Multidisciplinary Management of Craniopharyngiomas in Children: A Single Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Radiological Assessment
2.2. Surgery
2.3. Proton Beam Therapy
2.4. Ophthalmological Assessment
2.5. Endocrinological Assessment
2.6. Neuropsychological Evaluation
2.7. Statistical Analysis
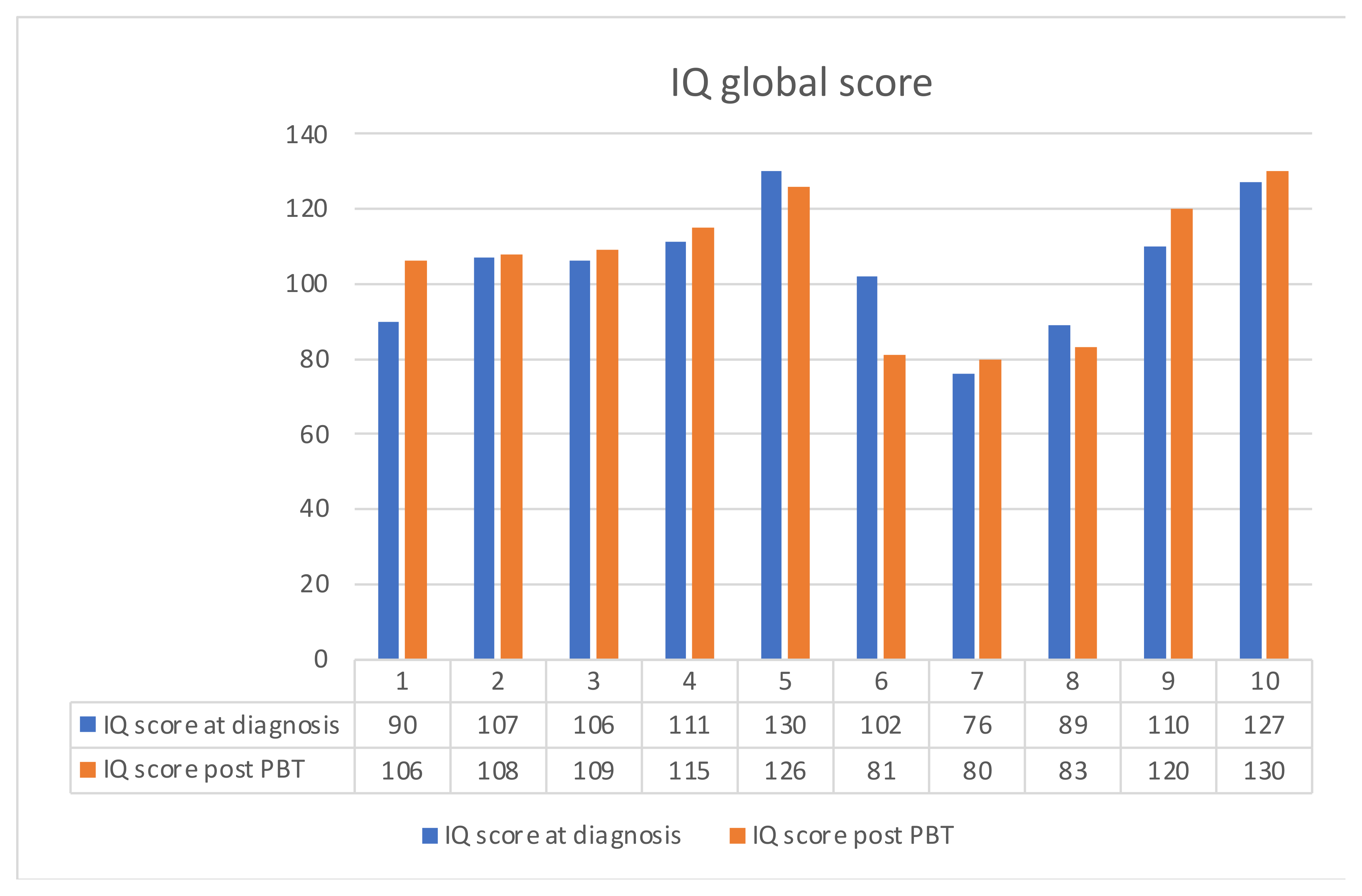
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zacharia, B.E.; Bruce, S.S.; Goldstein, H.; Malone, H.R.; Neugut, A.I.; Bruce, J.N. Incidence, treatment and survival of patients with craniopharyngioma in the surveillance, epidemiology and end results program. Neuro Oncol. 2012, 14, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, N.M.; Noormohamed, N.; Cote, D.J.; Alharthi, S.; Doucette, J.; Zaidi, H.A.; Mekary, R.A.; Smith, T.R. Physiologic Growth Hormone Replacement Therapy and Craniopharyngioma Recurrence in Pediatric Patients: A Meta-Analysis. World Neurosurg. 2018, 109, 487–496.e1. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Shao, Q.; Pan, Z.; You, J. Analysis and Long-Term Follow-Up of the Surgical Treatment of Children With Cranipharyngioma. J. Craniofac. Surg. 2016, 27, e763–e766. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.; Kortmann, R.D.; Skalej, M.; Bamberg, M. The role of radiotherapy in the treatment of craniopharyngioma--indications, results, side effects. Front. Radiat. Ther. Oncol. 1999, 33, 100–113. [Google Scholar]
- Clark, A.J.; Cage, T.A.; Aranda, D.; Parsa, A.T.; Sun, P.P.; Auguste, K.I.; Gupta, N. A systematic review of the results of surgery and radiotherapy on tumor control for pediatric craniopharyngioma. Childs Nerv. Syst. 2013, 29, 231–238. [Google Scholar] [CrossRef]
- Zhang, C.; Verma, V.; Lyden, E.R.; Horowitz, D.P.; Zacharia, B.E.; Lin, C.; Connolly, E.P. The Role of Definitive Radiotherapy in Craniopharyngioma: A SEER Analysis. Am. J. Clin. Oncol. 2018, 41, 807–812. [Google Scholar] [CrossRef]
