Hemoglobin Determination Using Pulse Co-Oximetry and Reduced-Volume Blood Gas Analysis in the Critically Ill: A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. PBM Measures in the Local ICU
2.2. Patients
2.3. Hb Measurements
2.4. Statistical Analysis
2.5. Ethics Committee Approval
3. Results
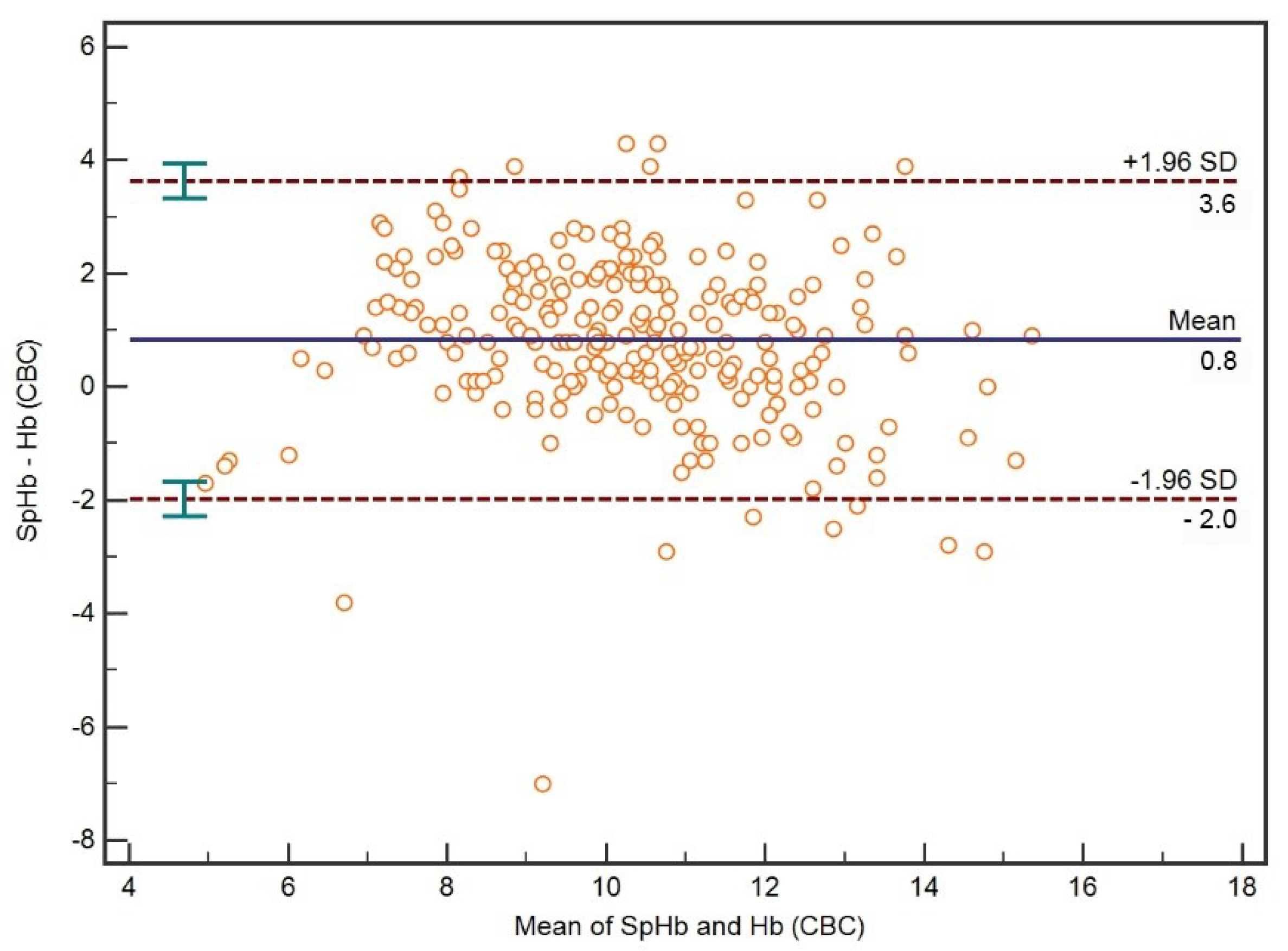
3.1. Comparison between SpHb and CBC Hb
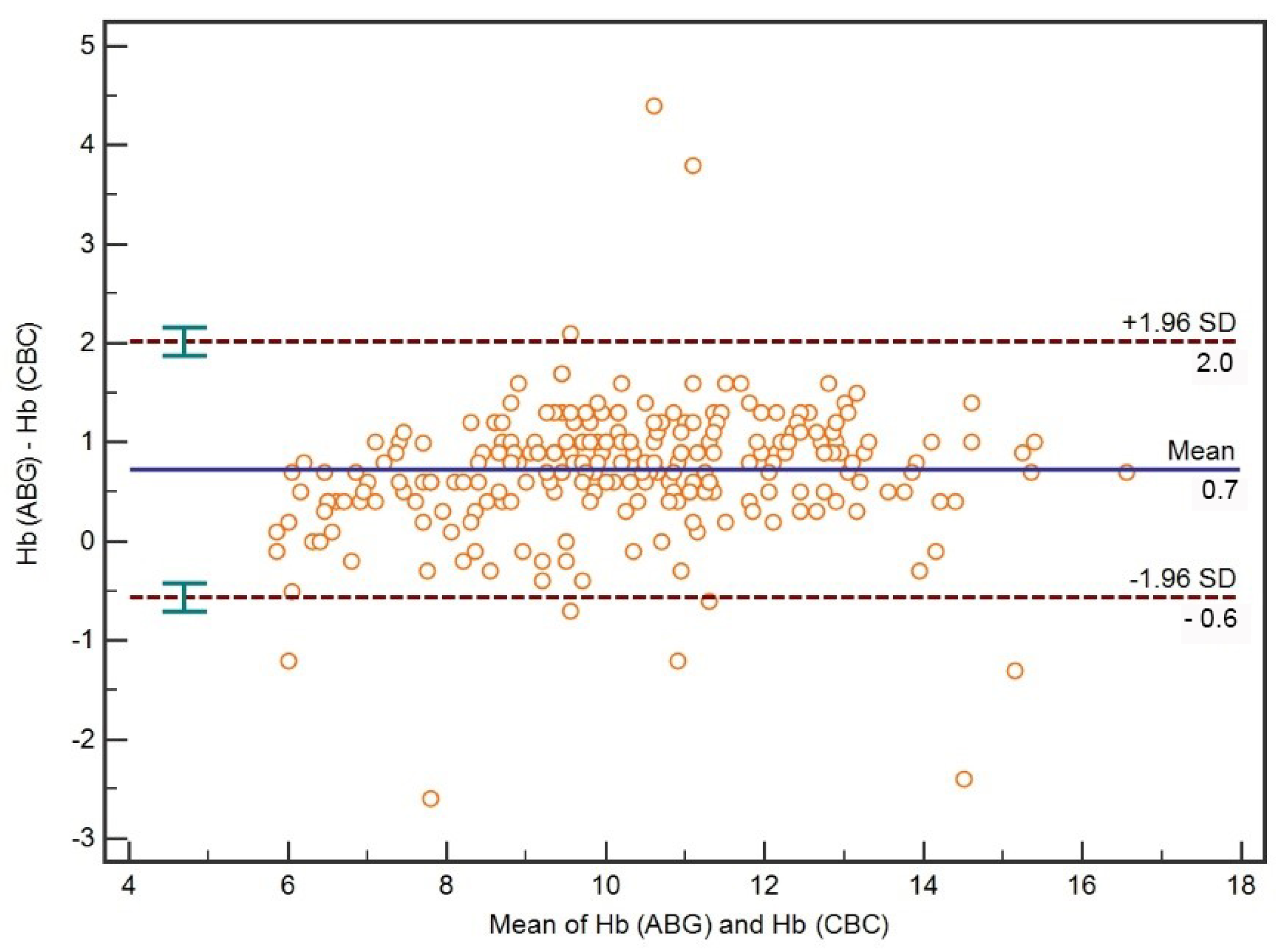
3.2. Comparison between ABG Hb and CBC Hb
3.3. Hb Concentration Subgroup Analysis
4. Discussion
4.1. Feasibility of SpHb
4.2. Accuracy of SpHb
4.3. Accuracy of ABG Hb
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vincent, J.L.; Baron, J.F.; Reinhart, K.; Gattinoni, L.; Thijs, L.; Webb, A.; Meier-Hellmann, A.; Nollet, G.; Peres-Bota, D.; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA 2002, 288, 1499–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corwin, H.L.; Gettinger, A.; Pearl, R.G.; Fink, M.P.; Levy, M.M.; Abraham, E.; MacIntyre, N.R.; Shabot, M.M.; Duh, M.-S.; Shapiro, M.J. The CRIT Study: Anemia and blood transfusion in the critically ill—current clinical practice in the United States. Crit. Care Med. 2004, 32, 39–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, J.; Jensen, L.; Nahirniak, S.; Gibney, R.T. Anemia and blood transfusion practices in the critically ill: A prospective cohort review. Heart Lung 2010, 39, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.G.; Li, L.; Sun, Z.; Hixson, E.D.; Tang, A.; Phillips, S.C.; Blackstone, E.H.; Henderson, J.M. Hospital-acquired anemia: Prevalence, outcomes, and healthcare implications. J. Hosp. Med. 2013, 8, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.; Peralta, R.M.; Moss, R.L.; Feane, K.; Uprichard, J. A single-centre review of iatrogenic anaemia in adult intensive care. Transfus. Med. 2020, 30, 196–200. [Google Scholar] [CrossRef]
