A Machine Learning Approach for Detecting Idiopathic REM Sleep Behavior Disorder
Abstract
1. Introduction
2. Participants and Methods
2.1. Participants
2.2. Heart Rate Variability (HRV)
- -
- NN/RR ratio: the fraction of total RR intervals that are classified as normal-to-normal (NN) intervals and included in the calculation of HRV statistics;
- -
- AVNN: average of all NN intervals;
- -
- SDNN: standard deviation of all NN intervals;
- -
- rMSSD: square root of the mean of the squares of the differences between adjacent NN intervals;
- -
- pNN50: percentage of differences between adjacent NN intervals that are greater than 50 ms;
- -
- TOT_PWR: total spectral power of all NN intervals up to 0.04 Hz;
- -
- VLF_PWR: total spectral power of all NN intervals between 0.003 and 0.04 Hz;
- -
- LF_PWR: total spectral power of all NN intervals between 0.04 and 0.15 Hz;
- -
- HF_PWR: total spectral power of all NN intervals between 0.15 and 0.4 Hz;
- -
- LF/HF: ratio of low to high frequency power;
- -
- Sample entropy of RR intervals;
- -
- Largest Lyapunov exponent to quantify the amount of chaos in RR series;
- -
- Hurst coefficient, as a measure of long-term memory in RR series;
- -
- Alpha: scaling exponent from Detrended Fluctuation Analysis (DFA), for determining the statistical self-affinity of RR series;
- -
- Triangular index: a geometric measure of HRV, defined as the integral of the density distribution (i.e., the number of all RR intervals) divided by the maximum of the density distribution;
- -
- SD1: the standard deviation of the Poincaré plot perpendicular to the line-of-identity;
- -
- SD2: the standard deviation of the Poincaré plot along the line-of-identity;
- -
- SD2/SD1 ratio.
2.3. Machine Learning Approaches
3. Results
3.1. Features and HRV Analysis
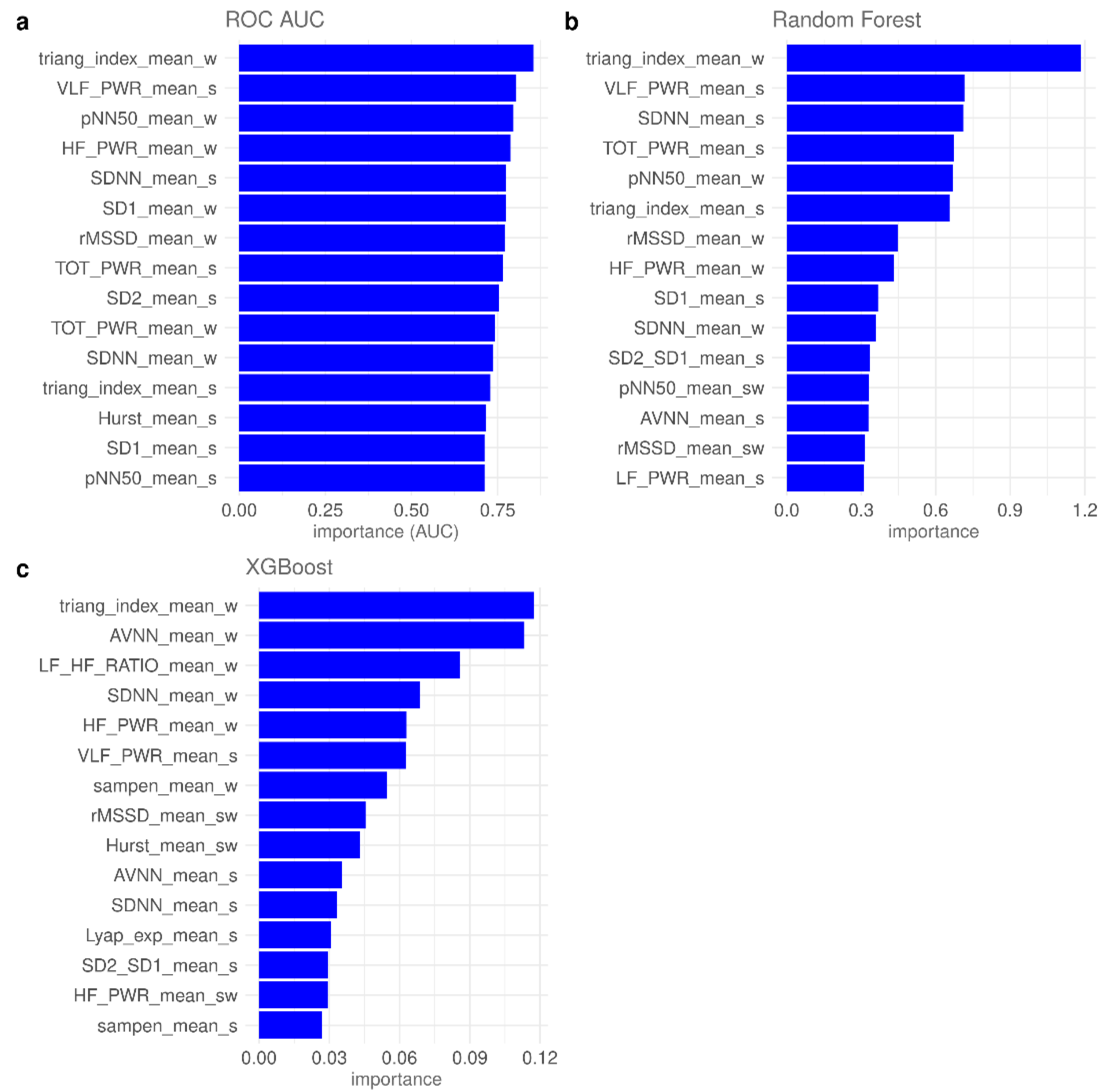
3.2. Feature Importance and Feature Selection
