, A.; et al. Risk factors of familial pancreatic cancer in Japan: Current smoking and recent onset of diabetes. Pancreas 2011, 40, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Tajima, K.; Takezaki, T.; Hamajima, N.; Hirose, K.; Ito, H.; Tominaga, S. Epidemiology of pancreatic cancer in Japan: A nested case-control study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC). Int. J. Epidemiol. 2003, 32, 257–262. [Google Scholar] [CrossRef] [Green Version]
- US Preventive Services Task Force; Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Curry, S.J.; Doubeni, C.A.; Epling, J.W., Jr.; et al. Screening for Pancreatic Cancer: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA 2019, 322, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Vital Statistics Japan Reported by the Ministry of Health, Labour and Welfare. Available online: http://www.mhlw.go.jp/toukei/saikin/hw/jinkou/suikei15/ (accessed on 1 August 2022).
- National Cancer Institute. Cancer Stat Facts: Pancreatic Cancer. 2021. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 23 December 2021).
- Egawa, S.; Takeda, K.; Fukuyama, S.; Motoi, F.; Sunamura, M.; Matsuno, S. Clinicopathological aspects of small pancreatic cancer. Pancreas 2004, 28, 235–240. [Google Scholar] [CrossRef]
- Egawa, S.; Toma, H.; Ohigashi, H.; Okusaka, T.; Nakao, A.; Hatori, T.; Maguchi, H.; Yanagisawa, A.; Tanaka, A. Japan pancreatic cancer Registry; 30th year anniversary: Japan pancreas society. Pancreas 2012, 41, 985–992. [Google Scholar] [CrossRef]
- Kanno, A.; Masamune, A.; Hanada, K.; Maguchi, H.; Shimizu, Y.; Ueki, T.; Hasebe, O.; Ohtsuka, T.; Nakamura, M.; Takenaka, M.; et al. Japan Study Group on the Early Detection of Pancreatic Cancer (JEDPAC) Multicenter study of early pancreatic cancer in Japan. Pancreatology 2018, 18, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Iiboshi, T.; Hanada, K.; Fukuda, T.; Yonehara, S.; Sasaki, T.; Chayama, K. Value of cytodiagnosis using endoscopic nasopancreatic drainage for early diagnosis of pancreatic cancer: Establishing a new method for the early detection of pancreatic carcinoma in situ. Pancreas 2012, 41, 523–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakaizumi, A.; Tatsuta, M.; Uehara, H.; Takenaka, A.; Iishi, H.; Kitamura, T.; Ohigashi, H.; Ishikawa, O.; Okuda, S.; Wada, A. Effectiveness of the cytologic examination of pure pancreatic juice in the diagnosis of early neoplasia of the pancreas. Cancer 1995, 76, 750–757. [Google Scholar] [CrossRef]
