Early Prediction and Monitoring of Treatment Response in Gastrointestinal Stromal Tumors by Means of Imaging: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Article Selection
2.3. Quality Assessment
2.4. Data Analysis
2.4.1. Response Prediction
2.4.2. Therapy Monitoring
3. Results
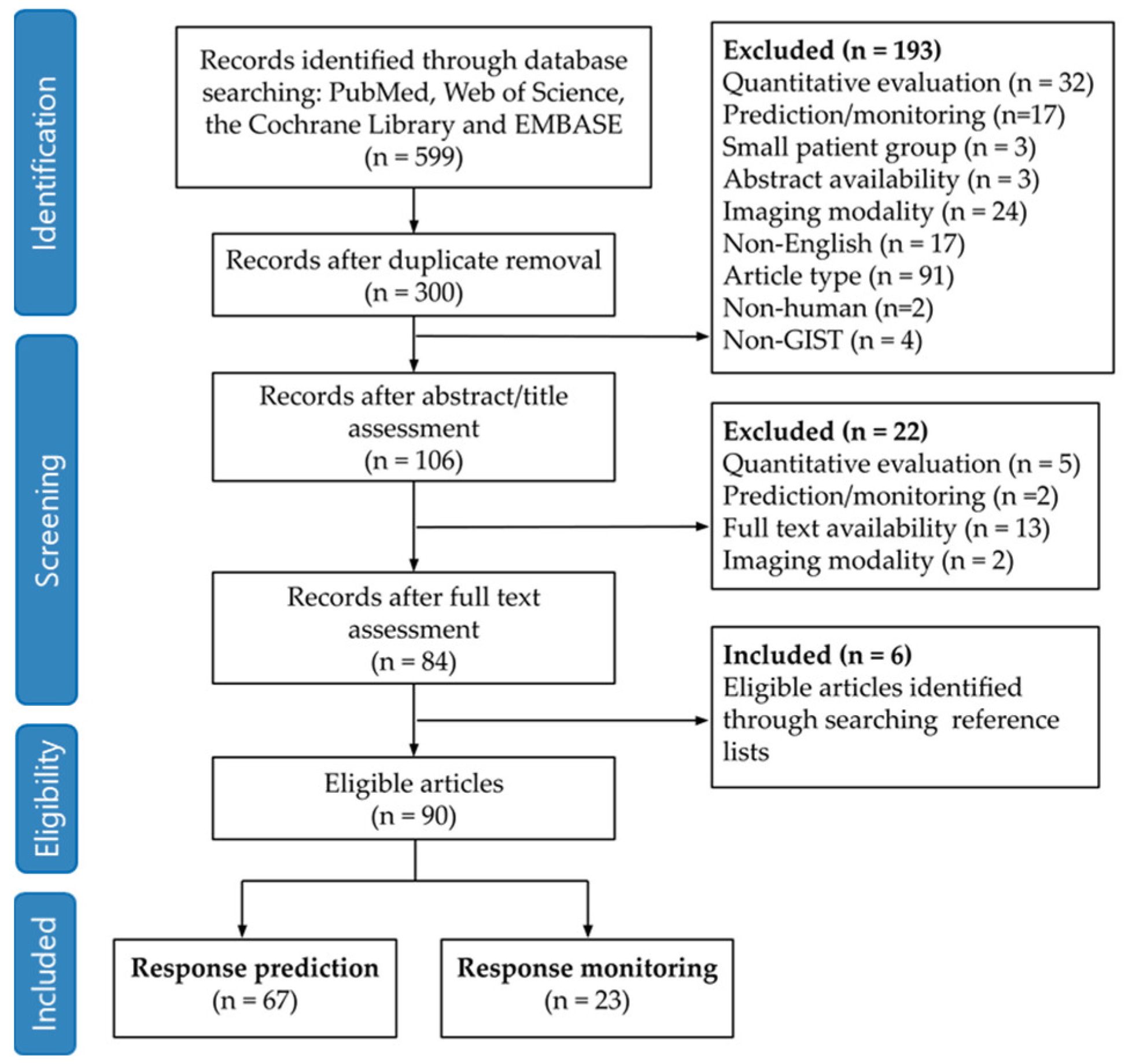
3.1. Search Strategy and Article Selection
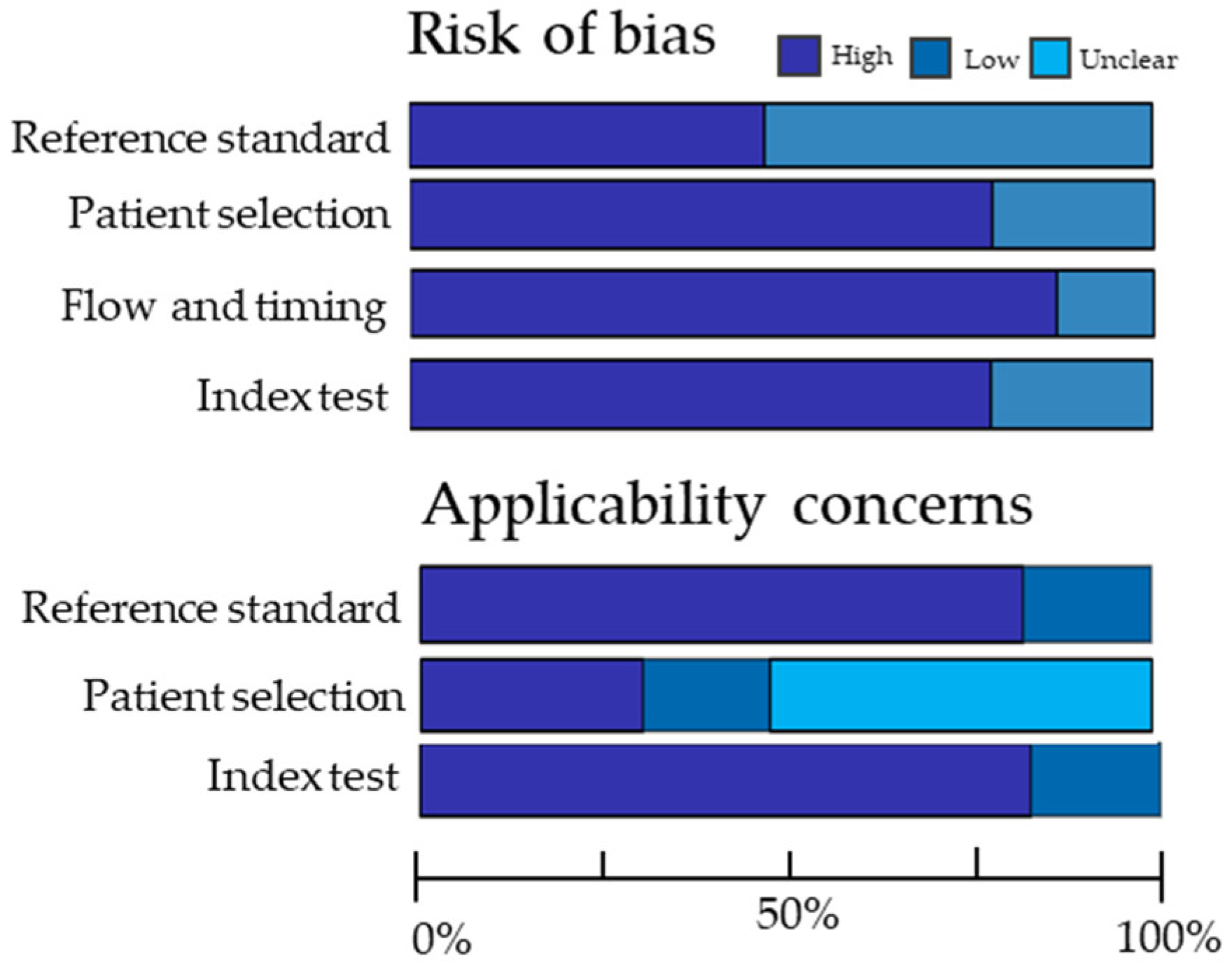
3.2. Quality Assessment
3.3. Response Prediction
3.3.1. Mutational Status
3.3.2. Proliferative Activity
3.3.3. Risk Stratification
3.3.4. Prediction of Radiological Response
3.3.5. Prognosis
3.4. Therapy Monitoring
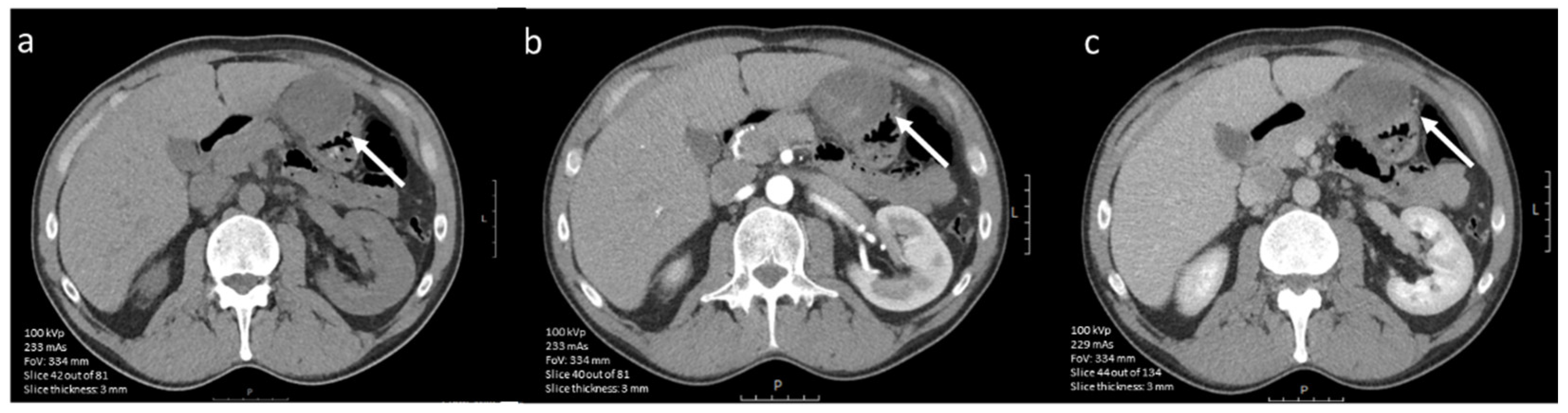
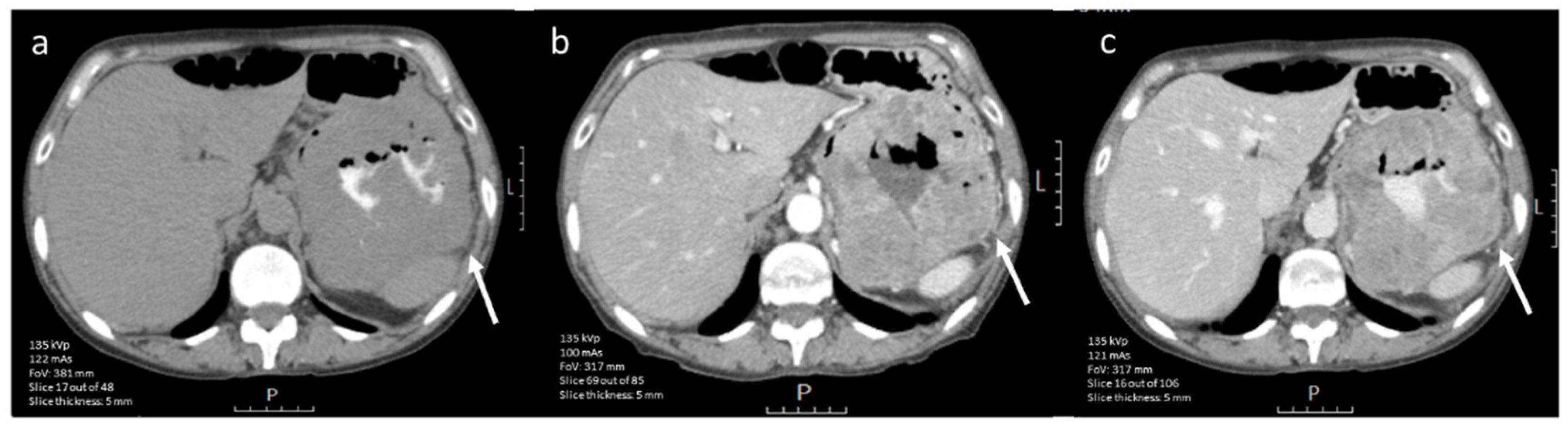
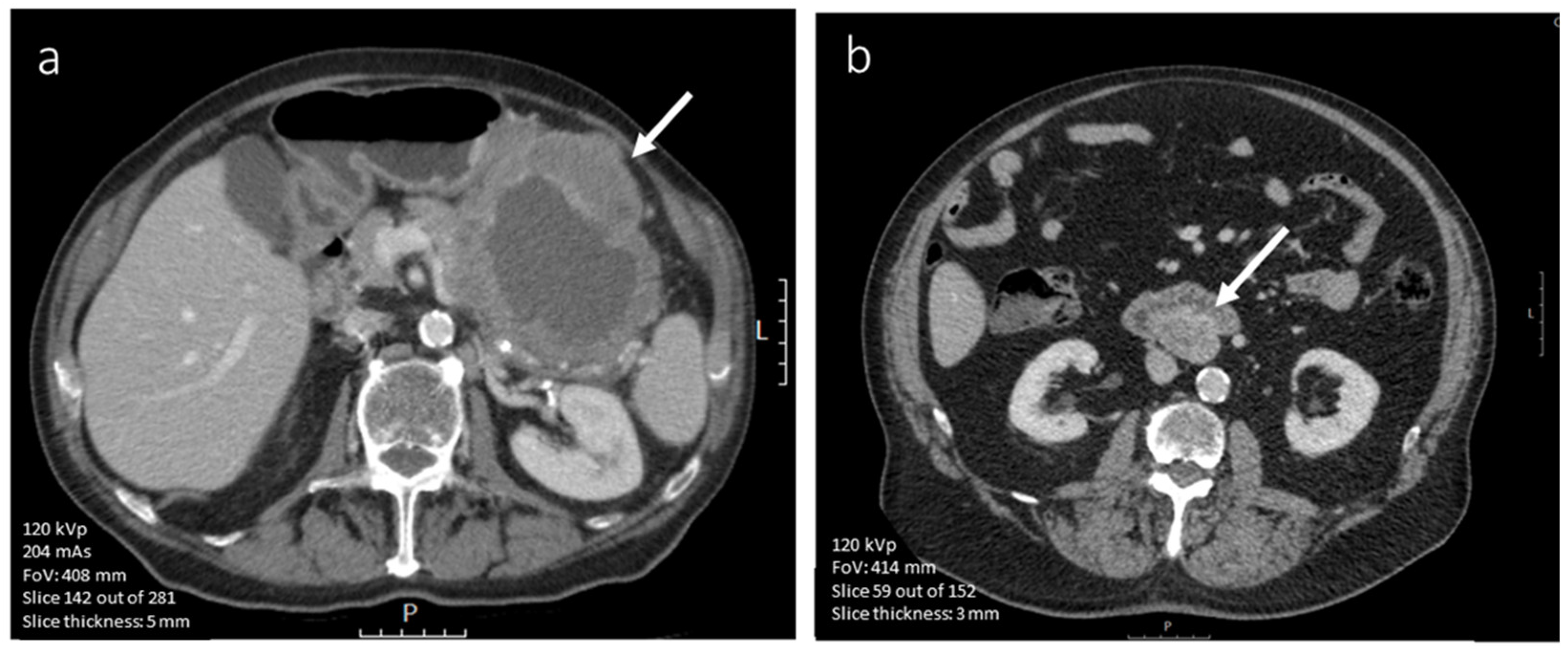
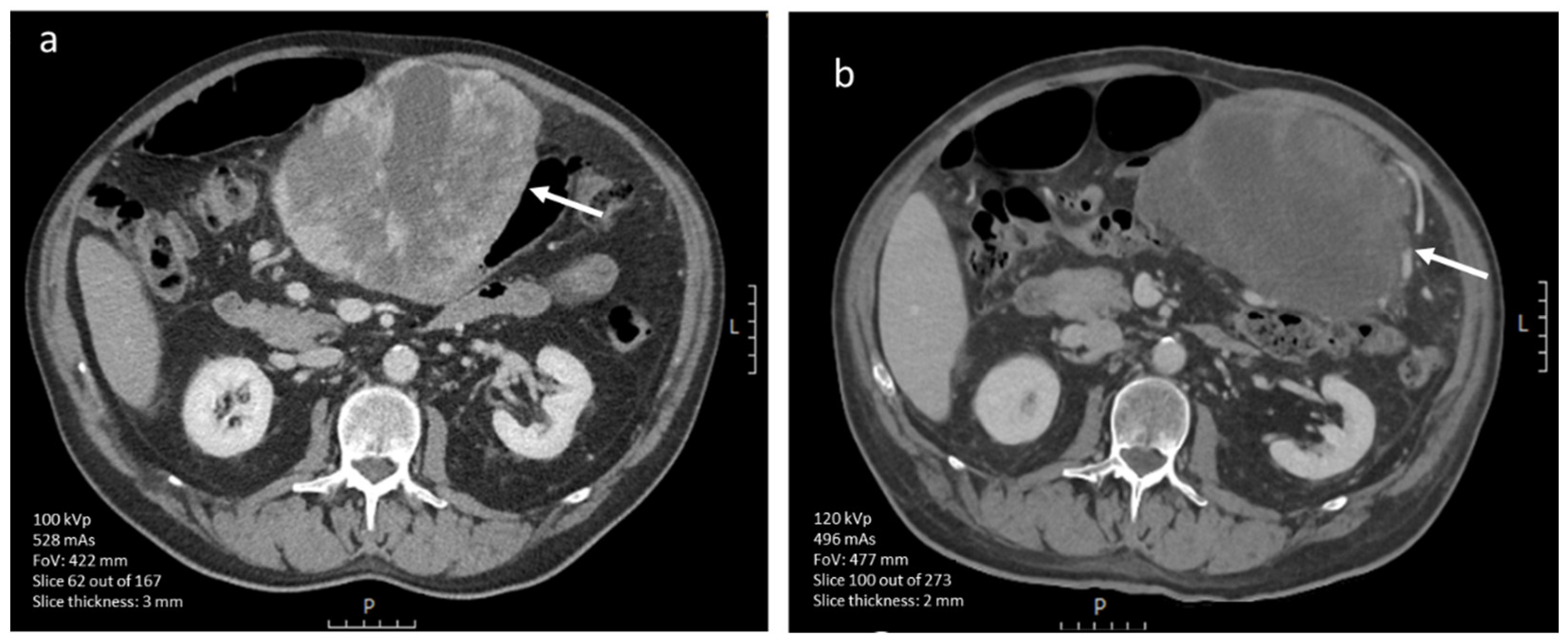
3.4.1. CE-CT Imaging
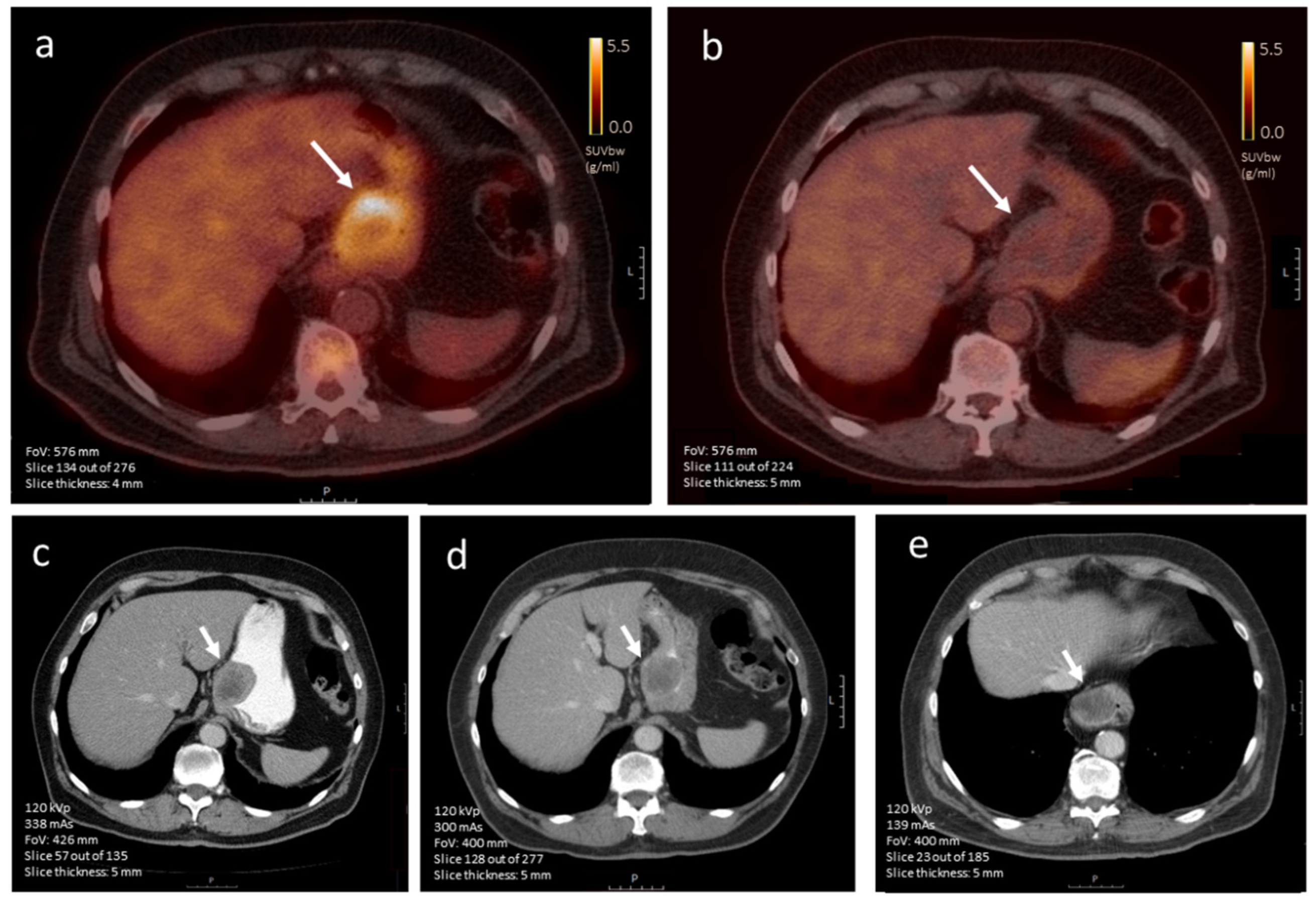
3.4.2. [18F]FDG-PET Imaging
3.4.3. CE-CT vs. [18F]FDG-PET Imaging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balachandran, V.P.; DeMatteo, R.P. Gastrointestinal stromal tumors: Who should get imatinib and for how long? Adv. Surg. 2014, 48, 165–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zappacosta, R.; Zappacosta, B.; Capanna, S.; D’Angelo, C.; Gatta, D.; Rosini, S. GISTs: From the History to the Tailored Therapy, Gastrointestinal Stromal Tumor; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Joensuu, H.; Hohenberger, P.; Corless, C.L. Gastrointestinal stromal tumour. Lancet 2013, 382, 973–983. [Google Scholar] [CrossRef]
- Wu, C.E.; Tzen, C.Y.; Wang, S.Y.; Yeh, C.N. Clinical Diagnosis of Gastrointestinal Stromal Tumor (GIST): From the Molecular Genetic Point of View. Cancers 2019, 11, 679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verweij, J.; Casali, P.G.; Zalcberg, J.; LeCesne, A.; Reichardt, P.; Blay, J.Y.; Issels, R.; van Oosterom, A.; Hogendoorn, P.C.; Van Glabbeke, M.; et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: Randomised trial. Lancet 2004, 364, 1127–1134. [Google Scholar] [CrossRef]
- Reichardt, P. The Story of Imatinib in GIST—A Journey through the Development of a Targeted Therapy. Oncol. Res. Treat. 2018, 41, 472–477. [Google Scholar] [CrossRef]
- Casali, P.G.; Blay, J.Y.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.; et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 20–33. [Google Scholar] [CrossRef]
- Casali, P.G.; Le Cesne, A.; Poveda Velasco, A.; Kotasek, D.; Rutkowski, P.; Hohenberger, P.; Fumagalli, E.; Judson, I.R.; Italiano, A.; Gelderblom, H.; et al. Time to Definitive Failure to the First Tyrosine Kinase Inhibitor in Localized GI Stromal Tumors Treated With Imatinib As an Adjuvant: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in Collaboration with the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J. Clin. Oncol. 2015, 33, 4276–4283. [Google Scholar] [CrossRef] [Green Version]
- Lopes, L.F.; Bacchi, C.E. Imatinib treatment for gastrointestinal stromal tumour (GIST). J. Cell Mol. Med. 2010, 14, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Reynoso, D.; Trent, J.C. Neoadjuvant and adjuvant imatinib treatment in gastrointestinal stromal tumor: Current status and recent developments. Curr. Opin. Oncol. 2010, 22, 330–335. [Google Scholar] [CrossRef]
- Eisenberg, B.L.; Trent, J.C. Adjuvant and neoadjuvant imatinib therapy: Current role in the management of gastrointestinal stromal tumors. Int. J. Cancer 2011, 129, 2533–2542. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Q.; Lin, G.; Zhang, B.; Cao, H.; Zhao, Y.; Xia, L.; Feng, F.; Xiong, Z.; Hu, J.; et al. The effect of neoadjuvant imatinib therapy on outcome and survival in rectal gastrointestinal stromal tumors: A multiinstitutional study. J. Surg. Oncol. 2021, 124, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors: Review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch. Pathol. Lab. Med. 2006, 130, 1466–1478. [Google Scholar] [CrossRef] [PubMed]