- Merchant, T.E.; Kiehna, E.N.; Sanford, R.A.; Mulhern, R.K.; Thompson, S.J.; Wilson, M.W.; Lustig, R.H.; Kun, L.E. Craniopharyngioma: The St. Jude Children’s Research Hospital experience 1984–2001. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 533–542. [Google Scholar] [CrossRef]
- Beltran, C.; Roca, M.; Merchant, T.E. On the benefits and risks of proton therapy in pediatric craniopharyngioma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e281–e287. [Google Scholar] [CrossRef]
- Boehling, N.S.; Grosshans, D.R.; Bluett, J.B.; Palmer, M.T.; Song, X.; Amos, R.A.; Sahoo, N.; Meyer, J.J.; Mahajan, A.; Woo, S.Y. Dosimetric comparison of three-dimensional conformal proton radiotherapy, intensity-modulated proton therapy, and intensity-modulated radiotherapy for treatment of pediatric craniopharyngiomas. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 643–652. [Google Scholar] [CrossRef]
- Conroy, R.; Gomes, L.; Owen, C.; Buchsbaum, J.; Ahern, V. Clinical equipoise: Protons and the child with craniopharyngioma. J. Med. Imaging Radiat. Oncol. 2015, 59, 379–385. [Google Scholar] [CrossRef]
- Paganetti, H.; Niemierko, A.; Ancukiewicz, M.; E Gerweck, L.; Goitein, M.; Loeffler, J.S.; Suit, H.D. Relative biological effectiveness (RBE) values for proton beam therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 407–421. [Google Scholar] [CrossRef]
- Ajithkumar, T.; Mazhari, A.L.; Stickan-Verfürth, M.; Kramer, P.H.; Fuentes, C.S.; Lambert, J.; Thomas, H.; Müller, H.; Fleischhack, G.; Timmermann, B. Proton Therapy for Craniopharyngioma—An Early Report from a Single European Centre. Clin. Oncol. 2018, 30, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_8.5x11.pdf (accessed on 18 September 2022).