- Hanada, K.; Okazaki, A.; Hirano, N.; Izumi, Y.; Teraoka, Y.; Ikemoto, J.; Kanemitsu, K.; Hino, F.; Fukuda, T.; Yonehara, S. Diagnostic strategies for early pancreatic cancer. J. Gastroenterol. 2015, 50, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kikuyama, M.; Hanada, K.; Ueki, T. Pancreatic carcinoma in situ presenting prominent fatty change of the pancreas body on CT: Expression from 3 cases. J. Jpn. Pancreas Soc. 2015, 30, 626–632. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Nakahodo, J.; kikuyama, M.; Nojiri, S.; Chiba, K.; Yoshimoto, K.; Kamisawa, T.; Horiguchi, S.I.; Honda, G. Focal parenchymal atrophy of pancreas: An important sign of underlying high-grade pancreatic intraepithelial neoplasia without invasive carcinoma, i.e., carcinoma in situ. Pancreatology 2020, 20, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Uesaka, K.; Boku, N.; Fukutomi, A.; Okamura, Y.; Konishi, M.; Matsumoto, I.; Kaneoka, Y.; Shimizu, Y.; Nakamori, S.; Sakamoto, H.; et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: A phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016, 388, 214–215. [Google Scholar] [CrossRef]
- Unno, M.; Motoi, F.; Matsuyama, Y.; Yamaue, H.; Satoi, S.; Sho, M.; Honda, G.; Matsumoto, I.; Wada, K.; Furuse, J.; et al. Randomized phase Ⅱ/Ⅲ trial of neoadjuvant chemotherapy for resectable pancreatic cancer (Prep-02/JSAP-05). J. Clin. Oncol. 2019, 37, 190–194. [Google Scholar] [CrossRef]
- Gooiker, G.A.; Lemmens, V.E.P.P.; Besselink, M.G.; Busch, O.R.; Bonsing, B.A.; Molenaar, I.Q.; Tollenaar, R.A.E.M.; de Hingh, I.H.J.T.; Wouters, M.W.J.M. Impact of centralization of pancreatic cancer surgery on resection rates and survival. Br. J. Surg. 2014, 101, 1000–1005. [Google Scholar] [CrossRef]
- Lidsky, M.E.; Sun, Z.; Nussbaum, D.P.; Adam, M.A.; Speicher, P.J.; Blazer, D.G., 3rd. Going the extra mile: Improved survival for Pancreatic cancer patients traveling to high-volume centers. Ann. Surg. 2017, 266, 333–338. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.B.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer Information Service, National Cancer Center, Japan. Available online: http://www.mhlw.go.jp/toukei/saikin/hw/jinkou/suikei15/ (accessed on 1 August 2022).
- Ben, Q.; Xu, M.; Ning, X.; Liu, J.; Hong, S.; Huang, W.; Zhang, H.; Li, Z. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur. J. Cancer 2011, 47, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Pergolini, I.; Sahora, K.; Ferrone, C.R.; Morales-Oyarvide, V.; Wolpin, B.M.; Mucci, L.A.; Brugge, W.R.; Mino-Kenudson, M.; Patino, M.; Sahani, D.V.; et al. Long-term risk of pancreatic malignancy in patients with branch duct intraductal papillary mucinous neoplasm in a referral center. Gastroenterology 2017, 153, 1284–1294. [Google Scholar] [CrossRef]
- Klein, A.P.; Brune, K.A.; Petersen, G.M.; Goggins, M.; Tersmette, A.C.; Offerhaus, G.J.A.; Griffin, C.; Cameron, J.L.; Yeo, C.J.; Kern, S.; et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004, 64, 2634–2638. [Google Scholar] [CrossRef] [Green Version]