- Betz, M.; Kopp, H.G.; Spira, D.; Claussen, C.D.; Horger, M. The benefit of using CT-perfusion imaging for reliable response monitoring in patients with gastrointestinal stromal tumor (GIST) undergoing treatment with novel targeted agents. Acta Radiol. 2013, 54, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Cassier, P.A.; Fumagalli, E.; Rutkowski, P.; Schöffski, P.; Van Glabbeke, M.; Debiec-Rychter, M.; Emile, J.F.; Duffaud, F.; Martin-Broto, J.; Landi, B.; et al. Outcome of patients with platelet-derived growth factor receptor alpha-mutated gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Clin. Cancer Res. 2012, 18, 4458–4464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Jüni, P.; Witschi, A.; Bloch, R.; Egger, M. The Hazards of Scoring the Quality of Clinical Trials for Meta-analysis. JAMA 1999, 282, 1054–1060. [Google Scholar] [CrossRef] [Green Version]
- Whiting, P.; Harbord, R.; Kleijnen, J. No role for quality scores in systematic reviews of diagnostic accuracy studies. BMC Med. Res. Methodol. 2005, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Pretze, M.; Reffert, L.; Diehl, S.; Schönberg, S.O.; Wängler, C.; Hohenberger, P.; Wängler, B. GMP-compliant production of [(68)Ga]Ga-NeoB for positron emission tomography imaging of patients with gastrointestinal stromal tumor. EJNMMI Radiopharm. Chem. 2021, 6, 22. [Google Scholar] [CrossRef]
- Hedenström, P.; Andersson, C.; Sjövall, H.; Enlund, F.; Nilsson, O.; Nilsson, B.; Sadik, R. Pretreatment Tumor DNA Sequencing of KIT and PDGFRA in Endosonography-Guided Biopsies Optimizes the Preoperative Management of Gastrointestinal Stromal Tumors. Mol. Diagn. Ther. 2020, 24, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Cai, P.Q.; Lv, X.F.; Tian, L.; Luo, Z.P.; Mitteer, R.A., Jr.; Fan, Y.; Wu, Y.P. CT Characterization of Duodenal Gastrointestinal Stromal Tumors. AJR Am. J. Roentgenol. 2015, 204, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Nannini, M.; Pantaleo, M.A.; Maleddu, A.; Saponara, M.; Mandrioli, A.; Lolli, C.; Pallotti, M.C.; Gatto, L.; Santini, D.; Paterini, P.; et al. Duration of adjuvant treatment following radical resection of metastases from gastrointestinal stromal tumours. Oncol. Lett. 2012, 3, 677–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Balas, H.A.; Shaib, Y.H. Gastrointestinal stromal tumours: Role of computed tomography in predicting tumour behaviour. Hong Kong J. Radiol. 2012, 15, 155–161. [Google Scholar]
- Albano, D.; Bosio, G.; Tomasini, D.; Bonù, M.; Giubbini, R.; Bertagna, F. Metabolic behavior and prognostic role of pretreatment 18F-FDG PET/CT in gist. Asia Pac. J. Clin. Oncol. 2020, 16, e207–e215. [Google Scholar] [CrossRef] [PubMed]
- Cannella, R.; Tabone, E.; Porrello, G.; Cappello, G.; Gozzo, C.; Incorvaia, L.; Grignani, G.; Merlini, A.; D’Ambrosio, L.; Badalamenti, G.; et al. Assessment of morphological CT imaging features for the prediction of risk stratification, mutations, and prognosis of gastrointestinal stromal tumors. Eur. Radiol. 2021, 31, 8554–8564. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xu, L.; Dong, X.; Li, Y.; Yu, J.; Xiong, W.; Li, G. The roles of CT and EUS in the preoperative evaluation of gastric gastrointestinal stromal tumors larger than 2 cm. Eur. Radiol. 2019, 29, 2481–2489. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.S.; Shan, Y.C.; Dong, S.Y.; Wang, W.T.; Yang, Y.T.; Liu, L.H.; Xu, Z.H.; Zeng, M.S.; Rao, S.X. Utility of preoperative computed tomography features in predicting the Ki-67 labeling index of gastric gastrointestinal stromal tumors. Eur. J. Radiol. 2021, 142, 109840. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, J.; Sun, J.; Wang, P. Gastric gastrointestinal stromal tumours (2–5 cm): Correlation of CT features with malignancy and differential diagnosis. Eur. J. Radiol. 2020, 123, 108783. [Google Scholar] [CrossRef]
- Cho, M.H.; Park, C.K.; Park, M.; Kim, W.K.; Cho, A.; Kim, H. Clinicopathologic Features and Molecular Characteristics of Glucose Metabolism Contributing to ¹⁸F-fluorodeoxyglucose Uptake in Gastrointestinal Stromal Tumors. PLoS ONE 2015, 10, e0141413. [Google Scholar] [CrossRef]
- Choi, I.Y.; Yeom, S.K.; Cha, J.; Cha, S.H.; Lee, S.H.; Chung, H.H.; Lee, C.M.; Choi, J. Feasibility of using computed tomography texture analysis parameters as imaging biomarkers for predicting risk grade of gastrointestinal stromal tumors: Comparison with visual inspection. Abdom. Radiol. 2019, 44, 2346–2356. [Google Scholar] [CrossRef]