3.3. LOO-CV Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dauvilliers, Y.; Schenck, C.H.; Postuma, R.B.; Iranzo, A.; Luppi, P.H.; Plazzi, G.; Montplaisir, J.; Boeve, B. REM sleep behaviour disorder. Nat. Rev. Dis. Prim. 2018, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Högl, B.; Stefani, A.; Videnovic, A. Idiopathic REM sleep behaviour disorder and neurodegeneration—An update. Nat. Rev. Neurol. 2018, 14, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Lang, A.E.; Massicotte-Marquez, J.; Montplaisir, J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology 2006, 66, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Arnulf, I.; Hogl, B.; Iranzo, A.; Miyamoto, T.; Dauvilliers, Y.; Oertel, W.; Ju, Y.E.; Puligheddu, M.; Jennum, P.; et al. A Single-Question Screen for REM Sleep Behavior Disorder: A Multicenter Validation Study. Mov. Disord. 2012, 27, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Salsone, M.; Vescio, B.; Fratto, A.; Sturniolo, M.; Arabia, G.; Gambardella, A.; Quattrone, A. Cardiac sympathetic index identifies patients with Parkinson’s disease and REM behavior disorder. Park. Relat Disord. 2016, 26, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Lanfranchi, P.A.; Fradette, L.; Gagnon, J.F.; Colombo, R.; Montplaisir, J. Cardiac autonomic regulation during sleep in idiopathic REM sleep behavior disorder. Sleep 2007, 30, 1019–1025. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Valappil, R.A.; Black, J.E.; Broderick, M.J.; Carrillo, O.; Frenette, E.; Sullivan, S.S.; Goldman, S.M.; Tanner, C.M.; Langston, J.W. Exploring the electrocardiogram as a potential tool to screen for premotor Parkinson’s disease. Mov. Disord. 2010, 25, 2296–2303. [Google Scholar] [CrossRef] [PubMed]
- Ministeri, C.M.; Modica, D.A.A.; Maccora, L.C.S. Decreased heart rate variability in REM sleep behavior disorder. Clin. Neurophysiol. 2016, 107, 144. [Google Scholar] [CrossRef]