- Brune, K.A.; Lau, B.; Palmisano, E.; Canto, M.; Goggins, M.G.; Hruban, R.H.; Klein, A.P. Importance of age of onset in pancreatic cancer kindreds. J. Natl. Cancer Inst. 2010, 102, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Committee for Pancreatic Cancer Registry. Japan Pancreas Society Pancreatic Cancer Registry Report 2007. Suizo 2007, 22, e1–e427. (In Japanese) [Google Scholar]
- Oyama, H.; Tada, M.; Takagi, K.; Tateishi, K.; Hamada, T.; Nakai, Y.; Hakuta, R.; Ijichi, H.; Ishigaki, K.; Kanai, S.; et al. Long-term risk of malignancy in branch-duct intraductal papillary mucinous neoplasms. Gastroenterology 2020, 158, 226–237. [Google Scholar] [CrossRef] [Green Version]
- Vege, S.S.; Ziring, B.; Jain, R.; Moayyedi, P.; Clinical Guidelines Committee. American Gastroenterology Association. American Gastroenterological Association Institute Guidelines on the Diagnosis and Management of Asymptomatic Neoplastic Pancreatic Cysts. Gastroenterology 2015, 148, 819–822. [Google Scholar] [CrossRef] [Green Version]
- Scheiman, J.M.; Hwang, J.H.; Moayyedi, P. American Gastroenterological Association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015, 148, 824–848. [Google Scholar] [CrossRef] [Green Version]
- The European Study Group on Cystic Tumors of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Fernandez-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Kudo, M.; Maekawa, K.; Suetomi, Y.; Sakamoto, H.; Fukuta, N.; Nakaoka, R.; Kawasaki, T. Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography. Gut 2004, 53, 854–859. [Google Scholar] [CrossRef]
- Böttger, T.C.; Boddin, J.; Düber, C.; Heintz, A.; Küchle, R.; Junginger, T. Diagnosing and staging of pancreatic carcinoma-what is necessary? Oncology 1998, 55, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Rösch, T.; Lorenz, R.; Braig, C.; Feuerbach, S.; Siewert, J.R.; Schusdziarra, V.; Classen, M. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest. Endosc. 1991, 37, 347–352. [Google Scholar] [CrossRef]
- Niederau, C.; Grendell, J.H. Diagnosis of pancreatic carcinoma. Imaging techniques and tumor markers. Pancreas 1992, 7, 66–86. [Google Scholar] [CrossRef]
- Ikemoto, J.; Serikawa, M.; Hanada, K.; Eguchi, N.; Sasaki, T.; Fujimoto, Y.; Sugiyama, S.; Yamaguchi, A.; Noma, B.; Kamigaki, M.; et al. Clinical analysis of early-stage pancreatic cancer and proposal for a new diagnostic algorithm: A multicenter observational study. Diagnostics 2021, 11, 287. [Google Scholar] [CrossRef]
- Sener, S.F.; Fremgen, A.; Menck, H.R.; Winchester, D.P. Pancreatic cancer: A report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J. Am. Coll. Surg. 1999, 189, 1–7. [Google Scholar] [CrossRef]
- Ashida, R.; Tanaka, S.; Ioka, T.; Katayama, K. Pancreatic cancer screening by abdominal ultrasonography. Suizo 2017, 32, 30–37. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Hanada, K.; Shimizu, A.; Minami, T. Social programs for early diagnosis of pancreatic cancer—Establishment of network between special doctors and practicing doctors. Nihon Shokakibyo Gakkai Zasshi 2018, 115, 327–333. (In Japanese) [Google Scholar]