- Ekert, K.; Hinterleitner, C.; Horger, M. Prognosis assessment in metastatic gastrointestinal stromal tumors treated with tyrosine kinase inhibitors based on CT-texture analysis. Eur. J. Radiol. 2019, 116, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Fuster, D.; Ayuso, J.R.; Poveda, A.; Cubedo, R.; Casado, A.; Martinez-Trufero, J.; Lopez-Pousa, A.; Del Muro, X.G.; Lomena, F.; Maurel, J.; et al. Value of FDG-PET for monitoring treatment response in patients with advanced GIST refractory to high-dose imatinib. A multicenter GEIS study. Q. J. Nucl. Med. Mol. Imaging 2011, 55, 680–687. [Google Scholar]
- Grazzini, G.; Guerri, S.; Cozzi, D.; Danti, G.; Gasperoni, S.; Pradella, S.; Miele, V. Gastrointestinal stromal tumors: Relationship between preoperative CT features and pathologic risk stratification. Tumori 2021, 107, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Jung, M.; Jeong, Y.H.; Jo, K.; Kim, S.; Wang, J.; Cho, A. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative (18)F-FDG PET/CT in patients with localized primary gastrointestinal stromal tumors. Cancer Metab. 2021, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Iannicelli, E.; Carbonetti, F.; Federici, G.F.; Martini, I.; Caterino, S.; Pilozzi, E.; Panzuto, F.; Briani, C.; David, V. Evaluation of the Relationships between Computed Tomography Features, Pathological Findings, and Prognostic Risk Assessment in Gastrointestinal Stromal Tumors. J. Comput. Assist. Tomogr. 2017, 41, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Lee, S.M.; Kim, Y.C.; Byun, J.; Park, J.Y.; Oh, B.Y.; Kwon, M.J.; Kim, J. Gastrointestinal stromal tumours: Preoperative imaging features to predict recurrence after curative resection. Eur. J. Radiol. 2022, 149, 110193. [Google Scholar] [CrossRef]
- Kim, H.C.; Lee, J.M.; Kim, K.W.; Park, S.H.; Kim, S.H.; Lee, J.Y.; Han, J.K.; Choi, B.I. Gastrointestinal stromal tumors of the stomach: CT findings and prediction of malignancy. AJR Am. J. Roentgenol. 2004, 183, 893–898. [Google Scholar] [CrossRef]
- Kamiyama, Y.; Aihara, R.; Nakabayashi, T.; Mochiki, E.; Asao, T.; Kuwano, H.; Oriuchi, N.; Endo, K. 18F-fluorodeoxyglucose positron emission tomography: Useful technique for predicting malignant potential of gastrointestinal stromal tumors. World J. Surg. 2005, 29, 1429–1435. [Google Scholar] [CrossRef]
- Kim, H.C.; Lee, J.M.; Kim, S.H.; Park, S.H.; Lee, J.W.; Lee, M.; Han, J.K.; Choi, B.I. Small gastrointestinal stromal tumours with focal areas of low attenuation on CT: Pathological correlation. Clin. Radiol. 2005, 60, 384–388. [Google Scholar] [CrossRef]
- Kurata, Y.; Hayano, K.; Ohira, G.; Narushima, K.; Aoyagi, T.; Matsubara, H. Fractal analysis of contrast-enhanced CT images for preoperative prediction of malignant potential of gastrointestinal stromal tumor. Abdom. Radiol. 2018, 43, 2659–2664. [Google Scholar] [CrossRef]
- Kwon, Y.; Park, E.; Pahk, K.; Kim, S.; Kim, M.J.; Graf, D.; Park, S. Preoperative assessment of malignant potential of gastrointestinal stromal tumor by dual-time-point 18F-fluorodeoxyglucose positron emission tomography imaging: Usefulness of standardized uptake value and retention index. J. Cancer Res. Ther. 2019, 15, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ren, G.; Cai, R.; Chen, J.; Wu, X.; Zhao, J. A correlation research of Ki67 index, CT features, and risk stratification in gastrointestinal stromal tumor. Cancer Med. 2018, 7, 4467–4474. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Fu, W.; Huang, L.; Chen, Y.; Xiang, P.; Guan, J.; Sun, C. A CT-based nomogram for predicting the malignant potential of primary gastric gastrointestinal stromal tumors preoperatively. Abdom. Radiol. 2021, 46, 3075–3085. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Pan, X.; Liu, R.; Zheng, H.; Chen, L.; Guan, W.; Wang, H.; Sun, Y.; Tang, L.; Guan, Y.; et al. Texture analysis of CT images in predicting malignancy risk of gastrointestinal stromal tumours. Clin. Radiol. 2018, 73, 266–274. [Google Scholar] [CrossRef]
- Mazzei, M.A.; Cioffi Squitieri, N.; Vindigni, C.; Guerrini, S.; Gentili, F.; Sadotti, G.; Mercuri, P.; Righi, L.; Lucii, G.; Mazzei, F.G.; et al. Gastrointestinal stromal tumors (GIST): A proposal of a “CT-based predictive model of Miettinen index” in predicting the risk of malignancy. Abdom. Radiol. 2020, 45, 2989–2996. [Google Scholar] [CrossRef]
- Miyake, K.K.; Nakamoto, Y.; Mikami, Y.; Tanaka, S.; Higashi, T.; Tadamura, E.; Saga, T.; Minami, S.; Togashi, K. The predictive value of preoperative (18)F-fluorodeoxyglucose PET for postoperative recurrence in patients with localized primary gastrointestinal stromal tumour. Eur. Radiol. 2016, 26, 4664–4674. [Google Scholar] [CrossRef]