- Hayden, S.J.; Albert, T.J.; Watkins, T.R.; Swenson, E.R. Anemia in critical illness: Insights into etiology, consequences, and management. Am. J. Respir. Crit. Care Med. 2012, 185, 1049–1057. [Google Scholar] [CrossRef] [Green Version]
- Czempik, P.F.; Wilczek, D.; Herzyk, J.; Krzych, Ł.J. Hospital-Acquired Anemia in Patients Hospitalized in the Intensive Care Unit: A Retrospective Cohort Study. J. Clin. Med. 2022, 11, 3939. [Google Scholar] [CrossRef] [PubMed]
- Meybohm, P.; Richards, T.; Isbister, J.; Hofmann, A.; Shander, A.; Goodnough, L.T.; Muñoz, M.; Gombotz, H.; Weber, C.F.; Choorapoikayil, S.; et al. Patient Blood Management Bundles to Facilitate Implementation. Transfus. Med. Rev. 2017, 31, 62–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gayat, E.; Bodin, A.; Sportiello, C.; Boisson, M.; Dreyfus, J.-F.; Mathieu, E.; Fischler, M. Performance evaluation of a noninvasive hemoglobin monitoring device. Ann. Emerg. Med. 2011, 57, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Berkow, L.; Rotolo, S.; Mirski, E. Continuous noninvasive hemoglobin monitoring during complex spine surgery. Anesth. Analg. 2011, 113, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Applegate, R.L.; Barr, S.J.; Collier, C.E.; Rook, J.L.; Mangus, D.B.; Allard, M.W. Evaluation of pulse cooximetry in patients undergoing abdominal or pelvic surgery. J. Am. Soc. Anesthesiol. 2012, 116, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.; Shih, B.; Tsai, Y.-F.; Chung, P.; Liu, F.; Yu, H.; Lee, W.; Chang, C.; Lin, C. Accuracy and Trending of Continuous Noninvasive Hemoglobin Monitoring in Patients Undergoing Liver Transplantation. Transplant. Proc. 2016, 48, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Pandit, V.; Aziz, H.; Kulvatunyou, N.; Zangbar, B.; Tang, A.; Keeffe, T.O.; Jehangir, Q.; Snyder, K.; Rhee, P. Transforming hemoglobin measurement in trauma patients: Noninvasive spot check hemoglobin. J. Am. Coll. Surg. 2015, 220, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Awada, W.N.; Mohmoued, M.F.; Radwan, T.M.; Hussien, G.Z.; Elkady, H.W. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: A prospective cohort study. Int. J. Clin. Monit. Comput. 2015, 29, 733–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riess, M.L.; Pagel, P.S. Noninvasively Measured Hemoglobin Concentration Reflects Arterial Hemoglobin Concentration Before but Not After Cardiopulmonary Bypass in Patients Undergoing Coronary Artery or Valve Surgery. J. Cardiothorac. Vasc. Anesth. 2016, 30, 1167–1171. [Google Scholar] [CrossRef] [Green Version]
- Welker, E.; Novak, J.; Jelsma, L.; Koehler, T.; Davis, A.; DeCou, J.; Durkin, E. Continuous hemoglobin monitoring in pediatric trauma patients with solid organ injury. J. Pediatr. Surg. 2018, 53, 2055–2058. [Google Scholar] [CrossRef]
- Frasca, D.; Dahyot-Fizelier, C.; Catherine, K.; Levrat, Q.; Debaene, B.; Mimoz, O. Accuracy of a continuous noninvasive hemoglobin monitor in intensive care unit patients. Crit. Care Med. 2011, 39, 2277–2282. [Google Scholar] [CrossRef]
- Raikhel, M. Accuracy of noninvasive and invasive point-of-care total blood hemoglobin measurement in an outpatient setting. Postgrad. Med. 2012, 124, 250–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBarros, M.; Shawhan, R.; Bingham, J.; Sokol, K.; Izenberg, S.; Martin, M. Assessing serum hemoglobin levels without venipuncture: Accuracy and reliability of Pronto-7 noninvasive spot-check device. Am. J. Surg. 2015, 209, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Czempik, P.F.; Spień, A.; Oleksa, M.; Wiśniewski, D.; Krzych, L.J. Inpatient recipients of packed red blood cells in a university medical center in Poland in 2018–2019. J. Transfus. Med. 2022, 15, 1–7. [Google Scholar] [CrossRef]
