Vitamin D Status and Potential Therapeutic Options in Critically Ill Patients: A Narrative Review of the Clinical Evidence
Abstract
1. Introduction
2. Physiology of Vitamin D
2.1. Anabolic and Catabolic Pathways
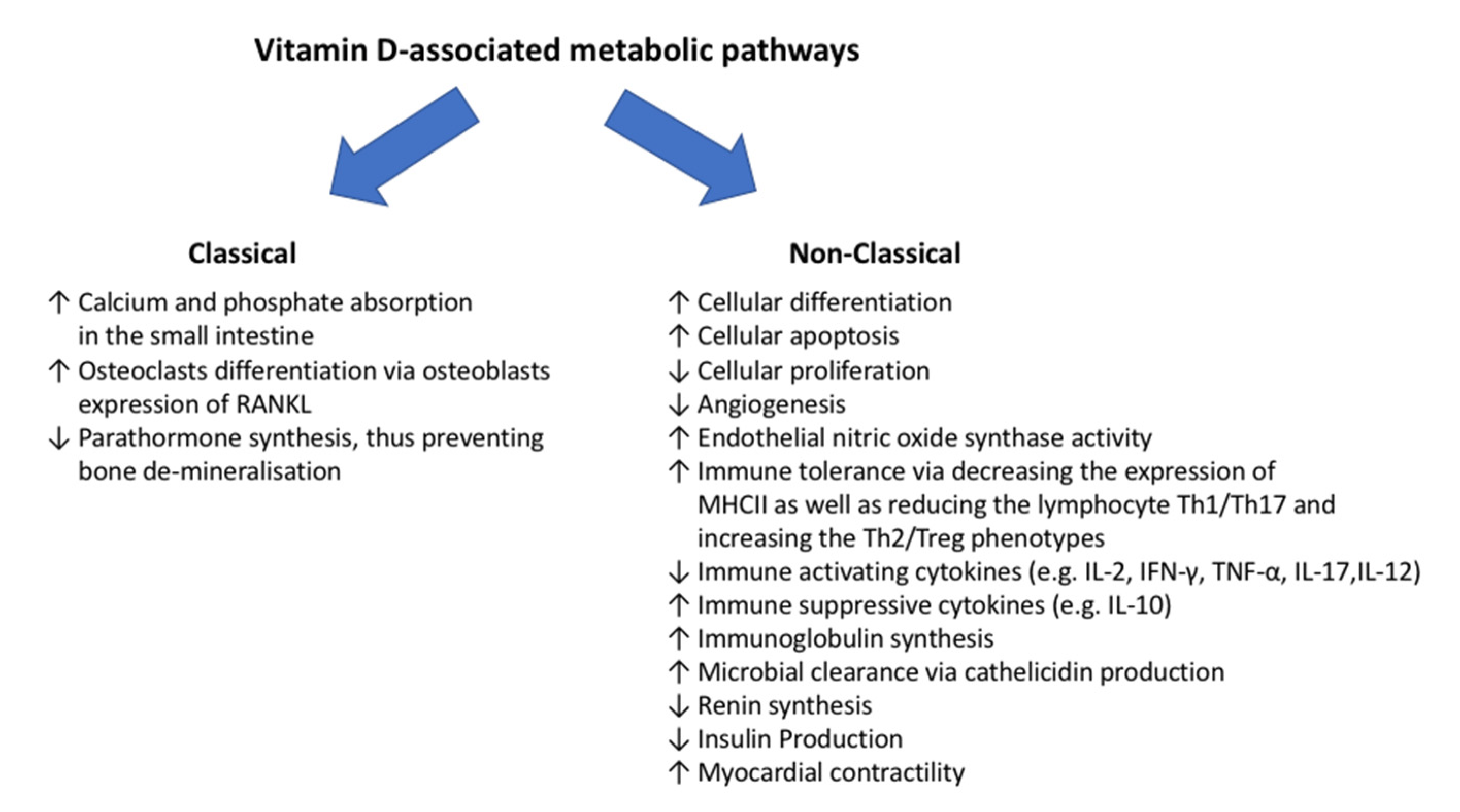
2.2. Vitamin-D-Associated Metabolic Pathways
3. Epidemiology of Vitamin D Status Alterations
3.1. Grading
3.2. Epidemiology
3.3. Settings
3.4. Associated Diseases
4. Therapeutic Options of Vitamin D Supplementation
4.1. Routes of Vitamin D Supplementation
4.2. Isoforms of Vitamin D Compounds
5. Vitamin D Supplementation in Critically Ill Patients
5.1. General Population
5.2. Acute Liver Dysfunction
5.3. Acute Kidney Injury
5.4. Acute Respiratory Failure
5.5. Sepsis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holick, M. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C. Clinical practice. Vitamin d insufficiency. N. Engl. J. Med. 2011, 364, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.; Dowling, K.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; Henauw, S.D.; Moreno, L.; Damsgaard, C.; Michaelsen, K.; Mølgaard, C.; et al. Vitamin D deficiency in europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Herrick, K.; Storandt, R.; Afful, J.; Pfeiffer, C.; Schleicher, R.; Gahche, J.; Potischman, N. Vitamin D status in the United States, 2011–2014. Am. J. Clin. Nutr. 2019, 110, 150–157. [Google Scholar] [CrossRef]
- Amrein, K.; Papinutti, A.; Mathew, E.; Vila, G.; Parekh, D. Vitamin D and critical illness: What endocrinology can learn from intensive care and vice versa. Endocr. Connect 2018, 7, R304–R315. [Google Scholar] [CrossRef] [PubMed]
- de Haan, K.; Groeneveld, A.; de Geus, H.; Egal, M.; Struijs, A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: Systematic review and meta-analysis. Crit. Care 2014, 18, 660. [Google Scholar] [CrossRef]
- McNally, J.; Nama, N.; O’Hearn, K.; Sampson, M.; Amrein, K.; Iliriani, K.; McIntyre, L.; Fergusson, D.; Menon, K. Vitamin D deficiency in critically ill children: A systematic review and meta-analysis. Crit. Care 2017, 21, 287. [Google Scholar] [CrossRef]
- Parekh, D.; Patel, J.; Scott, A.; Lax, S.; Dancer, R.; D’Souza, V.; Greenwood, H.; Fraser, W.; Gao, F.; Sapey, E.; et al. Vitamin D deficiency in human and murine sepsis. Crit. Care Med. 2017, 45, 282–289. [Google Scholar] [CrossRef]
- De Pascale, G.; Vallecoccia, M.; Schiattarella, A.; Di Gravio, V.; Cutuli, S.; Bello, G.; Montini, L.; Pennisi, M.; Spanu, T.; Zuppi, C.; et al. Clinical and microbiological outcome in septic patients with extremely low 25-hydroxyvitamin D levels at initiation of critical care. Clin. Microbiol. Infect. 2016, 22, 456.e7–456.e13. [Google Scholar] [CrossRef]
- Quraishi, S.; De Pascale, G.; Needleman, J.; Nakazawa, H.; Kaneki, M.; Bajwa, E.; Jr, C.C.; Bhan, I. Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in sepsis: A randomized, placebo-controlled trial. Crit. Care Med. 2015, 43, 1928–1937. [Google Scholar] [CrossRef]