- O’Neill, A.C.; Shinagare, A.B.; Kurra, V.; Tirumani, S.H.; Jagannathan, J.P.; Baheti, A.D.; Hornick, J.L.; George, S.; Ramaiya, N.H. Assessment of metastatic risk of gastric GIST based on treatment-naïve CT features. Eur. J. Surg. Oncol. 2016, 42, 1222–1228. [Google Scholar] [CrossRef]
- Pelandre, G.L.; Djahjah, M.C.; Gasparetto, E.L.; Nacif, M.S.; Marchiori, E.; De Mello, E.L.R. To mographic findings of gastric gastrointestinal stromal tumor and correlation with the mitotic index. Arq. Gastroenterol. 2013, 50, 244–250. [Google Scholar] [CrossRef] [Green Version]
- Palatresi, D.; Fedeli, F.; Danti, G.; Pasqualini, E.; Castiglione, F.; Messerini, L.; Massi, D.; Bettarini, S.; Tortoli, P.; Busoni, S.; et al. Correlation of CT radiomic features for GISTs with pathological classification and molecular subtypes: Preliminary and monocentric experience. Radiol. Med. 2022, 127, 117–128. [Google Scholar] [CrossRef]
- Peng, G.; Huang, B.; Yang, X.; Pang, M.; Li, N. Preoperative CT feature of incomplete overlying enhancing mucosa as a high-risk predictor in gastrointestinal stromal tumors of the stomach. Eur. Radiol. 2021, 31, 3276–3285. [Google Scholar] [CrossRef]
- Pinaikul, S.; Woodtichartpreecha, P.; Kanngurn, S.; Leelakiatpaiboon, S. 1189 Gastrointestinal stromal tumor (GIST): Computed tomographic features and correlation of CT findings with histologic grade. J. Med. Assoc. Thai. 2014, 97, 1189–1198. [Google Scholar] [PubMed]
- Tang, B.; Feng, Q.X.; Liu, X.S. Comparison of Computed Tomography Features of Gastric and Small Bowel Gastrointestinal Stromal Tumors with Different Risk Grades. J. Comput. Assist. Tomogr. 2022, 46, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, U.; Hasegawa, T.; Satake, M.; Moriyama, N. Gastrointestinal stromal tumor. Correlation of computed tomography findings with tumor grade and mortality. J. Comput. Assist. Tomogr. 2003, 27, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Tokumoto, N.; Tanabe, K.; Misumi, T.; Fujikuni, N.; Suzuki, T.; Ohdan, H. The usefulness of preoperative 18FDG positron-emission tomography and computed tomography for predicting the malignant potential of gastrointestinal stromal tumors. Dig. Surg. 2014, 31, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ulusan, S.; Koc, Z.; Kayaselcuk, F. Gastrointestinal stromal tumours: CT findings. Br. J. Radiol. 2008, 81, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Verde, F.; Hruban, R.H.; Fishman, E.K. Small Bowel Gastrointestinal Stromal Tumors: Multidetector Computed Tomography Enhancement Pattern and Risk of Progression. J. Comput. Assist. Tomogr. 2017, 41, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C.; Xu, L.; Li, W.H.; Li, Y.; Guo, S.F.; Sun, X.R.; Li, W.W. Risk stratification in GIST: Shape quantification with CT is a predictive factor. Eur. Radiol. 2020, 30, 1856–1865. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Si, G.Y.; He, Q.Z. Correlation analysis of multi-slice computed tomography (MSCT) findings, clinicopathological factors, and prognosis of gastric gastrointestinal stromal tumors. Transl. Cancer Res. 2020, 9, 1787–1794. [Google Scholar] [CrossRef]
- Yang, T.H.; Hwang, J.I.; Yang, M.S.; Hung, S.W.; Chan, S.W.; Wang, J.; Tyan, Y.S. Gastrointestinal stromal tumors: Computed tomographic features and prediction of malignant risk from computed tomographic imaging. J. Chin. Med. Assoc. 2007, 70, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.W.; Liu, X.J.; Zhao, L.; Che, F.; Yin, Y.; Chen, H.J.; Zhang, B.; Wu, M.; Song, B. Preoperative prediction of gastrointestinal stromal tumors with high Ki-67 proliferation index based on CT features. Ann. Transl. Med. 2021, 9, 1556. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Shimada, M.; Kurita, N.; Sato, H.; Iwata, T.; Morimoto, S.; Miyatani, T.; Kashihara, H.; Takasu, C.; Matsumoto, N. Efficacy of PET-CT for predicting the malignant potential of gastrointestinal stromal tumors. Surg. Today 2013, 43, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Duan, X.; Zhang, X.; Hu, H.; Wang, D.; Shen, J. Predictive features of CT for risk stratifications in patients with primary gastrointestinal stromal tumour. Eur. Radiol. 2016, 26, 3086–3093. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.P.; Ding, Q.L.; Xu, J.X.; Jiang, C.Y.; Wang, J.; Wang, C.; Yu, R.S. Building contrast-enhanced CT-based models for preoperatively predicting malignant potential and Ki67 expression of small intestine gastrointestinal stromal tumors (GISTs). Abdom. Radiol. 2022, 47, 3161–3173. [Google Scholar] [CrossRef] [PubMed]
