Comparative Analysis of In-House RT-qPCR Detection of SARS-CoV-2 for Resource-Constrained Settings
Abstract
1. Introduction
2. Materials and Methods
2.1. Detection Limit Assay
2.2. Sensitivity, Specificity, and Accuracy Assay
2.3. Statistical Analysis
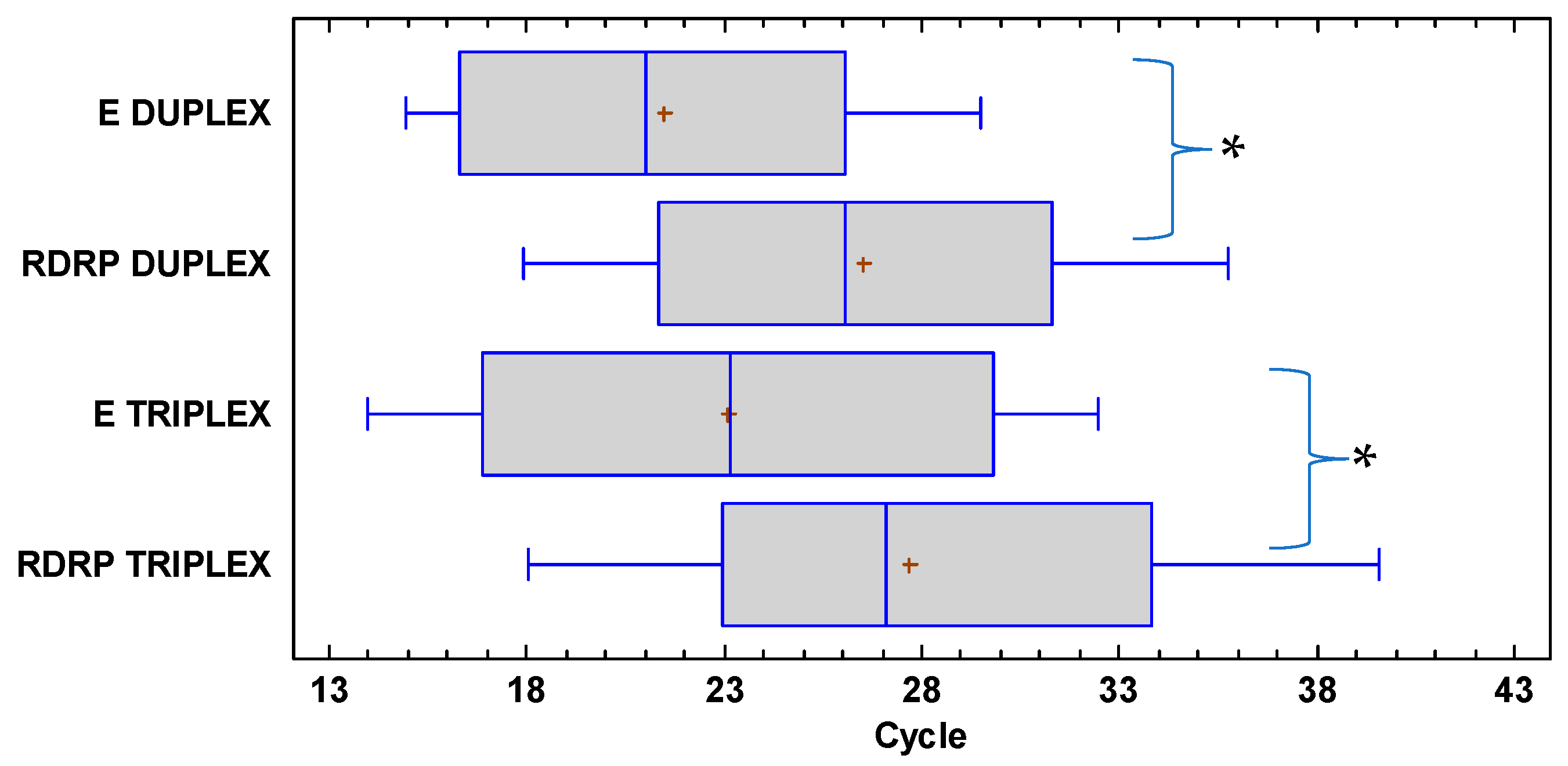
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO-Convened Global Study of Origins of SARS-CoV-2: China Part; World Health Organization: Geneva, Switzerland, 2021; pp. 16–92. [Google Scholar]
- Ryu, S.; Chun, B.C. An Interim Review of the Epidemiological Characteristics of 2019 Novel Coronavirus. Epidemiol. Health 2020, 42. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.C.; Goldstein, S.A.; Rasmussen, A.L.; Robertson, D.L.; Crits-Christoph, A.; Wertheim, J.O.; Anthony, S.J.; Barclay, W.S.; Boni, M.F.; Doherty, P.C.; et al. The Origins of SARS-CoV-2: A Critical Review. Cell 2021, 184, 4848–4856. [Google Scholar] [CrossRef] [PubMed]
- Biondi Zoccai, G.; Landoni, G.; Carnevale, R.; Cavarretta, E.; Sciarretta, S.; Frati, G. SARS-CoV-2 and COVID-19: Facing the Pandemic Together as Citizens and Cardiovascular Practitioners. Minerva Cardioangiol. 2020, 68, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Characteristics of SARS-CoV-2 and COVID-19 | Nature Reviews Microbiology. Available online: https://www.nature.com/articles/s41579-020-00459-7 (accessed on 25 October 2022).