- Amrein, K.; Sourij, H.; Wagner, G.; Holl, A.; Pieber, T.; Smolle, K.; Stojakovic, T.; Schnedl, C.; Dobnig, H. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin d deficient patients: A randomized, double-blind, placebo-controlled pilot study. Crit. Care 2011, 15, R104. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Schnedl, C.; Holl, A.; Riedl, R.; Christopher, K.; Pachler, C.; Purkart, T.U.; Waltensdorfer, A.; Münch, A.; Warnkross, H.; et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin d deficiency: The vitdal-icu randomized clinical trial. JAMA 2014, 312, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute PETAL Clinical Trials Network; Ginde, A.A.; Brower, R.; Caterino, J.; Finck, L.; Banner-Goodspeed, V.; Grissom, C.; Hayden, D.; Hough, C.; Hyzy, R.; et al. Early high-dose vitamin D 3 for critically ill, vitamin D-deficient patients. N. Engl. J. Med. 2019, 381, 2529–2540. [Google Scholar] [PubMed]
- Leaf, D.; Raed, A.; Donnino, M.; Ginde, A.; Waikar, S. Randomized controlled trial of calcitriol in severe sepsis. Am. J. Respir. Crit. Care Med. 2014, 190, 533–541. [Google Scholar] [CrossRef]
- Murai, I.; Fernandes, A.; Sales, L.; Pinto, A.; Goessler, K.; Duran, C.; Silva, C.; Franco, A.; Macedo, M.; Dalmolin, H.; et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: A randomized clinical trial. JAMA 2021, 325, 1053–1060. [Google Scholar] [CrossRef]
- Demer, L.; Hsu, J.; Tintut, Y. Steroid hormone vitamin D: Implications for cardiovascular disease. Circ. Res. 2018, 122, 1576–1585. [Google Scholar] [CrossRef]
- Komba, S.; Kotake-Nara, E.; Tsuzuki, W. Simultaneous synthesis of vitamins D 2, D 4, D 5, D 6, and D 7 from commercially available phytosterol, β-sitosterol, and identification of each vitamin d by hsqc nmr. Metabolites 2019, 9, 107. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Śliwińska, A. Analysis of association between vitamin D deficiency and insulin resistance. Nutrients 2019, 11, 794. [Google Scholar] [CrossRef]
- De Pascale, G.; Quraishi, S. Vitamin D status in critically ill patients: The evidence is now bioavailable! Crit. Care 2014, 18, 449. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Berndt, T.; Kumar, R. Phosphatonins and the regulation of phosphate homeostasis. Annu. Rev. Physiol. 2007, 69, 341–359. [Google Scholar] [CrossRef]
- Prentice, A.; Goldberg, G.; Schoenmakers, I. Vitamin D across the lifecycle: Physiology and biomarkers. Am. J. Clin. Nutr. 2008, 88, 500S–506S. [Google Scholar] [CrossRef] [PubMed]
- Norman, A. From vitamin D to hormone d: Fundamentals of the vitamin d endocrine system essential for good health. Am. J. Clin. Nutr. 2008, 88, 491S–499S. [Google Scholar] [CrossRef]
- Quraishi, S.; Camargo, C.J. Vitamin D in acute stress and critical illness. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog. Biophys. Mol. Biol. 2006, 92, 39–48. [Google Scholar] [CrossRef]