- Otomi, Y.; Otsuka, H.; Morita, N.; Terazawa, K.; Furutani, K.; Harada, M.; Nishitani, H. Relationship between FDG uptake and the pathological risk category in gastrointestinal stromal tumors. J. Med. Investig. 2010, 57, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-W.; Cho, C.-H.; Jeong, D.-S.; Chae, H.-D. Role of F-fluoro-2-deoxyglucose Positron Emission Tomography in Gastric GIST: Predicting Malignant Potential Pre-operatively. J. Gastric Cancer 2011, 11, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.Q.; Liu, C.J.; Zhang, B.; Wen, Y.; Yin, Y. Association between CT imaging features and KIT mutations in small intestinal gastrointestinal stromal tumors. Sci. Rep. 2019, 9, 7257. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.K. Predictive value and modeling analysis of MSCT signs in gastrointestinal stromal tumors (GISTs) to pathological risk degree. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 999–1005. [Google Scholar]
- Ao, W.; Cheng, G.; Lin, B.; Yang, R.; Liu, X.; Zhou, C.; Wang, W.; Fang, Z.; Tian, F.; Yang, G.; et al. A novel CT-based radiomic nomogram for predicting the recurrence and metastasis of gastric stromal tumors. Am. J. Cancer Res. 2021, 11, 3123–3134. [Google Scholar]
- Chen, T.; Liu, S.; Li, Y.; Feng, X.; Xiong, W.; Zhao, X.; Yang, Y.; Zhang, C.; Hu, Y.; Chen, H.; et al. Developed and validated a prognostic nomogram for recurrence-free survival after complete surgical resection of local primary gastrointestinal stromal tumors based on deep learning. EBioMedicine 2019, 39, 272–279. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Ning, Z.; Xu, L.; Feng, X.; Han, S.; Roth, H.R.; Xiong, W.; Zhao, X.; Hu, Y.; Liu, H.; et al. Radiomics nomogram for predicting the malignant potential of gastrointestinal stromal tumours preoperatively. Eur. Radiol. 2019, 29, 1074–1082. [Google Scholar] [CrossRef]
- Chen, Z.H.; Xu, L.Y.; Zhang, C.M.; Huang, C.C.; Wang, M.H.; Feng, Z.; Xiong, Y. CT Radiomics Model for Discriminating the Risk Stratification of Gastrointestinal Stromal Tumors: A Multi-Class Classification and Multi-Center Study. Front. Oncol. 2021, 11, 654114. [Google Scholar] [PubMed]
- Chu, H.; Pang, P.; He, J.; Zhang, D.; Zhang, M.; Qiu, Y.; Li, X.; Lei, P.; Fan, B.; Xu, R. Value of radiomics model based on enhanced computed tomography in risk grade prediction of gastrointestinal stromal tumors. Sci. Rep. 2021, 11, 12009. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Yuan, X.; Wang, H.; Qin, S.; Song, X.; Yu, X.; Zhang, S.; Sun, C.; Zhou, Q.; Wei, Y.; et al. Preoperative CT-Based Deep Learning Model for Predicting Risk Stratification in Patients With Gastrointestinal Stromal Tumors. Front. Oncol. 2021, 11, 750875. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, H.; Zhang, L.; Song, Y.; Yang, S.; Zheng, Z.; Zhao, J.; Hou, F.; Zhang, J. Value of contrast-enhanced CT based radiomic machine learning algorithm in differentiating gastrointestinal stromal tumors with KIT exon 11 mutation: A two-center study. Diagn. Interv. Radiol. 2022, 28, 29–38. [Google Scholar] [CrossRef]
- Liu, X.; Yin, Y.; Wang, X.; Yang, C.; Wan, S.; Yin, X.; Wu, T.; Chen, H.; Xu, Z.; Li, X.; et al. Gastrointestinal stromal tumors: Associations between contrast-enhanced CT images and KIT exon 11 gene mutation. Ann. Transl. Med. 2021, 9, 1496. [Google Scholar] [CrossRef]
- Feng, Q.; Tang, B.; Zhang, Y.; Liu, X. Prediction of the Ki-67 expression level and prognosis of gastrointestinal stromal tumors based on CT radiomics nomogram. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 1167–1175. [Google Scholar] [CrossRef]
- Shao, M.; Niu, Z.; He, L.; Fang, Z.; He, J.; Xie, Z.; Cheng, G.; Wang, J. Building Radiomics Models Based on Triple-Phase CT Images Combining Clinical Features for Discriminating the Risk Rating in Gastrointestinal Stromal Tumors. Front. Oncol. 2021, 11, 737302. [Google Scholar] [CrossRef]
- Ren, C.; Wang, S.; Zhang, S. Development and validation of a nomogram based on CT images and 3D texture analysis for preoperative prediction of the malignant potential in gastrointestinal stromal tumors. Cancer Imaging 2020, 20, 5. [Google Scholar] [CrossRef] [Green Version]