- Riou, J.; Althaus, C.L. Pattern of Early Human-to-Human Transmission of Wuhan 2019 Novel Coronavirus (2019-NCoV), December 2019 to January 2020. Eurosurveillance 2020, 25, 2000058. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 21 October 2022).
- Dennison Himmelfarb, C.R.; Baptiste, D. Coronavirus Disease (COVID-19): Implications for Cardiovascular and Socially At-Risk Populations. J. Cardiovasc. Nurs. 2020, 35, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Sule, W.F.; Oluwayelu, D.O. Real-Time RT-PCR for COVID-19 Diagnosis: Challenges and Prospects. Pan Afr. Med. J. 2020, 35, 121. [Google Scholar] [CrossRef]
- Hadweh, P.; Orfanidou, T.; Tsiamita, M.; Timologos, G.; Papadopoulos, T. SARS-CoV2: Diagnostic Tests Available to the Clinician. Hell. J. Nucl. Med. 2020, 23, 8–14. [Google Scholar]
- Priyanka; Choudhary, O.P.; Singh, I. Diagnosis of SARS-CoV-2: A Review on the Current Scenario and Future Outlook. Acta Virol. 2020, 64, 396–408. [Google Scholar] [CrossRef]
- Younes, N.; Al-Sadeq, D.W.; AL-Jighefee, H.; Younes, S.; Al-Jamal, O.; Daas, H.I.; Yassine, H.M.; Nasrallah, G.K. Challenges in Laboratory Diagnosis of the Novel Coronavirus SARS-CoV-2. Viruses 2020, 12, 582. [Google Scholar] [CrossRef]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef]
- SARS-CoV-2 Diagnostic Pipeline. Available online: https://www.finddx.org/covid-19/pipeline/ (accessed on 11 June 2022).