- Molinari, C.; Uberti, F.; Grossini, E.; Vacca, G.; Carda, S.; Invernizzi, M.; Cisari, C. 1α,25-dihydroxycholecalciferol induces nitric oxide production in cultured endothelial cells. Cell. Physiol. Biochem. 2011, 27, 661–668. [Google Scholar] [CrossRef]
- Li, Y. Vitamin D regulation of the renin-angiotensin system. J. Cell. Biochem. 2003, 88, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.; Chu, A.; Go, V.; Saad, M. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am. J. Clin. Nutr. 2004, 79, 820–825. [Google Scholar] [CrossRef]
- Chandler, P.; Chen, W.; Ajala, O.; Hazra, A.; Cook, N.; Bubes, V.; Lee, I.; Giovannucci, E.; Willett, W.; Buring, J.; et al. Effect of vitamin D3 supplements on development of advanced cancer: A secondary analysis of the vital randomized clinical trial. JAMA Netw Open 2020, 3, e2025850. [Google Scholar] [CrossRef]
- Umar, M.; Sastry, K.; Chouchane, A. Role of vitamin D beyond the skeletal function: A review of the molecular and clinical studies. Int. J. Mol. Sci. 2018, 19, 1618. [Google Scholar] [CrossRef]
- Makris, K.; Sempos, C.; Cavalier, E. The measurement of vitamin D metabolites: Part i-metabolism of vitamin D and the measurement of 25-hydroxyvitamin D. Hormones 2020, 19, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US). Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- El Hajj Fuleihan, G.; Nabulsi, M.; Choucair, M.; Salamoun, M.; Shahine, C.H.; Kizirian, A.; Tannous, R. Hypovitaminosis D in healthy schoolchildren. Pediatrics 2001, 107, E53. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, S.; Di Nisio, A.; Mele, C.; Scappaticcio, L.; Savastano, S.; Colao, A.; Obesity Programs of nutrition, Education, Research and Assessment (OPERA) Group. Obesity and hypovitaminosis D: Causality or casualty? Int. J. Obes. Suppl. 2019, 9, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Zajic, P.; Schnedl, C.; Waltensdorfer, A.; Fruhwald, S.; Holl, A.; Purkart, T.; Wünsch, G.; Valentin, T.; Grisold, A.; et al. Vitamin D status and its association with season, hospital and sepsis mortality in critical illness. Crit. Care 2014, 18, R47. [Google Scholar] [CrossRef]
- Lee, J.; Smith, J.; Philipp, B.; Chen, T.; Mathieu, J.; Holick, M. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin. Pediatr. 2007, 46, 42–44. [Google Scholar] [CrossRef]
- Wagner, C.; Taylor, S.; Dawodu, A.; Johnson, D.; Hollis, B. Vitamin D and its role during pregnancy in attaining optimal health of mother and fetus. Nutrients 2012, 4, 208–230. [Google Scholar] [CrossRef]
- Lips, P.; Hosking, D.; Lippuner, K.; Norquist, J.; Wehren, L.; Maalouf, G.; Ragi-Eis, S.; Chandler, J. The prevalence of vitamin D inadequacy amongst women with osteoporosis: An international epidemiological investigation. J. Intern. Med. 2006, 260, 245–254. [Google Scholar] [CrossRef]