- Ruffini, G.; Ibañez, D.; Castellano, M.; Dubreuil-Vall, L.; Soria-Frisch, A.; Postuma, R.; Gagnon, J.F.; Montplaisir, J. Deep Learning with EEG Spectrograms in Rapid Eye Movement Behavior Disorder. Front. Neurol. 2019, 10, 806. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gong, X.; Chen, H.; Zhong, W.; Chen, Y.; Zhou, Y.; Zhang, W.; He, Y.; Lou, M. Causative Classification of Ischemic Stroke by the Machine Learning Algorithm Random Forests. Front. Aging Neurosci. 2022, 14, 788637. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Luo, W.; Liu, Z.; Liu, W.; Liu, C.; Liu, X.; Zhu, H.; Li, R.; Song, J.; Hu, X.; et al. Integration of the Extreme Gradient Boosting model with electronic health records to enable the early diagnosis of multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 47, 102632. [Google Scholar] [CrossRef] [PubMed]
- Rechichi, I.; Iadarola, A.; Zibetti, M.; Cicolin, A.; Olmo, G. Assessing REM Sleep Behaviour Disorder: From Machine Learning Classification to the Definition of a Continuous Dissociation Index. Int. J. Environ. Res. Public Health 2021, 19, 248. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.A.; Lee, H.J.; Kim, H.C.; Park, K.M. Application of machine learning analysis based on diffusion tensor imaging to identify REM sleep behavior disorder. Sleep Breath. 2022, 26, 633–640. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Hamilton, P.S. Open source ECG analysis. In Proceedings of the Computers in Cardiology, Memphis, TN, USA, 22–25 September 2002; pp. 101–104. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In KDD ′16, Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; Association for Computing Machinery: New York, NY, USA, 2016; pp. 785–794. [Google Scholar] [CrossRef]