- Gomaa, D.; Rodriquez, D.; Petro, M.; Blakeman, T.C.; Branson, R.D. Impact of Oxygenation Status on the Noninvasive Measurement of Hemoglobin. Mil. Med. 2017, 182, 87–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.W. Regression analysis for continuous independent variables in medical research: Statistical standard and guideline of Life Cycle Committee. Life Cycle 2022, 2, e3. [Google Scholar] [CrossRef]
- Hebert, P.C.; Wells, G.; Blajchman, M.A.; Marshall, J.; Martin, C.; Pagliarello, G.; Tweeddale, M.; Schweitzer, I.; Yetisir, E.; Transfusion Requirements in Critical Care Investigators for the Canadian Critical Care Trials Group. A Multicenter, Randomized, Controlled Clinical Trial of Transfusion Requirements in Critical Care. N. Engl. J. Med. 1999, 340, 409–417. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, Y.; Yu, K.; Gu, J. Liberal transfusion versus restrictive transfusion and outcomes in critically ill adults: A meta-analysis. Transfus. Med. Hemother. 2021, 48, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Shabaninejad, H.; Ghadimi, N.; Sayehmiri, K.; Hosseinifard, H.; Azarfarin, R.; Gorji, H.A. Comparison of invasive and noninvasive blood hemoglobin measurement in the operating room: A systematic review and meta-analysis. J. Anesth. 2019, 33, 441–453. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Value, Median, IQR 1 |
|---|---|
| Age [years] | 63 (45–70) |
| CBC 2 Hb 3 [g/dL] | 9.8 (8.5–11.4) |
| ABG 4 Hb [g/dL] | 10.7 (9.2–12.4) |
| SpHb 5 [g/dL] | 10.8 (9.7–11.9) |
| CBC Hct 6 [%] | 30.6 (26.2–34.5) |
| ABG Hct [%] | 31.0 (27.0–36.0) |
| SaO2 7 [%] | 97 (95–98) |
| SpO2 8 [%] | 96 (94–98) |
| PI 9 | 3.1 (1.3–5.7) |
| Comparisons | Spearman’s Coefficient (95%CI 1) | p | Concordance Correlation Coefficient (95%CI 1) |
|---|---|---|---|
| SpHb 2-CBC 3 Hb 4 | 0.78 (0.73–0.83) | <0.001 | 0.69 (0.63–0.75) |
| SpHb-CBC Hb (Hb ≤ 7 g/dL) | 0.34 (−0.04–0.63) | 0.1 | 0.11 (0.02–0.20) |