- Lips, P.; Schoor, N.V. The effect of vitamin D on bone and osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 585–591. [Google Scholar] [CrossRef]
- Alagacone, S.; Verga, E.; Verdolini, R.; Saifullah, S. The association between vitamin D deficiency and the risk of resistant hypertension. Clin. Exp. Hypertens 2020, 42, 177–180. [Google Scholar] [CrossRef]
- Han, Y.; Hsu, S.; Su, T. Association between vitamin D deficiency and high serum levels of small dense ldl in middle-aged adults. Biomedicines 2021, 9, 464. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, J.; Song, M. The association between vitamin D deficiency and metabolic syndrome in korean adolescents. J. Pediatr. Nurs. 2018, 38, e7–e11. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, Y.; Wang, Y.; Chen, C.; Lai, C.; Huang, K. Association between vitamin D and latent tuberculosis infection in the United States: Nhanes, 2011-2012. Infect. Drug Resist. 2019, 12, 2251–2257. [Google Scholar] [CrossRef]
- Furuya-Kanamori, L.; Wangdi, K.; Yakob, L.; McKenzie, S.; Doi, S.; Clark, J.; Paterson, D.; Riley, T.; Clements, A. 25-hydroxyvitamin D concentrations and clostridium difficile infection: A meta-analysis. JPEN J. Parenter. Enteral Nutr. 2017, 41, 890–895. [Google Scholar] [CrossRef]
- Oscanoa, T.; Amado, J.; Vidal, X.; Laird, E.; Ghashut, R.; Romero-Ortuno, R. The relationship between the severity and mortality of SARS-CoV-2 infection and 25-hydroxyvitamin D concentration—A metaanalysis. Adv. Respir. Med. 2021, 89, 145–157. [Google Scholar] [CrossRef]
- Kiani, A.; Abedini, A.; Adcock, I.; Mirenayat, M.; Taghavi, K.; Mortaz, E.; Kazempour-Dizaji, M. Association between vitamin D deficiencies in sarcoidosis with disease activity, course of disease and stages of lung involvements. J. Med. Biochem. 2018, 37, 103–109. [Google Scholar] [CrossRef]
- Sintzel, M.; Rametta, M.; Reder, A. Vitamin D and multiple sclerosis: A comprehensive review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef]
- Männistö, V.; Jääskeläinen, T.; Färkkilä, M.; Jula, A.; Männistö, S.; Lundqvist, A.; Zeller, T.; Blankenberg, S.; Salomaa, V.; Perola, M.; et al. Low serum vitamin D level associated with incident advanced liver disease in the general population—A prospective study. Scand J. Gastroenterol. 2021, 56, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Jean, G.; Souberbielle, J.; Chazot, C. Vitamin D in chronic kidney disease and dialysis patients. Nutrients 2017, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.; Wolf, M.; Waikar, S.; Chase, H.; Christov, M.; Cremers, S.; Stern, L. Fgf-23 levels in patients with AKI and risk of adverse outcomes. Clin. J. Am. Soc. Nephrol. 2012, 7, 1217–1223. [Google Scholar] [CrossRef]
- Bacchetta, J.; Sea, J.; Chun, R.; Lisse, T.; Wesseling-Perry, K.; Gales, B.; Adams, J.; Salusky, I.; Hewison, M. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J. Bone Miner. Res. 2013, 28, 46–55. [Google Scholar] [CrossRef]