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 Novel Coronavirus (2019-NCoV) by Real-Time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Alhamlan, F.S.; Al-Qahtani, A.A.; Bakheet, D.M.; Bohol, M.F.; Althawadi, S.I.; Mutabagani, M.S.; Almaghrabi, R.S.; Obeid, D.A. Development and Validation of an In-House, Low-Cost SARS-CoV-2 Detection Assay. J. Infect. Public Health 2021, 14, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Serrano, N.; Lope-Pari, P.; Huaringa-Nuñez, M.; Simas, P.V.M.; Palacios-Salvatierra, R.; Balbuena-Torres, J.; Rey, O.A.C.; Padilla-Rojas, C. Validación y evaluación de una prueba de RT-PCR en tiempo real in house para la detección de SARS-CoV-2 usando un gen específico RdRp y control endógeno GAPDH. Rev. Peru. De Med. Exp. Y Salud Pública 2021, 38, 595–600. [Google Scholar] [CrossRef]
- Álvarez-Díaz, D.A.; Franco-Muñoz, C.; Laiton-Donato, K.; Usme-Ciro, J.A.; Franco-Sierra, N.D.; Flórez-Sánchez, A.C.; Gómez-Rangel, S.; Rodríguez-Calderon, L.D.; Barbosa-Ramirez, J.; Ospitia-Baez, E.; et al. Molecular Analysis of Several In-House RRT-PCR Protocols for SARS-CoV-2 Detection in the Context of Genetic Variability of the Virus in Colombia. Infect. Genet. Evol. 2020, 84, 104390. [Google Scholar] [CrossRef] [PubMed]
- Nalla, A.K.; Casto, A.M.; Huang, M.-L.W.; Perchetti, G.A.; Sampoleo, R.; Shrestha, L.; Wei, Y.; Zhu, H.; Jerome, K.R.; Greninger, A.L. Comparative Performance of SARS-CoV-2 Detection Assays Using Seven Different Primer-Probe Sets and One Assay Kit. J. Clin. Microbiol. 2020, 58, e00557-20. [Google Scholar] [CrossRef]
- Kadam, S.B.; Sukhramani, G.S.; Bishnoi, P.; Pable, A.A.; Barvkar, V.T. SARS-CoV-2, the Pandemic Coronavirus: Molecular and Structural Insights. J. Basic Microbiol. 2021, 61, 180–202. [Google Scholar] [CrossRef]
- Li, Y.; Yao, L.; Li, J.; Chen, L.; Song, Y.; Cai, Z.; Yang, C. Stability Issues of RT-PCR Testing of SARS-CoV-2 for Hospitalized Patients Clinically Diagnosed with COVID-19. J. Med. Virol. 2020, 92, 903–908. [Google Scholar] [CrossRef]
- Villarreal-González, R.; Acosta-Hoyos, A.J.; Garzon-Ochoa, J.A.; Galán-Freyle, N.J.; Amar-Sepúlveda, P.; Pacheco-Londoño, L.C. Anomaly Identification during Polymerase Chain Reaction for Detecting SARS-CoV-2 Using Artificial Intelligence Trained from Simulated Data. Molecules 2020, 26, 20. [Google Scholar] [CrossRef]
- Sebastián Bravo-Grau, J.P.C. Estudios de Exactitud Diagnóstica: Herramientas Para Su Interpretación. Rev. Chil. Radiol. 2015, 21, 158–164. [Google Scholar]
- Pinilla, B.G.; Cruz, B.C.A.; Navarrete, O.J.; Pinilla, B.G.; Cruz, B.C.A.; Navarrete, O.J. Diagnóstico molecular de SARS-CoV-2. Nova 2020, 18, 35–41. [Google Scholar] [CrossRef]