- Avery, R.A.; Ferner, R.E.; Listernick, R.; Fisher, M.J.; Gutmann, D.H.; Liu, G.T. Visual acuity in children with low grade gliomas of the visual pathway: Implications for patient care and clinical research. J. Neurooncol. 2012, 110, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Abongwa, C.; Ashwal, S.; Deming, D.D.; Winter, T.W. Referral for Ophthalmology Evaluation and Visual Sequelae in Children With Primary Brain Tumors. JAMA Netw. Open 2019, 2, e198273. [Google Scholar] [CrossRef]
- Parrozzani, R.; Miglionico, G.; Leonardi, F.; Pulze, S.; Trevisson, E.; Clementi, M.; Opocher, E.; Licata, V.; Viscardi, E.; Pilotto, E.; et al. Correlation of peripapillary retinal nerve fibre layer thickness with visual acuity in paediatric patients affected by optic pathway glioma. Acta Ophthalmol. 2018, 96, e1004–e1009. [Google Scholar] [CrossRef]
- Donahue, S.P.; Porter, A. SITA visual field testing in children. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2001, 5, 114–117. [Google Scholar] [CrossRef]
- Greve, E.L.; Bakker, D.; de Boer, R.W.; Moed, J. Clinical evaluation of the Scoperimeter, an experimental automatic perimeter. Int. Ophthalmol. 1982, 5, 193–200. [Google Scholar] [CrossRef]
- Koenraads, Y.; Braun, K.P.J.; van der Linden, D.C.P.; Imhof, S.M.; Porro, G.L. Perimetry in Young and Neurologically Impaired Children: The Behavioral Visual Field (BEFIE) Screening Test Revisited. JAMA Ophthalmol. 2015, 133, 319. [Google Scholar] [CrossRef]
- Kolling, G.H.; Wabbels, B. Kinetic perimetry in neuroophthalmological practice. Strabismus 2000, 8, 215. [Google Scholar] [CrossRef]
- SAS Program (Ages 0 to < 20 Years), Resources, Growth Chart Training, Nutrition, DNPAO, CDC. Available online: https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm (accessed on 15 September 2022).
- Endocrine Testing Protocols: Hypothalamic Pituitary Adrenal Axis. Available online: https://pubmed.ncbi.nlm.nih.gov/25905177/ (accessed on 3 September 2022).
- Grizzle, R. Wechsler Intelligence Scale for Children, Fourth Edition. In Encyclopedia of Child Behavior and Development; Goldstein, S., Naglieri, J.A., Eds.; Springer: Boston, MA, USA, 2011; pp. 1553–1555. [Google Scholar] [CrossRef]
- Warschausky, S.; Raiford, S.E. Wechsler Preschool and Primary Scale of Intelligence. Encyclopedia of Clinical Neuropsychology; Springer: Cham, Switzerland, 2018; pp. 3705–3709. Available online: http://link.springer.com/10.1007/978-3-319-57111-9_1606 (accessed on 15 September 2022).
- Müller, H.L.; Merchant, T.E.; Warmuth-Metz, M.; Martinez-Barbera, J.P.; Puget, S. Craniopharyngioma. Nat. Rev. Dis. Primers 2019, 5, 75. [Google Scholar] [CrossRef]
- Grewal, M.R.; Spielman, D.B.; Safi, C.; Overdevest, J.B.; Otten, M.; Bruce, J.; Gudis, D.A. Gross Total Versus Subtotal Surgical Resection in the Management of Craniopharyngiomas. Allergy Rhinol. 2020, 11, 2152656720964158. [Google Scholar] [CrossRef] [PubMed]