- Holick, M. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin. Proc. 2006, 81, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Thanapluetiwong, S.; Chewcharat, A.; Takkavatakarn, K.; Praditpornsilpa, K.; Eiam-Ong, S.; Susantitaphong, P. Vitamin D supplement on prevention of fall and fracture: A meta-analysis of randomized controlled trials. Medicine 2020, 99, e21506. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, E.; Mascia, M.; Cipriani, C.; Fassino, V.; Mazzei, F.; D’Erasmo, E.; Carnevale, V.; Scillitani, A.; Minisola, S. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J. Clin. Endocrinol. Metab. 2008, 93, 3015–3020. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.; VG, S.; Vavia, P. Zero order controlled release delivery of cholecalciferol from injectable biodegradable microsphere: In-vitro characterization and in-vivo pharmacokinetic studies. Eur. J. Pharm. Sci. 2017, 107, 78–86. [Google Scholar] [CrossRef]
- Gupta, R.; Behera, C.; Paudwal, G.; Rawat, N.; Baldi, A.; Gupta, P. Recent advances in formulation strategies for efficient delivery of vitamin D. AAPS PharmSciTech 2018, 20, 11. [Google Scholar] [CrossRef]
- Heaney, R.; Recker, R.; Grote, J.; Horst, R.; Armas, L. Vitamin D(3) is more potent than vitamin D(2) in humans. J. Clin. Endocrinol. Metab. 2011, 96, E447–E452. [Google Scholar] [CrossRef]
- Bilezikian, J.; Formenti, A.; Adler, R.; Binkley, N.; Bouillon, R.; Lazaretti-Castro, M.; Marcocci, C.; Napoli, N.; Rizzoli, R.; Giustina, A. Vitamin D: Dosing, levels, form, and route of administration: Does one approach fit all? Rev. Endocr. Metab. Disord. 2021, 22, 1201–1218. [Google Scholar] [CrossRef]
- Chen, J.; Tang, Z.; Slominski, A.; Li, W.; Żmijewski, M.; Liu, Y.; Chen, J. Vitamin D and its analogs as anticancer and anti-inflammatory agents. Eur. J. Med. Chem. 2020; Online ahead of print. [Google Scholar]
- Cunningham, J.; Zehnder, D. New vitamin D analogs and changing therapeutic paradigms. Kidney Int. 2011, 79, 702–707. [Google Scholar] [CrossRef]
- Lan, S.; Lai, C.; Chang, S.; Lu, L.; Hung, S.; Lin, W. Vitamin D supplementation and the outcomes of critically ill adult patients: A systematic review and meta-analysis of randomized controlled trials. Sci. Rep. 2020, 10, 14261. [Google Scholar] [CrossRef]
- Menger, J.; Lee, Z.; Notz, Q.; Wallqvist, J.; Hasan, M.S.; Elke, G.; Dworschak, M.; Meybohm, P.; Heyland, D.; Stoppe, C. Administration of vitamin D and its metabolites in critically ill adult patients: An updated systematic review with meta-analysis of randomized controlled trials. Crit. Care 2022, 26, 268. [Google Scholar] [CrossRef]
- Saponaro, F.; Saba, A.; Zucchi, R. An update on vitamin D metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef]