- Fukasawa, M.; Yoda, Y.; Takayama, I.; Takano, S.; Kadokura, M.; Shindo, H.; Takahashi, E.; Yokota, Y.; Amemiya, F.; Hanawa, M.; et al. Regional medical cooperation aimed at early detection of pancreatic cancer. Shoukakinaika 2013, 57, 63–67. (In Japanese) [Google Scholar]
- Cancer Incidence of Japan. Available online: https://www.e-stat.go.jp/stat-search/files?page=1&toukei=00450173&tstat=000001133323 (accessed on 1 August 2022).
- Morelli, L.; Guadagni, S.; Borrelli, V.; Pisano, R.; Di Franco, G.; Palmeri, M.; Furbetta, N.; Gambaccini, D.; Marchi, S.; Boraschi, P.; et al. Role of abdominal ultrasound for the surveillance follow-up of pancreatic cystic neoplasms: A cost-effective safe alternative to the routine use of magnetic resonance imaging. World J. Gastroenterol. 2019, 25, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Kowada, A. Cost-effectiveness of abdominal ultrasound versus magnetic resonance imaging for pancreatic cancer screening in familial high-risk individuals in Japan. Pancreas 2020, 49, 1052–1056. [Google Scholar] [CrossRef]
- Tanaka, T. Diagnosis opportunities and clinical features of resectable pancreatic cancer: A single center retrospective analysis. J. Gastrointest. Cancer Screen. 2022, 60, 32–40. (In Japanese) [Google Scholar]



| All Patients | Symptomatic Group | Asymptomatic Group | p-Value # | |
|---|---|---|---|---|
| Patients’ number | 374 | 242 | 132 | |
| Male, n (%) | 192 (51.3) | 126 (52.0) | 66 (50) | 0.75 |
| Age, median (range), years | 74 (34–105) | 72 (34–105) | 76 (44–98) | <0.01 |
| Comorbidities | ||||
| Diabetes mellitus, n (%) | 120 (32.1) | 56 (23.1) | 64 (48.5) | <0.001 |
| Hypertension, n (%) | 162 (43.3) | 93 (38.4) | 69 (52.3) | <0.05 |
| Hyperlipidemia, n (%) | 76 (20.3) | 37 (15.3) | 39 (29.5) | <0.01 |
| Any of the above 3 diseases, n (%) | 265 (70.9) | 160 (66.1) | 105 (79.5) | <0.01 |
| History of other cancer, n (%) | 70 (17.7) | 34 (14.0) | 36 (26.9) | <0.01 |
| History of heavy drinking (ethanol ≥100 g/day) | 19 (5.1) | 9 (3.7) | 10 (7.6) | 0.13 |
| History of smoking, n/N (%) | 197/373 (52.8) | 115/241 (47.7) | 82/132 (62.1) | <0.01 |
| Family history of PC | ||||
| (≤1st degree), n/N (%) | 27/289 (9.3) | 15/180 (8.3) | 12/109 (11.0) | 0.53 |
| (≤2nd degree), n/N (%) | 30/289 (10.4) | 18/180 (10) | 12/109 (11.0) | 0.84 |
| PDAC, IPMN-derived carcinoma, n | 355, 19 | 235, 7 | 120, 12 | <0.05 |
| Localization of PC | ||||
| uncus, head, groove, head~body, body, body~tail, tail | 35, 119, 8, 11, 104, 30, 67 | 24, 81, 9, 6, 64, 19, 39 | 7, 38, 3, 5, 40, 11, 28 | |
| tail, n (%) | 67 (17.9) | 39 (16.1) | 28 (21.2) | 0.26 |
| Tumor size * median (range), mm | 34 (0–128) | 66 (0–128) | 25 (0–100) | <0.001 |
| Clinical or pathological Stage (UICC 8th) | ||||
| 0, 1, 2, 3, 4 | 12, 8, 144, 41, 169 | 1, 1, 66, 35, 139 | 11, 7, 78, 6, 30 | |