- Eveslage, M.; Calaminus, G.; Warmuth-Metz, M.; Kortmann, R.D.; Pohl, F.; Timmermann, B.; Schuhmann, M.U.; Flitsch, J.; Faldum, A.; Müller, H.L. The Postoperative Quality of Life in Children and Adolescents with Craniopharyngioma. Dtsch. Ärzteblatt Int. 2019, 116, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Castilla, L.; Russin, J.J.; Spetzler, R.F. Surgical management of skull base tumors. Rep. Pract. Oncol. Radiother. 2016, 21, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Özyurt, J.; Müller, H.L.; Thiel, C.M. A systematic review of cognitive performance in patients with childhood craniopharyngioma. J. Neurooncol. 2015, 125, 9–21. [Google Scholar] [CrossRef]
- Müller, H.L. Consequences of craniopharyngioma surgery in children. J. Clin. Endocrinol. Metab. 2011, 96, 1981–1991. [Google Scholar] [CrossRef]
- de Vile, C.J.; Grant, D.B.; Hayward, R.D.; Kendall, B.E.; Neville, B.G.; Stanhope, R. Obesity in childhood craniopharyngioma: Relation to post-operative hypothalamic damage shown by magnetic resonance imaging. J. Clin. Endocrinol. Metab. 1996, 81, 2734–2737. [Google Scholar]
- Duff, J.M.; Meyer, F.B.; Ilstrup, D.M.; Laws, E.R.; Schleck, C.D.; Scheithauer, B.W. Long-term Outcomes for Surgically Resected Craniopharyngiomas. Neurosurgery 2000, 46, 291–305. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, S.-H.; Seoul, H.J.; Nam, D.-H.; Lee, J.-I.; Park, K.; Kim, J.-H.; Kong, D.-S. Impact of maximal safe resection on the clinical outcome of adults with craniopharyngiomas. J. Clin. Neurosci. 2012, 19, 1005–1008. [Google Scholar] [CrossRef]
- Park, H.R.; Kshettry, V.R.; Farrell, C.J.; Lee, J.M.; Kim, Y.H.; Bin Won, T.; Han, D.H.; Do, H.; Nyguist, G.; Rosen, M.; et al. Clinical Outcome After Extended Endoscopic Endonasal Resection of Craniopharyngiomas: Two-Institution Experience. World Neurosurg. 2017, 103, 465–474. [Google Scholar] [CrossRef]
- Schoenfeld, A.; Pekmezci, M.; Barnes, M.J.; Tihan, T.; Gupta, N.; Lamborn, K.R.; Banerjee, A.; Mueller, S.; Chang, S.; Berger, M.S.; et al. The superiority of conservative resection and adjuvant radiation for craniopharyngiomas. J. Neuro-Oncol. 2012, 108, 133–139. [Google Scholar] [CrossRef]
- Tomita, T.; Bowman, R.M. Craniopharyngiomas in children: Surgical experience at Children’s Memorial Hospital. Childs Nerv. Syst. 2005, 21, 729–746. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A.J.; Greenfield, B.; Mahajan, A.; Paulino, A.C.; Okcu, M.F.; Allen, P.K.; Chintagumpala, M.; Kahalley, L.S.; McAleer, M.F.; McGovern, S.L.; et al. Proton Beam Therapy Versus Conformal Photon Radiation Therapy for Childhood Craniopharyngioma: Multi-institutional Analysis of Outcomes, Cyst Dynamics, and Toxicity. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Habrand, J.-L.; Ganry, O.; Couanet, D.; Rouxel, V.; Levy-Piedbois, C.; Pierre-Kahn, A.; Kalifa, C. The role of radiation therapy in the management of craniopharyngioma: A 25-year experience and review of the literature. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 255–263. [Google Scholar] [CrossRef]
- Habrand, J.L.; Saran, F.; Alapetite, C.; Noel, G.; El Boustany, R.; Grill, J. Radiation therapy in the management of craniopharyngioma: Current concepts and future developments. J. Pediatr. Endocrinol. Metab. 2006, 19 (Suppl. S1), 389–394. [Google Scholar] [PubMed]
- Minniti, G.; Esposito, V.; Amichetti, M.; Enrici, R.M. The role of fractionated radiotherapy and radiosurgery in the management of patients with craniopharyngioma. Neurosurg. Rev. 2009, 32, 125–132. [Google Scholar] [CrossRef]