- Doi, J.; Moro, A.; Fujiki, M.; Eghtesad, B.; Quintini, C.; Menon, K.N.; Hashimoto, K.; Sasaki, K. Nutrition support in liver transplantation and postoperative recovery: The effects of vitamin D level and vitamin D supplementation in liver transplantation. Nutrients 2020, 12, 3677. [Google Scholar] [CrossRef] [PubMed]
- Biggins, S.; Kim, W.; Terrault, N.; Saab, S.; Balan, V.; Schiano, T.; Benson, J.; Therneau, T.; Kremers, W.; Wiesner, R.; et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology 2006, 130, 1652–1660. [Google Scholar] [CrossRef]
- Martucci, G.; Volpes, R.; Panarello, G.; Tuzzolino, F.; Di Carlo, D.; Ricotta, C.; Gruttadauria, S.; Conaldi, P.; Luca, A.; Amrein, K.; et al. Vitamin D levels in liver transplantation recipients and early postoperative outcomes: Prospective observational dliverx study. Clin. Nutr. 2021, 40, 2355–2363. [Google Scholar] [CrossRef] [PubMed]
- Christov, M.; Waikar, S.; Pereira, R.; Havasi, A.; Leaf, D.; Goltzman, D.; Pajevic, P.; Wolf, M.; Jüppner, H. Plasma fgf23 levels increase rapidly after acute kidney injury. Kidney Int. 2013, 84, 776–785. [Google Scholar] [CrossRef]
- Cameron, L.; Lei, K.; Smith, S.; Doyle, N.; Doyle, J.; Flynn, K.; Purchase, N.; Smith, J.; Chan, K.; Kamara, F.; et al. Vitamin D levels in critically ill patients with acute kidney injury: A protocol for a prospective cohort study (vid-aki). BMJ Open 2017, 7, e016486. [Google Scholar] [CrossRef]
- Dancer, R.; Parekh, D.; Lax, S.; D’Souza, V.; Zheng, S.; Bassford, C.; Park, D.; Bartis, D.; Mahida, R.; Turner, A.; et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ards). Thorax 2015, 70, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Barnett, N.; Zhao, Z.; Koyama, T.; Janz, D.; Wang, C.; May, A.; Bernard, G.; Ware, L. Vitamin D deficiency and risk of acute lung injury in severe sepsis and severe trauma: A case-control study. Ann. Intensive Care 2014, 4, 5. [Google Scholar] [CrossRef]
- Fernandes, A.; Murai, I.; Reis, B.; Sales, L.; Santos, M.; Pinto, A.; Goessler, K.; Duran, C.; Silva, C.; Franco, A.; et al. Effect of a single high dose of vitamin D3 on cytokines, chemokines, and growth factor in patients with moderate to severe COVID-19. Am. J. Clin. Nutr. 2022, 115, 790–798. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.; Seymour, C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.; Chiche, J.; Coopersmith, C.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Cutuli, S.; Carelli, S.; Grieco, D.; De Pascale, G. Immune modulation in critically ill septic patients. Medicina 2021, 57, 552. [Google Scholar] [CrossRef] [PubMed]
- Moromizato, T.; Litonjua, A.; Braun, A.; Gibbons, F.; Giovannucci, E.; Christopher, K. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Crit. Care Med. 2014, 42, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, S. Serum 25-hydroxyvitamin D and the risk of mortality in adult patients with sepsis: A meta-analysis. BMC Infect. Dis. 2020, 20, 189. [Google Scholar] [CrossRef] [PubMed]