- Ren, C.; Wang, S.; Zhang, S.; Jiang, Z. Value of CT-Based Texture Analysis in Preoperative Prediction of the Grade of Gastrointestinal Stromal Tumors Compared to Conventional CT Imaging. Iran. J. Radiol. 2019, 16, e85703. [Google Scholar] [CrossRef] [Green Version]
- Starmans, M.P.A.; Timbergen, M.J.M.; Vos, M.; Renckens, M.; Grünhagen, D.J.; van Leenders, G.; Dwarkasing, R.S.; Willemssen, F.; Niessen, W.J.; Verhoef, C.; et al. Differential Diagnosis and Molecular Stratification of Gastrointestinal Stromal Tumors on CT Images Using a Radiomics Approach. J. Digit. Imaging 2022, 35, 127–136. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Jiaerken, Y.; Huang, P.; Sun, L.; Dong, F.; Huang, Y.; Dong, D.; Tian, J.; Zhang, M. Building CT Radiomics-Based Models for Preoperatively Predicting Malignant Potential and Mitotic Count of Gastrointestinal Stromal Tumors. Transl. Oncol. 2019, 12, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Feng, Z.; Zhou, L.; Zhang, L.; Hao, X.; Zhai, J. Computed-Tomography-Based Radiomics Model for Predicting the Malignant Potential of Gastrointestinal Stromal Tumors Preoperatively: A Multi-Classifier and Multicenter Study. Front. Oncol. 2021, 11, 582847. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Ma, X.; Wang, Y.; Tian, Y.; Tang, W.; Wang, M.; Wei, R.; Zhao, X. CT texture analysis can be a potential tool to differentiate gastrointestinal stromal tumors without KIT exon 11 mutation. Eur. J. Radiol. 2018, 107, 90–97. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, J.; Wang, X.; Fan, S.; Huang, X.; Xie, X.; Yu, R. A multi-class scoring system based on CT features for preoperative prediction in gastric gastrointestinal stromal tumors. Am. J. Cancer Res. 2020, 10, 3867–3881. [Google Scholar] [PubMed]
- Zhang, L.; Kang, L.; Li, G.; Zhang, X.; Ren, J.; Shi, Z.; Li, J.; Yu, S. Computed tomography-based radiomics model for discriminating the risk stratification of gastrointestinal stromal tumors. Radiol. Med. 2020, 125, 465–473. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Zhou, X.X.; Zhang, R.Y.; Chen, S.L.; Liu, Q.; Wang, J.; Zhang, Y.; Lin, J.; Xu, J.R.; Gao, Y.J.; et al. Comparison of malignancy-prediction efficiency between contrast and non-contract CT-based radiomics features in gastrointestinal stromal tumors: A multicenter study. Clin. Transl. Med. 2020, 10, e291. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Gao, Y.J.; Zhang, R.Y.; Zhou, X.X.; Chen, S.L.; Zhang, Y.; Liu, Q.; Xu, J.R.; Ge, Z.Z. Personalized CT-based radiomics nomogram preoperative predicting Ki-67 expression in gastrointestinal stromal tumors: A multicenter development and validation cohort. Clin. Transl. Med. 2020, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Feng, M.; Wang, M.; Zhang, L.; Li, M.; Huang, C. CT Radiomics for the Preoperative Prediction of Ki67 Index in Gastrointestinal Stromal Tumors: A Multi-Center Study. Front. Oncol. 2021, 11, 689136. [Google Scholar] [CrossRef]
- Zheng, J.; Xia, Y.; Xu, A.; Weng, X.; Wang, X.; Jiang, H.; Li, Q.; Li, F. Combined model based on enhanced CT texture features in liver metastasis prediction of high-risk gastrointestinal stromal tumors. Abdom. Radiol. 2022, 47, 85–93. [Google Scholar] [CrossRef]
- Antoch, G.; Kanja, J.; Bauer, S.; Kuehl, H.; Renzing-Koehler, K.; Schuette, J.; Bockisch, A.; Debatin, J.F.; Freudenberg, L.S. Comparison of PET, CT, and dual-modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. J. Nucl. Med. 2004, 45, 357–365. [Google Scholar]
- Beheshti, M.; Li, S.R.; Vali, R.; Schima, W.; Dudczak, R.; Langsteger, W. The Potential Value of F-18 FDG PET in Comparison to CT in Early Prediction of Response to Imatinib (STI571) Therapy in Patients with Gastrointestinal Stromal Tumors. Iran. J. Nucl. Med. 2007, 15, 34–42. [Google Scholar]
- Chacón, M.; Eleta, M.; Espindola, A.R.; Roca, E.; Méndez, G.; Rojo, S.; Pupareli, C. Assessment of early response to imatinib 800 mg after 400 mg progression by ¹⁸F-fluorodeoxyglucose PET in patients with metastatic gastrointestinal stromal tumors. Future Oncol. 2015, 11, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Charnsangavej, C.; de Castro Faria, S.; Tamm, E.P.; Benjamin, R.S.; Johnson, M.M.; Macapinlac, H.A.; Podoloff, D.A. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: A quantitative analysis correlated with FDG PET findings. AJR Am. J. Roentgenol. 2004, 183, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Charnsangavej, C.; Faria, S.C.; Macapinlac, H.A.; Burgess, M.A.; Patel, S.R.; Chen, L.L.; Podoloff, D.A.; Benjamin, R.S. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J. Clin. Oncol. 2007, 25, 1753–1759. [Google Scholar] [CrossRef]