| 0, 1, 2, n (%) | 164 (43.9) | 68 (28.1) | 96 (72.7) | <0.001 |
| Therapy | ||||
| BST, n (%) | 76 (20.3) | 57 (23.6) | 19 (14.4) | <0.05 |
| Chemotherapy | 158 | 128 | 30 | |
| Radiation | 2 | 1 | 1 | |
| Excision, n (%) | 138 (36.9) | 56 (23.1) | 82 (62.1) | <0.001 |
| BMI, median (range), kg/mm2 | 21.9 (13.6–35.2) n = 371 | 21.6 (14.3–35.2) n = 240 | 22.5 (13.6–34.3) n = 131 | 0.13 |
| BMI < 18.5, n (%) | 67 (18.1) | 48 (20) | 19 (8.2) | 0.06 |
| 18.5 ≤ BMI < 25 | 323 | 155 | 77 | |
| 25 ≤ BMI | 72 | 37 | 35 | |
| CA19-9, median (range), U/mL | 239 (<2–26,165,454) | 557 (<2–26,165,454) | 104 (<2–7,575,434) | <0.01 |
| AMY, median (range), U/L | 68 (12–902) n = 373 | 63 (12–902) n = 242 | 77 (13–372) n = 131 | <0.05 |
| Alb, median (range), g/dL | 4.0 (12–90.2) n = 371 | 4.0 (2.2–5) n = 241 | 4.1 (2.5–5.2) n = 130 | <0.01 |
| NLR, median (range) | 3.4 (0.6–27.7) n = 372 | 3.6 (0.6–27.7) n = 241 | 2.8 (0.69–12.5) n = 131 | <0.001 |
| PNI, median (range) | 47.6 (26.6–80.1) n = 370 | 46.5 (26.6–61.7) n = 240 | 48.8 (28.3–80.1) n = 130 | <0.01 |
| Group | 1 | 2 | 1, 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|
| Patients’ number | 68 | 174 | 242 | 22 | 16 | 53 | 13 | 17 |
| Male, n (%) | 29 (42.6) | 97 (55.7) | 126 (52.1) | 9 (40.9) | 4 (25) * | 32 (60.4) | 10 (76.9) | 8 (47.1) |
| Age, median (range), years | 76 (41–105) | 71 (34–93) | 71 (34–105) | 74 (44–87) | 83 (66–89) * | 76 (45–89) | 81 (71–86) * | 75 (59–86) |
| IPMN-derived carcinoma, (vs. PDAC), n (%) | 1 (1.5) | 6 (3.4) | 7 (2.9) | 0 (0) | 1 (1.9) | 6 (11.3) | 3 (23.1) * | 1 (5.9) |
| Localization of PC | ||||||||
| uncus, head, groove, head~body, body, body~tail, tail | 3, 49, 7, 3, 5, 1, 0 | 21, 32, 2, 3, 59, 18, 39 | 24, 81, 9, 6, 42, 19, 39 | 0, 8, 0, 2, 5, 2, 5 | 2, 4, 0, 0, 5, 1, 4 | 3, 18, 0, 1, 17, 6, 8 | 0, 4, 0, 0, 4, 0, 5 | 2, 2, 1, 2, 7, 0, 3 |
| tail, n (%) | 0 (0) | 39 (22.4) | 39 (16.1) | 5 (22.7) | 4 (25) | 8 (15.1) | 5 (38.5) | 3 (17.6) |
| Tumor size **, median (range), mm | 30 (0–63) | 36 (0–128) | 34 (0–128) | 25 (0–77) # | 28 (18–55) * | 26 (0–100) ● | 17 (0–40) # | 20 (0–52) |
| Clinical or pathological stage (UICC 8th) | ||||||||
| 0, 1, 2, 3, 4 | 0, 0, 37, 7, 24 | 1, 1, 29, 28, 115 | 1, 1, 66, 35, 139 | 1, 1, 15, 1, 4 | 0, 0, 13, 2, 1 | 4, 5, 27, 3, 14 | 3, 0, 8, 0, 2 | 2, 1, 9, 0, 5 |
| 0, 1, 2, n (%) | 37 (54.4) | 31 (17.8) | 68 (28.1) | 17 (77.3) ● | 13 (81.3) ● | 36 (67.9) ● | 11 (84.6) ● | 12 (70.6) ● |
| Therapy | ||||||||
| BST, n (%) | 28 (41.2) | 29 (16.7) | 57 (23.6) | 1 (4.5) | 2 (12.5) | 12 (22.6) | 2 (15.4) | 0 (0) * |
| Chemotherapy | 14 | 114 | 128 | 6 | 2 | 12 | 0 | 7 |
| Radiation | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| Excision, n (%) | 26 (38.2) | 30 (17.2) | 56 (23.1) | 15 (68.2) ● | 12 (75) ● | 28 (52.8) ● | 11 (84.6) ● | 10 (58.8) ● |