- Merchant, T.E.; Kiehna, E.N.; Kun, L.E.; Mulhern, R.K.; Li, C.; Xiong, X.; Boop, F.A.; Sanford, R.A. Phase II trial of conformal radiation therapy for pediatric patients with craniopharyngioma and correlation of surgical factors and radiation dosimetry with change in cognitive function. J Neurosurg. 2006, 104 (Suppl. S2), 94–102. [Google Scholar] [CrossRef]
- Merchant, T.E.; Kun, L.E.; Hua, C.-H.; Wu, S.; Xiong, X.; Sanford, R.A.; Boop, F.A. Disease control after reduced volume conformal and intensity-modulated radiation therapy for childhood craniopharyngioma. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, e187–e192. [Google Scholar] [CrossRef]
- Greenfield, B.J.; Okcu, M.F.; Baxter, P.A.; Chintagumpala, M.; Teh, B.S.; Dauser, R.C.; Su, J.; Desai, S.S.; Paulino, A.C. Long-term disease control and toxicity outcomes following surgery and intensity modulated radiation therapy (IMRT) in pediatric craniopharyngioma. Radiother. Oncol. 2015, 114, 224–229. [Google Scholar] [CrossRef]
- Indelicato, D.J.; Merchant, T.; Laperriere, N.; Lassen, Y.; Vennarini, S.; Wolden, S.; Hartsell, W.; Pankuch, M.; Brandal, P.; Law, C.-C.K.; et al. Consensus Report From the Stockholm Pediatric Proton Therapy Conference. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 387–392. [Google Scholar] [CrossRef]
- Kiehna, E.N.; Merchant, T.E. Radiation therapy for pediatric craniopharyngioma. Neurosurg. Focus 2010, 28, E10. [Google Scholar] [CrossRef]
- Luu, Q.T.; Loredo, L.N.; Archambeau, J.O.; Yonemoto, L.T.; Slater, J.M.; Slater, J.D. Fractionated proton radiation treatment forpediatric craniopharyngioma: Preliminary report. Cancer J. 2006, 12, 155–159. [Google Scholar] [PubMed]
- Winkfield, K.M.; Linsenmeier, C.; Yock, T.I.; Grant, P.E.; Yeap, B.Y.; Butler, W.E.; Tarbell, N.J. Surveillance of craniopharyngioma cyst growth in children treated with proton radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Sklar, C.A. Craniopharyngioma: Endocrine Abnormalities at Presentation. Pediatr. Neurosurg. 1994, 21 (Suppl. S1), 18–20. [Google Scholar] [CrossRef]
- Capatina, C.; Vintila, M.; Gherlan, I.; Dumitraşcu, A.; Caragheorgheopol, A.; Procopiuc, C.; Ciubotaru, V.; Poiana, C. Craniopharyngioma—Clinical and therapeutic outcome data in a mixed cohort of adult and paediatric cases. Acta Endocrinol. 2018, 14, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Paja, M.; Lucas, T.; García-Uría, J.; Salamé, F.; Barceló, B.; Estrada, J. Hypothalamic-pituitary dysfunction in patients with craniopharyngioma. Clin. Endocrinol. 1995, 42, 467–473. [Google Scholar] [CrossRef]
- Müller, H.L.; Emser, A.; Faldum, A.; Bruhnken, G.; Etavard-Gorris, N.; Gebhardt, U.; Oeverink, R.; Kolb, R.; Sörensen, N. Longitudinal study on growth andbody mass index before and after diagnosis of childhood craniopharyngioma. J. Clin. Endocrinol. Metab. 2004, 89, 3298–3305. [Google Scholar] [CrossRef]
- Müller, H.L.; Faldum, A.; Etavard-Gorris, N.; Gebhardt, U.; Oeverink, R.; Kolb, R.; Sörensen, N. Functional Capacity, Obesity and Hypothalamic Involvement: Cross-Sectional Study on 212 Patients with Childhood Craniopharyngioma. Klin. Padiatr. 2003, 215, 310–314. [Google Scholar]