- Palacio Rua, K.; García Correa, J.F.; Aguilar-Jiménez, W.; Afanador Ayala, C.; Rugeles, M.T.; Zuluaga, A.F. Validación de Una Técnica de PCR Dúplex Usando El Gen E y RNasa P Para El Diagnóstico de SARS-CoV-2. Enferm. Infecc. Microbiol. Clínica 2021, 40, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Vogels, C.B.F.; Brito, A.F.; Wyllie, A.L.; Fauver, J.R.; Ott, I.M.; Kalinich, C.C.; Petrone, M.E.; Casanovas-Massana, A.; Catherine Muenker, M.; Moore, A.J.; et al. Analytical Sensitivity and Efficiency Comparisons of SARS-CoV-2 RT–QPCR Primer–Probe Sets. Nat. Microbiol. 2020, 5, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Mair, T.; Ivankovic, M.; Paar, C.; Salzer, H.J.F.; Heissl, A.; Lamprecht, B.; Schreier-Lechner, E.; Tiemann-Boege, I. Processing Hundreds of SARS-CoV-2 Samples with an In-House PCR-Based Method without Robotics. Viruses 2021, 13, 1712. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.U.; Iqbal, J. An Update on Molecular Diagnostics for COVID-19. Front. Cell. Infect. Microbiol. 2020, 10, 560616. [Google Scholar] [CrossRef] [PubMed]
- Wernike, K.; Keller, M.; Conraths, F.J.; Mettenleiter, T.C.; Groschup, M.H.; Beer, M. Pitfalls in SARS-CoV-2 PCR Diagnostics. Transbound. Emerg. Dis. 2021, 68, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.; Neumann, M.; Krause, E.; Rinner, T.; Muzeniek, T.; Grossegesse, M.; Hille, G.; Schwarz, F.; Puyskens, A.; Förster, S.; et al. Resource-Efficient Internally Controlled in-House Real-Time PCR Detection of SARS-CoV-2. Virol. J. 2021, 18, 110. [Google Scholar] [CrossRef]
- Kucirka, L.M.; Lauer, S.A.; Laeyendecker, O.; Boon, D.; Lessler, J. Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction–Based SARS-CoV-2 Tests by Time Since Exposure. Ann. Intern. Med. 2020, 173, 262–267. [Google Scholar] [CrossRef]


| Gene Name | Oligonucleotide ID | Sequence (5′–3′) [15] | TRIPLEX * E:RdRp:RNase P | DUPLEX * E:RNase P | DUPLEX * RdRp:RNase P |
|---|---|---|---|---|---|
| RdRp | Forward | GTGARATGGTCATGTGTGGCGG | 600 nM | - | 600 nM |
| Reverse | CARATGTTAAASACACTATTAGCATA | 800 nM | - | 800 nM | |
| Probe_P2 | FAM-CAGGTGGAACCTCATCAGGAGATGC-BBQ-1 | 200 nM | - | 200 nM | |
| E | Forward | ACAGGTACGTTAATAGTTAATAGCGT | 400 nM | 200 nM | - |
| Reverse | ATATTGCAGCAGTACGCACACA | 400 nM | 200 nM | - | |
| Probe_P1 | CAL FLUOR RED 610-ACACTAGCCATCCTTACTGCGCTTCG-BBQ-2 | 100 nM | 200 nM | - | |
| RNase P | Forward | AGATTTGGACCTGCGAGCG | 200 nM | 100 nM | 100 nM |
| Reverse | GAGCGGCTGTCTCCACAAGT | 200 nM | 100 nM | 100 nM | |
| Probe_Pro1 | HEX-TTCTGACCT-Nova-GAAGGCTCTGCGCG- BHQ-1 | 100 nM | 100 nM | 100 nM |
| RT-qPCR TRIPLEX | RT-qPCR E DUPLEX | RT-qPCR RdRp DUPLEX | |||
|---|---|---|---|---|---|
| Component | Stock Concentration | Volume per 20 μL Reaction | Volume per 16 μL Reaction | Volume per 16 μL Reaction | Final Concentration |
| 2 × SuperScript™ RB | - | 10 | 8 | 8 | - |
| RT/Taq | - | 0.5 | 0.3 | 0.3 | - |
| Forward primer RdRp | 48 µM | 0.25 | - | 0.2 | 0.6 µM |