| BMI, median (range), kg/mm2 | 21.8 (14.4–32.7) | 22.1 (14.3–35.2) n = 173 | 21.6 (14.3–35.2) n = 241 | 22.1 (15.3–28.8) | 24.1 (16.3–34.3) | 21.2 (13.6–31.9) | 21.7 (16.2–25.6) | 21.6 (18.0–29.8) |
| BMI < 18.5, n (%) | 15 (22.1) | 15 (8.6) | 30 (12.4) | 5 (22.7) | 1 (6.3) | 11 (20.8) | 1 (7.7) | 1 (5.9) |
| CA19-9, median (range), U/mL | 231 (<2–194,660) n = 67 | 824 (<2–26,165,454) n = 172 | 557 (<2–26,165,454) n = 239 | 312 (<2–10,334) | 391 (43–3618) | 28 (<2–7,574,431) | 15 (<2–25,550) ● | 9 (<2–21,945) ● |
| AMY, median (range), U/mL | 72 (15–517) | 61 (12–902) | 63 (12–902) | 68 (26–267) n = 21 | 86 (33–124) | 88 (27–372) * | 77 (20–192) | 60 (27–286) |
| Alb, median (range), g/dL | 3.7 (2.2–4.9) | 3.9 (2.2–5.0) n = 173 | 4.0 (2.2–5.0) n = 241 | 4.2 (3.2–5.1) n = 21 | 4.2 (3.5–4.5) | 4.0 (3.2–5.2) | 4.2 (3.2–4.4) n = 12 | 4.0 (2.9–4.8) |
| NLR, median (range) | 3.9 (0.9–27.7) | 3.6 (0.6–22.6) n = 173 | 3.6 (0. 6–27.7) n = 241 | 2.5 (0.9–11) | 2.9 (1.2–4.0) * | 2.8 (0.7–12.5) # | 1.9 (0.7–3.6) n = 12 # | 2 (0.9–5.3) * |
| PNI, median (range) | 44.7 (27.6–57.2) n = 66 | 47.7 (7.7–61.7) n = 172 | 46.5 (7.7–61.7) n = 238 | 49.1 (38.9–62.2) n = 21 * | 49.9 (39.9–58) | 48.1 (28.3–80.1) | 50.2 (45.9–59.5) n = 12 # | 47.2 (39–60.5) |
| Unresected | Resected | Univariate Analysis (p-Value) | Multivariate Analysis | ||
|---|---|---|---|---|---|
| p-Value | Odds Ratio (95%CI) | ||||
| Patients’ number | 193 | 66 | |||
| Female, n (%) | 94 (48.7) | 40 (60.6) | 0.045 | <0.01 | 2.36 (1.26–4.45) |
| Age, median (range), years | 73 (34–105) | 72 (42–86) | 0.78 | ||
| Age, ≥75 years old, n (%) | 87 (45.1) | 25 (37.9) | 0.32 | 0.20 | 0.66 (0.34–1.26) |
| Group 7 (vs. Group1,2), n (%) | 7 (3.6) | 10 (15.2) | <0.01 | 0.04 | 3.31 (1.08–10.11) |
| Diabetes mellitus, n (%) | 43 (22.3) | 16 (9.1) | 0.74 | ||
| Any of the 3 diseases (diabetes mellitus, hypertension, hyperlipidemia), n (%) | 105 (54.4) | 40 (24.2) | 0.09 | ||
| History of other cancer, n (%) | 26 (13.5) | 11 (16.7) | 0.39 | ||
| History of heavy drinking (ethanol ≥100 g/day) | 7 (3.6) | 3 (4.5) | 0.72 | ||
| History of smoking, n/N (%) | 96/192 (50) | 27 (40.9) | 0.25 | ||
| Family history of PC (≤1st degree), n/N (%) | 13/145 (9.0) | 3/50 (6) | 0.77 | ||
| IPMN-derived carcinoma, PDAC, n | 3, 190 | 5, 61 | 0.03 | 0.10 | 3.81 (0.78–18.67) |
| Localization of PC tail, n (%) | 36 (18.7) | 6 (9.1) | 0.08 | ||
| BMI, median (range), kg/mm2 | 21.1 (14.3–35.2) n = 191 | 23.0 (15.0–32.7) | <0.001 | ||
| BMI (kg/mm2) ≥ 18.5, n/N (%) | 146/191 (76.4) | 62/66 (93.9) | <0.01 | 0.02 | 3.76 (1.24–11.43) |
| CA19-9, median (range), U/mL | 1352 (1–215,454) n = 190 | 165 (1–4941) | <0.001 | ||
| CA19-9 (U/mL) ≥ 425 | 109/190 (5 7.4) | 17/66 (25.8) | < 0.001 | <0.001 | 0.31 (0.16–0.59) |
| AMY (U/L), median (range), | 62.0 (12–902) | 68.5 (24–664) | 0.15 | ||
| Multivariate Analysis | ||
|---|---|---|
| p-Value | Hazard Ratio (95%CI) | |