- Müller, H.L.; Bueb, K.; Bartels, U.; Roth, C.; Harz, K.; Graf, N.; Korinthenberg, R.; Bettendorf, M.; Kühl, J.; Gutjahr, P.; et al. Obesity after childhood craniopharyngioma—German multicenter study on pre-operative risk factors and quality of life. Klin. Padiatr. 2001, 213, 244–249. [Google Scholar] [CrossRef]
- Lustig, R.H.; Post, S.R.; Srivannaboon, K.; Rose, S.R.; Danish, R.K.; Burghen, G.A.; Xiong, X.; Wu, S.; Merchant, T.E. Risk factors for the development of obesity in children surviving brain tumors. J. Clin. Endocrinol. Metab. 2003, 88, 611–616. [Google Scholar] [CrossRef]
- Srinivasan, S.; Ogle, G.D.; Garnett, S.P.; Briody, J.N.; Lee, J.W.; Cowell, C.T. Features of the metabolic syndrome after childhood craniopharyngioma. J. Clin. Endocrinol. Metab. 2004, 89, 81–86. [Google Scholar] [CrossRef]
- Müller, H.L.; Schneider, P.; Bueb, K.; Etavard-Gorris, N.; Gebhardt, U.; Kolb, R.; Sörensen, N. Volumetric bone mineral density in patients with childhood craniopharyngioma. Exp. Clin. Endocrinol. Diabetes 2003, 111, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.L.; Heinrich, M.; Bueb, K.; Oeverink, R.; Kolb, R.; Sörensen, N. Perioperative dexamethasone treatment in childhood craniopharyngioma--influence on short-term and long-term weight gain. Exp. Clin. Endocrinol. Diabetes 2003, 111, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Holmer, H.; Pozarek, G.; Wirfalt, E.; Popovic, V.; Ekman, B.; Bjork, J.; Erfurth, E.M. Reduced energy expenditure and impaired feeding-related signals but not high energy intake reinforces hypothalamic obesity in adults with childhood onset craniopharyngioma. J. Clin. Endocrinol. Metab. 2010, 95, 5395–5402. [Google Scholar] [CrossRef] [PubMed]
- Holmer, H.; Ekman, B.; Björk, J.; Nordstöm, C.H.; Popovic, V.; Siversson, A.; Erfurth, E.M. Hypothalamic involvement predicts cardiovascular risk in adults with childhood onset craniopharyngioma on long-term GH therapy. Eur. J. Endocrinol. 2009, 161, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Nuijts, M.A.; Veldhuis, N.; Stegeman, I.; van Santen, H.M.; Porro, G.L.; Imhof, S.M.; Schouten–van Meeteren, A.Y. Visual functions in children with craniopharyngioma at diagnosis: A systematic review. PLoS ONE 2020, 15, e0240016. [Google Scholar] [CrossRef] [PubMed]
- Peragallo, J.H.; Vasseneix, C.F.; Jariyakosol, S.; Newman, N.J.; Biousse, V.; Bruce, B.B. The relationship of vision and quality of life (QOL) in patients with pediatric primary brain tumors (PBT). Invest. Ophthalmol. Vis. Sci. 2016, 57, 5604. [Google Scholar] [CrossRef]
- Castle-Kirszbaum, M.; Shi, M.D.Y.; Goldschlager, T. Quality of Life in Craniopharyngioma: A Systematic Review. World Neurosurg. 2022, 164, 424–435.e2. [Google Scholar] [CrossRef]
- Bakhsheshian, J.; Jin, D.L.; Chang, K.-E.; Strickland, B.A.; Donoho, D.A.; Cen, S.; Mack, W.J.; Attenello, F.; Christian, E.A.; Zada, G. Risk factors associated with the surgical management of craniopharyngiomas in pediatric patients: Analysis of 1961 patients from a national registry database. Neurosurg. Focus 2016, 41, E8. [Google Scholar] [CrossRef]
- Choux, M.; Lena, G.; Genitori, L. Craniopharyngioma in Children: Surgical Considerations. In Craniopharyngioma; Springer: Milan, Italy, 1995; pp. 20–30. Available online: http://link.springer.com/10.1007/978-88-470-2291-1_3 (accessed on 12 August 2022).