- Dudeck, O.; Zeile, M.; Reichardt, P.; Pink, D. Comparison of RECIST and Choi criteria for computed tomographic response evaluation in patients with advanced gastrointestinal stromal tumor treated with sunitinib. Ann. Oncol. 2011, 22, 1828–1833. [Google Scholar] [CrossRef]
- Gayed, I.; Vu, T.; Iyer, R.; Johnson, M.; Macapinlac, H.; Swanston, N.; Podoloff, D. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J. Nucl. Med. 2004, 45, 17–21. [Google Scholar]
- Goerres, G.W.; Stupp, R.; Barghouth, G.; Hany, T.F.; Pestalozzi, B.; Dizendorf, E.; Schnyder, P.; Luthi, F.; von Schulthess, G.K.; Leyvraz, S. The value of PET, CT and in-line PET/CT in patients with gastrointestinal stromal tumours: Long-term outcome of treatment with imatinib mesylate. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Holdsworth, C.H.; Badawi, R.D.; Manola, J.B.; Kijewski, M.F.; Israel, D.A.; Demetri, G.D.; Van den Abbeele, A.D. CT and PET: Early prognostic indicators of response to imatinib mesylate in patients with gastrointestinal stromal tumor. AJR Am. J. Roentgenol. 2007, 189, W324–W330. [Google Scholar] [CrossRef]
- Jager, P.L.; Gietema, J.A.; van der Graaf, W.T. Imatinib mesylate for the treatment of gastrointestinal stromal tumours: Best monitored with FDG PET. Nucl. Med. Commun. 2004, 25, 433–438. [Google Scholar] [CrossRef]
- Phongkitkarun, S.; Phaisanphrukkun, C.; Jatchavala, J.; Sirachainan, E. Assessment of gastrointestinal stromal tumors with computed tomography following treatment with imatinib mesylate. World J. Gastroenterol. 2008, 14, 892–898. [Google Scholar] [CrossRef] [Green Version]
- Prior, J.O.; Montemurro, M.; Orcurto, M.V.; Michielin, O.; Luthi, F.; Benhattar, J.; Guillou, L.; Elsig, V.; Stupp, R.; Delaloye, A.B.; et al. Early prediction of response to sunitinib after imatinib failure by 18F-fluorodeoxyglucose positron emission tomography in patients with gastrointestinal stromal tumor. J. Clin. Oncol. 2009, 27, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.H.; Lee, J.L.; Chang, H.M.; Kim, T.W.; Kang, H.J.; Sohn, H.J.; Lee, J.S.; Kang, Y.K. Patterns of progression in gastrointestinal stromal tumor treated with imatinib mesylate. Jpn. J. Clin. Oncol. 2006, 36, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindler, E.; Amantea, M.A.; Karlsson, M.O.; Friberg, L.E. PK-PD modeling of individual lesion FDG-PET response to predict overall survival in patients with sunitinib-treated gastrointestinal stromal tumor. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Schramm, N.; Englhart, E.; Schlemmer, M.; Hittinger, M.; Übleis, C.; Becker, C.R.; Reiser, M.F.; Berger, F. Tumor response and clinical outcome in metastatic gastrointestinal stromal tumors under sunitinib therapy: Comparison of RECIST, Choi and volumetric criteria. Eur. J. Radiol. 2013, 82, 951–958. [Google Scholar] [CrossRef]
- Shinagare, A.B.; Barysauskas, C.M.; Braschi-Amirfarzan, M.; O’Neill, A.C.; Catalano, P.J.; George, S.; Ramaiya, N.H. Comparison of performance of various tumor response criteria in assessment of sunitinib activity in advanced gastrointestinal stromal tumors. Clin. Imaging 2016, 40, 880–884. [Google Scholar] [CrossRef]
- Van den Abbeele, A.D.; Gatsonis, C.; de Vries, D.J.; Melenevsky, Y.; Szot-Barnes, A.; Yap, J.T.; Godwin, A.K.; Rink, L.; Huang, M.; Blevins, M.; et al. ACRIN 6665/RTOG 0132 phase II trial of neoadjuvant imatinib mesylate for operable malignant gastrointestinal stromal tumor: Monitoring with 18F-FDG PET and correlation with genotype and GLUT4 expression. J. Nucl. Med. 2012, 53, 567–574. [Google Scholar] [CrossRef]
- Stroobants, S.; Goeminne, J.; Seegers, M.; Dimitrijevic, S.; Dupont, P.; Nuyts, J.; Martens, M.; van den Borne, B.; Cole, P.; Sciot, R.; et al. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec). Eur. J. Cancer 2003, 39, 2012–2020. [Google Scholar] [CrossRef]