| Female sex | 0.16 | 0.81 (0.60–1.09) |
| Age, ≥75 years old | 0.10 | 1.29 (0.98–1.75) |
| Group7 (vs. group 1or 2) | <0.01 | 0.36 (0.17–0.78) |
| IPMN-derived carcinoma (vs. PDAC) | 0.13 | 0.49 (0.19–1.22) |
| BMI (kg/mm2) ≥18.5 | <0.01 | 0.60 (0.42–0.87) |
| CA19-9 (U/mL) ≥425 | <0.001 | 1.69 (1.24–2.30) |
| NLR ≥ 3.6 | <0.01 | 1.58 (1.18–2.11) |
| Patients’ Number | 17 |
|---|---|
| Place where PC was found | |
| Clinic going regularly | 12 |
| Health screening center | 3 |
| Referral center (our hospital) | 2 |
| Specialty of doctors in clinic | |
| Internal medicine, n/N | 12/12 |
| Subspecialty | |
| Gastroenterology | 10 |
| Respiratory medicine | 1 |
| Unknown | 1 |
| Comorbidities | |
| Diabetes Mellitus | 3 |
| Hypertension | 9 |
| Hyperlipidemia | 10 |
| Each of above 3 diseases, n (%) | 13 (76.5) |
| Performance status | |
| 0, 1, 2, 3, 4 | 17, 0, 0, 0, 0 |
| Findings of ultrasonography | |
| Tumor in pancreas | 6 |
| Main pancreatic duct dilatation | 10 |
| Cyst in pancreas | 1 |
| Tumor in liver | 2 |
| Patients with operation | 11 |
| Pathological stage, 0, 1, 2a, 2b, 3, 4 (UICC 8th) | 2, 1, 4, 4, 0, 0 |
| No relapse in stage 2, n/N (%) | 7/8 (87.5) |
| Days after surgery in 7 patients with no relapse in stage 2 | 539, 642, 834, 992, 2619, 2621, 2690 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, A.; Kato, N.; Sugata, S.; Hamada, T.; Furuya, N.; Mizumoto, T.; Tamaru, Y.; Kusunoki, R.; Kuwai, T.; Kouno, H.; et al. Effectiveness of Abdominal Ultrasonography for Improving the Prognosis of Pancreatic Cancer during Medical Checkup: A Single Center Retrospective Analysis. Diagnostics 2022, 12, 2913. https://doi.org/10.3390/diagnostics12122913
Yamaguchi A, Kato N, Sugata S, Hamada T, Furuya N, Mizumoto T, Tamaru Y, Kusunoki R, Kuwai T, Kouno H, et al. Effectiveness of Abdominal Ultrasonography for Improving the Prognosis of Pancreatic Cancer during Medical Checkup: A Single Center Retrospective Analysis. Diagnostics. 2022; 12(12):2913. https://doi.org/10.3390/diagnostics12122913
Chicago/Turabian StyleYamaguchi, Atsushi, Naohiro Kato, Shuhei Sugata, Takuro Hamada, Nao Furuya, Takeshi Mizumoto, Yuzuru Tamaru, Ryusaku Kusunoki, Toshio Kuwai, Hirotaka Kouno, and et al. 2022. "Effectiveness of Abdominal Ultrasonography for Improving the Prognosis of Pancreatic Cancer during Medical Checkup: A Single Center Retrospective Analysis" Diagnostics 12, no. 12: 2913. https://doi.org/10.3390/diagnostics12122913
APA StyleYamaguchi, A., Kato, N., Sugata, S., Hamada, T., Furuya, N., Mizumoto, T., Tamaru, Y., Kusunoki, R., Kuwai, T., Kouno, H., Toyota, N., Sudo, T., Kuraoka, K., & Kohno, H. (2022). Effectiveness of Abdominal Ultrasonography for Improving the Prognosis of Pancreatic Cancer during Medical Checkup: A Single Center Retrospective Analysis. Diagnostics, 12(12), 2913. https://doi.org/10.3390/diagnostics12122913