- Mende, K.C.; Kellner, T.; Petersenn, S.; Honegger, J.; Evangelista-Zamora, R.; Droste, M.; Stalla, G.; Deutschbein, T.; Wang, Y.; Moskopp, D.; et al. Clinical Situation, Therapy, and Follow-Up of Adult Craniopharyngioma. J. Clin. Endocrinol. Metab. 2020, 105, 252–265. [Google Scholar] [CrossRef]
- Karavitaki, N.; Brufani, C.; Warner, J.T.; Adams, C.B.T.; Richards, P.; Ansorge, O.; Shine, B.; Turner, H.E.; Wass, J.A.H. Craniopharyngiomas in children and adults: Systematic analysis of 121 cases with long-term follow-up. Clin. Endocrinol. 2005, 62, 397–409. [Google Scholar] [CrossRef]
- Clark, A.J.; Cage, T.A.; Aranda, D.; Parsa, A.T.; Auguste, K.I.; Gupta, N. Treatment-related morbidity and the management of pediatric craniopharyngioma: A systematic review. J. Neurosurg. Pediatr. 2012, 10, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Caldarelli, M.; Massimi, L.; Tamburrini, G.; Cappa, M.; Di Rocco, C. Long-term results of the surgical treatment of craniopharyngioma: The experience at the Policlinico Gemelli, Catholic University, Rome. Childs Nerv. Syst. 2005, 21, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.E.; Jane, J.A.; Wisoff, J.H. Surgical management of craniopharyngiomas in children: Meta-analysis and comparison of transcranial and transsphenoidal approaches. Neurosurgery 2011, 69, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L. Childhood craniopharyngioma. Recent advances in diagnosis, treatment and follow-up. Horm. Res. 2008, 69, 193–202. [Google Scholar] [PubMed]
- Hoffman, H.J.; De Silva, M.; Humphreys, R.P.; Drake, J.M.; Smith, M.L.; Blaser, S.I. Aggressive surgical management of craniopharyngiomas in children. J. Neurosurg. 1992, 76, 47–52. [Google Scholar] [CrossRef]
- Mueller, S.; Fullerton, H.; Stratton, K.; Leisenring, W.; Weathers, R.E.; Stovall, M.; Armstrong, G.T.; Goldsby, R.E.; Packer, R.J.; Sklar, C.A.; et al. Radiation, Atherosclerotic Risk Factors and Stroke Risk in Survivors of Pediatric Cancer: A Report from the Childhood Cancer Survivor Study. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 649–655. [Google Scholar] [CrossRef]
- Liu, A.K.; Bagrosky, B.; Fenton, L.Z.; Gaspar, L.E.; Handler, M.H.; McNatt, S.A.; Foreman, N.K. Vascular abnormalities in pediatric craniopharyngioma patients treated with radiation therapy. Pediatr. Blood Cancer 2009, 52, 227–230. [Google Scholar] [CrossRef]
- Lo, A.C.; Howard, F.; Nichol, A.; Sidhu, K.; Abdulsatar, F.; Hasan, H.; Goddard, K. Long-term outcomes and complications in patients with craniopharyngioma: The British Columbia Cancer Agency experience. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 1011–1018. [Google Scholar] [CrossRef]
- Lo, A.C.; Howard, A.F.; Nichol, A.; Hasan, H.; Martin, M.; Heran, M.K.S.; Goddard, K. A Cross-Sectional Cohort Study of Cerebrovascular Disease and Late Effects After Radiation Therapy for Craniopharyngioma. Pediatr. Blood Cancer 2016, 63, 786–793. [Google Scholar] [CrossRef]
| Characteristics | n (%) |
|---|---|
| Age at diagnosis | Median 8.7 years (range, 3.3–13.3) |
| Gender | Male 6 (60%); female 4 (40%) |
| Clinical characteristics at diagnosis: | |
| Endocrine impairment | GHD: 1 (10%); panhypopituitarism: 4 (40%) |
| Obesity | 3 (30%) |
| Visual impairment | 7 (70%) |
| Surgical details: | |
| Shunt implant for hydrocephalus | 2 (20%) |
| Single surgical approach | 4 (40%) |
| Multiple surgeries | 6 (60%) |