| Reverse primer RdRp | 64 µM | 0.25 | - | 0.2 | 0.8 µM |
| Forward primer E gene | 32 µM | 0.25 | 0.2 | - | 0.4 µM |
| Reverse primer E gene | 32 µM | 0.25 | 0.2 | - | 0.4 µM |
| Forward primer RNase P | 16 µM | 0.25 | 0.2 | 0.2 | 0.2 µM |
| Reverse primer RNase P | 16 µM | 0.25 | 0.2 | 0.2 | 0.2 µM |
| Probe_P2 RdRp | 4 µM | 1 | - | 0.8 | 0.2 µM |
| Probe_P1 E | 2 µM | 1 | 0.8 | - | 0.1 µM |
| Probe_Pro1 RNase P | 2 µM | 1 | 0.8 | 0.8 | 0.1 µM |
| RNA (add at step 4) | - | 5 | 5 | 5 | - |
| H20 nuclease free | - | - | 0.3 | 0.3 | - |
| DuplexRdRp:RNaseP | |||||||
|---|---|---|---|---|---|---|---|
| Total | Ct 13–15 | Ct 16–20 | Ct 21–25 | Ct 26–30 | Ct 31–35 | Ct 36–38 | |
| Sensitivity (%) | 83.1 | 100 | 100 | 100 | 100 | 68.2 | 54.5 |
| Specificity (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| PPV (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| NPV (%) | 87.6 | 100 | 100 | 100 | 100 | 91.3 | 93.6 |
| Accuracy (%) | 92.3 | 100 | 100 | 100 | 100 | 92.6 | 94.0 |
| TriplexE:RdRp:RNaseP | |||||||
| Sensitivity (%) | 98.3 | 100 | 100 | 100 | 100 | 100 | 75.0 |
| Specificity (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| PPV (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| NPV (%) | 98.7 | 100 | 100 | 100 | 100 | 100 | 97.3 |
| Accuracy (%) | 99.2 | 100 | 100 | 100 | 100 | 100 | 97.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bello-Lemus, Y.; Anaya-Romero, M.; Gómez-Montoya, J.; Árquez, M.; González-Torres, H.J.; Navarro-Quiroz, E.; Pacheco-Londoño, L.; Pacheco-Lugo, L.; Acosta-Hoyos, A.J. Comparative Analysis of In-House RT-qPCR Detection of SARS-CoV-2 for Resource-Constrained Settings. Diagnostics 2022, 12, 2883. https://doi.org/10.3390/diagnostics12112883
Bello-Lemus Y, Anaya-Romero M, Gómez-Montoya J, Árquez M, González-Torres HJ, Navarro-Quiroz E, Pacheco-Londoño L, Pacheco-Lugo L, Acosta-Hoyos AJ. Comparative Analysis of In-House RT-qPCR Detection of SARS-CoV-2 for Resource-Constrained Settings. Diagnostics. 2022; 12(11):2883. https://doi.org/10.3390/diagnostics12112883
Chicago/Turabian StyleBello-Lemus, Yesit, Marco Anaya-Romero, Janni Gómez-Montoya, Moisés Árquez, Henry J. González-Torres, Elkin Navarro-Quiroz, Leonardo Pacheco-Londoño, Lisandro Pacheco-Lugo, and Antonio J. Acosta-Hoyos. 2022. "Comparative Analysis of In-House RT-qPCR Detection of SARS-CoV-2 for Resource-Constrained Settings" Diagnostics 12, no. 11: 2883. https://doi.org/10.3390/diagnostics12112883
APA StyleBello-Lemus, Y., Anaya-Romero, M., Gómez-Montoya, J., Árquez, M., González-Torres, H. J., Navarro-Quiroz, E., Pacheco-Londoño, L., Pacheco-Lugo, L., & Acosta-Hoyos, A. J. (2022). Comparative Analysis of In-House RT-qPCR Detection of SARS-CoV-2 for Resource-Constrained Settings. Diagnostics, 12(11), 2883. https://doi.org/10.3390/diagnostics12112883