- Schiavon, G.; Ruggiero, A.; Bekers, D.J.; Barry, P.A.; Sleijfer, S.; Kloth, J.; Krestin, G.P.; Schöffski, P.; Verweij, J.; Mathijssen, R.H. The effect of baseline morphology and its change during treatment on the accuracy of Response Evaluation Criteria in Solid Tumours in assessment of liver metastases. Eur. J. Cancer 2014, 50, 972–980. [Google Scholar] [CrossRef]
- Schiavon, G.; Ruggiero, A.; Schöffski, P.; van der Holt, B.; Bekers, D.J.; Eechoute, K.; Vandecaveye, V.; Krestin, G.P.; Verweij, J.; Sleijfer, S.; et al. Tumor volume as an alternative response measurement for imatinib treated GIST patients. PLoS ONE 2012, 7, e48372. [Google Scholar] [CrossRef] [Green Version]
- Farag, S.; Geus-Oei, L.F.; van der Graaf, W.T.; van Coevorden, F.; Grunhagen, D.; Reyners, A.K.L.; Boonstra, P.A.; Desar, I.; Gelderblom, H.; Steeghs, N. Early Evaluation of Response Using (18)F-FDG PET Influences Management in Gastrointestinal Stromal Tumor Patients Treated with Neoadjuvant Imatinib. J. Nucl. Med. 2018, 59, 194–196. [Google Scholar] [CrossRef] [Green Version]
- Farag, S.; NS, I.J.; Houdijk, M.P.M.; Reyners, A.K.L.; Arens, A.I.; Grünhagen, D.J.; Desar, I.M.E.; Gelderblom, H.; Steeghs, N.; de Geus-Oei, L.F. Early response evaluation using 18F-FDG-PET/CT does not influence management of patients with metastatic gastrointestinal stromal tumors (GIST) treated with palliative intent. Nuklearmedizin 2021, 60, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.K.; Chow, P.K.; Chuah, K.L.; Yap, W.M.; Wong, W.K. Pathologic, radiologic and PET scan response of gastrointestinal stromal tumors after neoadjuvant treatment with imatinib mesylate. Eur. J. Surg. Oncol. 2006, 32, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Arshad, J.; Ahmed, J.; Subhawong, T.; Trent, J.C. Progress in determining response to treatment in gastrointestinal stromal tumor. Expert Rev. Anticancer Ther. 2020, 20, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Ollivier, L. The RECIST (Response Evaluation Criteria in Solid Tumors) criteria: Implications for diagnostic radiologists. Br. J. Radiol. 2001, 74, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Faivre, S.; Demetri, G.; Sargent, W.; Raymond, E. Molecular basis for sunitinib efficacy and future clinical development. Nat. Rev. Drug Discov. 2007, 6, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Nakatani, H.; Taguchi, T.; Nakano, T.; Okabayashi, T.; Sugimoto, T.; Kobayashi, M.; Araki, K. STI571 (Glivec) suppresses the expression of vascular endothelial growth factor in the gastrointestinal stromal tumor cell line, GIST-T1. World J. Gastroenterol. 2006, 12, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Young, H.; Baum, R.; Cremerius, U.; Herholz, K.; Hoekstra, O.; Lammertsma, A.A.; Pruim, J.; Price, P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur. J. Cancer 1999, 35, 1773–1782. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weeda, Y.A.; Kalisvaart, G.M.; van Velden, F.H.P.; Gelderblom, H.; van der Molen, A.J.; Bovee, J.V.M.G.; van der Hage, J.A.; Grootjans, W.; de Geus-Oei, L.-F. Early Prediction and Monitoring of Treatment Response in Gastrointestinal Stromal Tumors by Means of Imaging: A Systematic Review. Diagnostics 2022, 12, 2722. https://doi.org/10.3390/diagnostics12112722
Weeda YA, Kalisvaart GM, van Velden FHP, Gelderblom H, van der Molen AJ, Bovee JVMG, van der Hage JA, Grootjans W, de Geus-Oei L-F. Early Prediction and Monitoring of Treatment Response in Gastrointestinal Stromal Tumors by Means of Imaging: A Systematic Review. Diagnostics. 2022; 12(11):2722. https://doi.org/10.3390/diagnostics12112722
Chicago/Turabian StyleWeeda, Ylva. A., Gijsbert M. Kalisvaart, Floris H. P. van Velden, Hans Gelderblom, Aart. J. van der Molen, Judith V. M. G. Bovee, Jos A. van der Hage, Willem Grootjans, and Lioe-Fee de Geus-Oei. 2022. "Early Prediction and Monitoring of Treatment Response in Gastrointestinal Stromal Tumors by Means of Imaging: A Systematic Review" Diagnostics 12, no. 11: 2722. https://doi.org/10.3390/diagnostics12112722
APA StyleWeeda, Y. A., Kalisvaart, G. M., van Velden, F. H. P., Gelderblom, H., van der Molen, A. J., Bovee, J. V. M. G., van der Hage, J. A., Grootjans, W., & de Geus-Oei, L.-F. (2022). Early Prediction and Monitoring of Treatment Response in Gastrointestinal Stromal Tumors by Means of Imaging: A Systematic Review. Diagnostics, 12(11), 2722. https://doi.org/10.3390/diagnostics12112722