| PBT details: | |
| Age at PBT | Median 10.7 years (range, 6.1–16.6) |
| Relapse/progression after surgery | 7 (70%) |
| Residual disease after surgery | 3 (30%) |
| Residual type before PBT | Solid: 2 (20%) |
| Cystic-solid: 8 (80%) | |
| Time from diagnosis to PBT | Median 24.17 months (range, 4.7–77.8) |
| Follow-up data: | |
| Status after PBT | SD: 3 (30%); PR: 7 (70%) |
| Median duration PR | 26.7 months (range, 13.5–75.6) |
| Median duration SD | 41.3 months (range, 25.6–77.9) |
| Survival | 78.9 months (range, 20.8–108.8) |
| Patient | Age at Diagnosis (Years) | Sex | Visual Impairment at Diagnosis | Endocrine Dysfunction at Diagnosis | HC | More than One Surgery before PBT | Status before PBT | Visual Impairment after Surgery, before PBT | Endocrine Dysfunction after Surgery, before PBT | Age at PBT (Years) | Status after PBT (Duration in Months) | New Visual Impairment after PBT | New Endocrine Dysfunction after PBT | OS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6.2 | M | YES (in one eye) | PH | NO | NO | PD | YES (in one eye) | PH | 6.8 | SD (77.9) | NO | NO | 86.9 |
| 2 | 12.7 | M | NO | PH | NO | NO | PD | NO | PH | 14.2 | PR (75.5) | NO | NO | 95.2 |
| 3 | 9.3 | F | NO | GHD | YES | YES | PD | NO | PH | 11.1 | SD (25.6) | NO | NO | 48.2 |
| 4 | 8.1 | F | YES (in one eye) | PH | NO | NO | PD | YES (in one eye) | PH | 10.3 | PR (13.5) | NO | NO | 42.2 |
| 5 | 2.6 | M | YES (in both eyes) | PH | NO | YES | PD | YES (in both eyes) | PH | 8.2 | PR (41.8) | NO | NO | 108.9 |
| 6 | 13.3 | F | YES (in both eyes) | NO | YES | YES | RD | YES (in both eyes) | PH | 15.7 | SD (40.9) | NO | NO | 71 |
| 7 | 11.1 | M | YES (in one eye) | NO | NO | YES | RD | YES (in one eye) | PH | 16.6 | PR (29.2) | NO | NO | 97.2 |
| 8 | 4.4 | F | YES (in both eyes) | NO | YES | YES | PD | YES (in both eyes) | PH | 6.1 | PR (26.7) | NO | NO | 43.9 |
| 9 | 3.3 | M | YES (in both eyes) | NO | NO | YES | RD | YES (in both eyes) | PH | 9.8 | PR (26.8) | NO | NO | 105.9 |
| 10 | 11.2 | M | NO | NO | NO | NO | PD | NO | PH | 11.6 | PR (14.7) | NO | NO | 20.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Baldo, G.; Vennarini, S.; Cacchione, A.; Amelio, D.; De Ioris, M.A.; Fabozzi, F.; Colafati, G.S.; Mastronuzzi, A.; Carai, A. Multidisciplinary Management of Craniopharyngiomas in Children: A Single Center Experience. Diagnostics 2022, 12, 2745. https://doi.org/10.3390/diagnostics12112745
Del Baldo G, Vennarini S, Cacchione A, Amelio D, De Ioris MA, Fabozzi F, Colafati GS, Mastronuzzi A, Carai A. Multidisciplinary Management of Craniopharyngiomas in Children: A Single Center Experience. Diagnostics. 2022; 12(11):2745. https://doi.org/10.3390/diagnostics12112745
Chicago/Turabian StyleDel Baldo, Giada, Sabina Vennarini, Antonella Cacchione, Dante Amelio, Maria Antonietta De Ioris, Francesco Fabozzi, Giovanna Stefania Colafati, Angela Mastronuzzi, and Andrea Carai. 2022. "Multidisciplinary Management of Craniopharyngiomas in Children: A Single Center Experience" Diagnostics 12, no. 11: 2745. https://doi.org/10.3390/diagnostics12112745
APA StyleDel Baldo, G., Vennarini, S., Cacchione, A., Amelio, D., De Ioris, M. A., Fabozzi, F., Colafati, G. S., Mastronuzzi, A., & Carai, A. (2022). Multidisciplinary Management of Craniopharyngiomas in Children: A Single Center Experience. Diagnostics, 12(11), 2745. https://doi.org/10.3390/diagnostics12112745