| Grading | Institute of Medicine [32] |
|---|---|
| Vitamin D | |
| Deficiency | 25(OH)D blood level ≤ 20 ng/mL |
| Insufficiency | 25(OH)D blood level 21–29 ng/mL |
| Sufficiency | 25(OH)D blood level ≥ 30 ng/mL |
| Authors, Year of Publication | Study Sites | Study Duration | Number of Patients | Inclusion Criteria | Intervention | Primary Outcome | Patients Characteristics | Main Result |
|---|---|---|---|---|---|---|---|---|
| Amrein et al. (the VITdAL-ICU trial), 2014 [12] | Single centre, Austria | 2012–2015 | 475 | Adult white critically ill patients, expected length of ICU stay ≥48 h and with 25-hydroxyvitamin D blood level of ≤20 ng/mL | Enteral vitamin D3 protocol administration: 540,000 IUs followed by monthly 90,000 IU for 5 months vs. Placebo | Length of hospital stay | Surgical patients were prevalent Mean body mass index about 27 kg/m2 Mean eGRF slightly above 60 mL/min/1.73 m2 | No difference for the primary outcome |
| Ginde et al. (the VIOLET trial), 2019 [13] | 44 centres, USA | 2017–2018 | 1078 | Adult patients with with >1 risk factors for death or lung injury, deemed to be managed in the ICU and with 25-hydroxyvitamin D blood level ≤20 ng/mL | Enteral vitamin D3 protocol administration: 540,000 Ius vs. Placebo | 90-day mortality rate | Medical patients were prevalent Black patients about 20% Mean body mass index about 30 kg/m2 Mean eGRF slightly about 60 mL/min/1.73 m2 | No difference for the primary outcome |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutuli, S.L.; Cascarano, L.; Tanzarella, E.S.; Lombardi, G.; Carelli, S.; Pintaudi, G.; Grieco, D.L.; De Pascale, G.; Antonelli, M. Vitamin D Status and Potential Therapeutic Options in Critically Ill Patients: A Narrative Review of the Clinical Evidence. Diagnostics 2022, 12, 2719. https://doi.org/10.3390/diagnostics12112719
Cutuli SL, Cascarano L, Tanzarella ES, Lombardi G, Carelli S, Pintaudi G, Grieco DL, De Pascale G, Antonelli M. Vitamin D Status and Potential Therapeutic Options in Critically Ill Patients: A Narrative Review of the Clinical Evidence. Diagnostics. 2022; 12(11):2719. https://doi.org/10.3390/diagnostics12112719
Chicago/Turabian StyleCutuli, Salvatore L., Laura Cascarano, Eloisa S. Tanzarella, Gianmarco Lombardi, Simone Carelli, Gabriele Pintaudi, Domenico L. Grieco, Gennaro De Pascale, and Massimo Antonelli. 2022. "Vitamin D Status and Potential Therapeutic Options in Critically Ill Patients: A Narrative Review of the Clinical Evidence" Diagnostics 12, no. 11: 2719. https://doi.org/10.3390/diagnostics12112719
APA StyleCutuli, S. L., Cascarano, L., Tanzarella, E. S., Lombardi, G., Carelli, S., Pintaudi, G., Grieco, D. L., De Pascale, G., & Antonelli, M. (2022). Vitamin D Status and Potential Therapeutic Options in Critically Ill Patients: A Narrative Review of the Clinical Evidence. Diagnostics, 12(11), 2719. https://doi.org/10.3390/diagnostics12112719