| ABG 5 Hb-CBC Hb | 0.96 (0.95–0.97) | <0.001 | 0.91 (0.88–0.92) |
| ABG Hb-CBC Hb (Hb ≤ 7 g/dL) | 0.74 (0.51–0.87) | <0.001 | 0.47 (0.24–0.64) |
| ABG Hct 6-CBC Hct | 0.91 (0.89–0.93) | <0.001 | 0.86 (0.83–0.89) |
| ABG Hct-CBC Hct (Hb ≤ 7 g/dL) | 0.71 (0.45–0.85) | <0.001 | 0.65 (0.40–0.81) |
| SpHb-ABG Hb | 0.78 (0.73–0.83) | <0.001 | 0.74 (0.69–0.79) |
| SpHb-ABG Hb (Hb ≤ 7 g/dL) | 0.17 (−0.21–0.51) | 0.4 | 0.15 (−0.02–0.3) |
| Parameter | Value, Median, IQR 1 |
|---|---|
| Age [years] | 66 (51–73) |
| CBC 2 Hb 3 [g/dL] | 6.5 (6.2–6.7) |
| ABG 4 Hb [g/dL] | 6.7 (6.4–7.3) |
| SpHb 5 [g/dL] | 8.4 (7.4–9.3) |
| CBC Hct 6 [%] | 21 (19–21) |
| ABG Hct [%] | 20 (19–21) |
| SaO2 7 [%] | 98 (97–99) |
| SpO2 8 [%] | 97 (94–99) |
| PI 9 | 3.2 (2.1–5.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czempik, P.F.; Pluta, M.P.; Krzych, Ł.J. Hemoglobin Determination Using Pulse Co-Oximetry and Reduced-Volume Blood Gas Analysis in the Critically Ill: A Prospective Cohort Study. Diagnostics 2022, 12, 2908. https://doi.org/10.3390/diagnostics12122908
Czempik PF, Pluta MP, Krzych ŁJ. Hemoglobin Determination Using Pulse Co-Oximetry and Reduced-Volume Blood Gas Analysis in the Critically Ill: A Prospective Cohort Study. Diagnostics. 2022; 12(12):2908. https://doi.org/10.3390/diagnostics12122908
Chicago/Turabian StyleCzempik, Piotr F., Michał P. Pluta, and Łukasz J. Krzych. 2022. "Hemoglobin Determination Using Pulse Co-Oximetry and Reduced-Volume Blood Gas Analysis in the Critically Ill: A Prospective Cohort Study" Diagnostics 12, no. 12: 2908. https://doi.org/10.3390/diagnostics12122908
APA StyleCzempik, P. F., Pluta, M. P., & Krzych, Ł. J. (2022). Hemoglobin Determination Using Pulse Co-Oximetry and Reduced-Volume Blood Gas Analysis in the Critically Ill: A Prospective Cohort Study. Diagnostics, 12(12), 2908. https://doi.org/10.3390/diagnostics12122908