- PhysioNet: Heart Rate Variability Analysis with the HRV Toolkit. Available online: https://archive.physionet.org/tutorials/hrv-toolkit/ (accessed on 11 August 2022).
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 21 September 2022).
- Salsone, M.; Nistico’, R.; Vescio, B.; Novellino, F.; Morelli, M.; Lupo, A.; Arabia, G.; Quattrone, A. Heart rate variability in patients with essential tremor: A cross sectional study. Park. Relat Disord. 2016, 33, 134–137. [Google Scholar] [CrossRef]
- Sauvageot, N.; Vaillant, M.; Diederich, N.J. Reduced sympathetically driven heart rate variability during sleep in Parkinson’s disease: A case-control polysomnography-based study. Mov. Disord. 2011, 26, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Pursiainen, V.; Haapaniemi, T.H.; Korpelainen, J.T.; Huikuri, H.V.; Sotaniemi, K.A.; Myllylä, V.V. Circadian heart rate variability in Parkinson’s disease. J. Neurol. 2002, 249, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Vescio, B.; Salsone, M.; Gambardella, A.; Quattrone, A. Comparison between Electrocardiographic and Earlobe Pulse Photoplethysmographic Detection for Evaluating Heart Rate Variability in Healthy Subjects in Short- and Long-Term Recordings. Sensors 2018, 18, 844. [Google Scholar] [CrossRef] [PubMed]
| HC | iRBD | p Value | |
|---|---|---|---|
| N | 12 | 20 | -- |
| Sex * (M/F) | 7/5 | 14/6 | 0.70 |
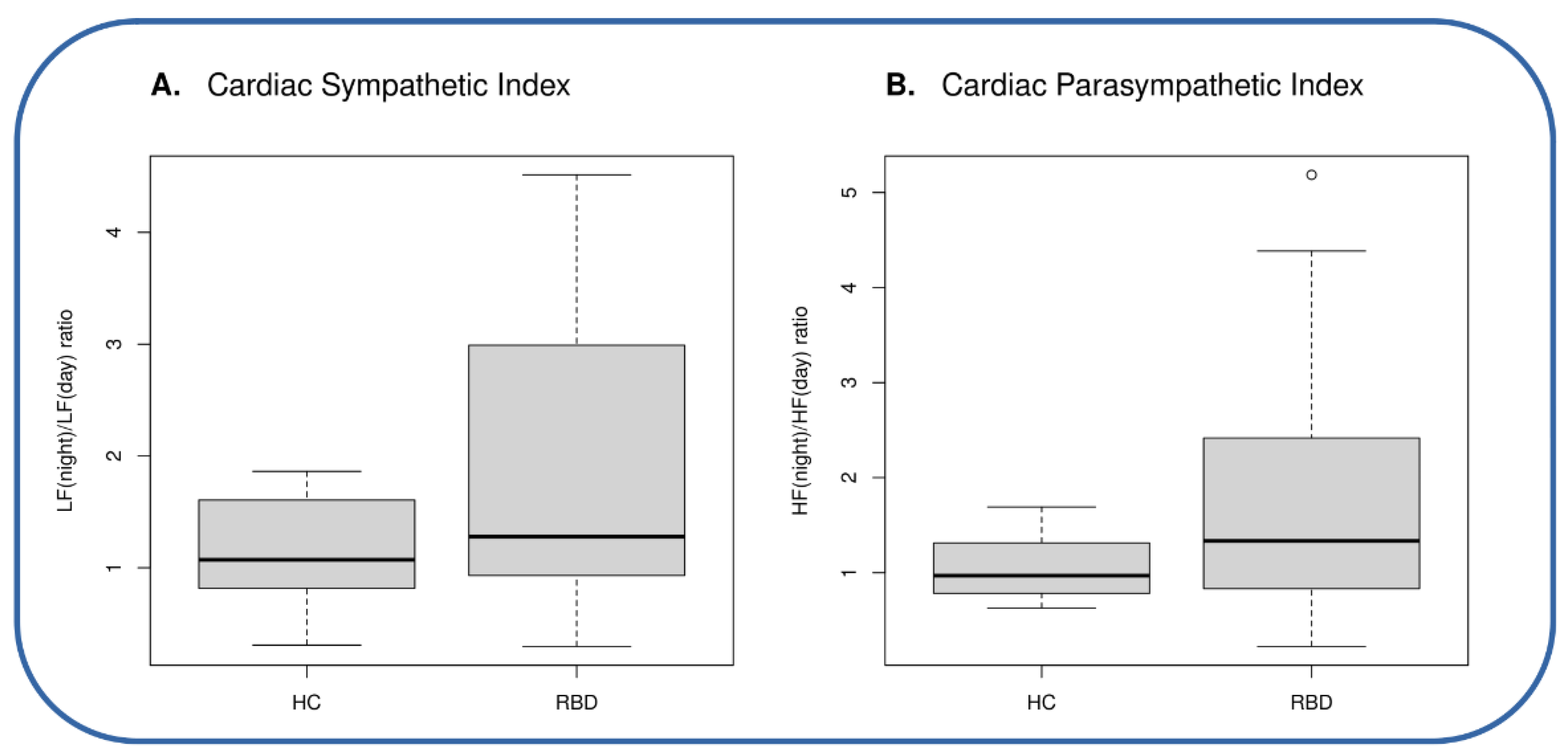
| Age # Sympathetic Index # Parasympathetic Index # | 54.2 ± 15.1 1.15 ± 0.52 1.05 ± 0.33 | 54.9 ± 9.1 1.86 ± 1.33 1.83 ± 1.42 | 0.89 0.04 0.03 |
| Autonomic Indices | Accuracy (95% conf. int.) | AUC (95% conf. int.) | Sensitivity (95% conf. int.) | Specificity (95% conf. int.) |
|---|---|---|---|---|
| Sympathetic | 0.63 (0.50–0.81) | 0.62 (0.53–0.81) | 0.45 (0.20–0.90) | 1 (0.42–1) |
| Parasympathetic | 0.69 (0.53–0.81) | 0.65 (0.46–0.84) | 0.55 (0.25–0.85) | 1 (0.58–1) |
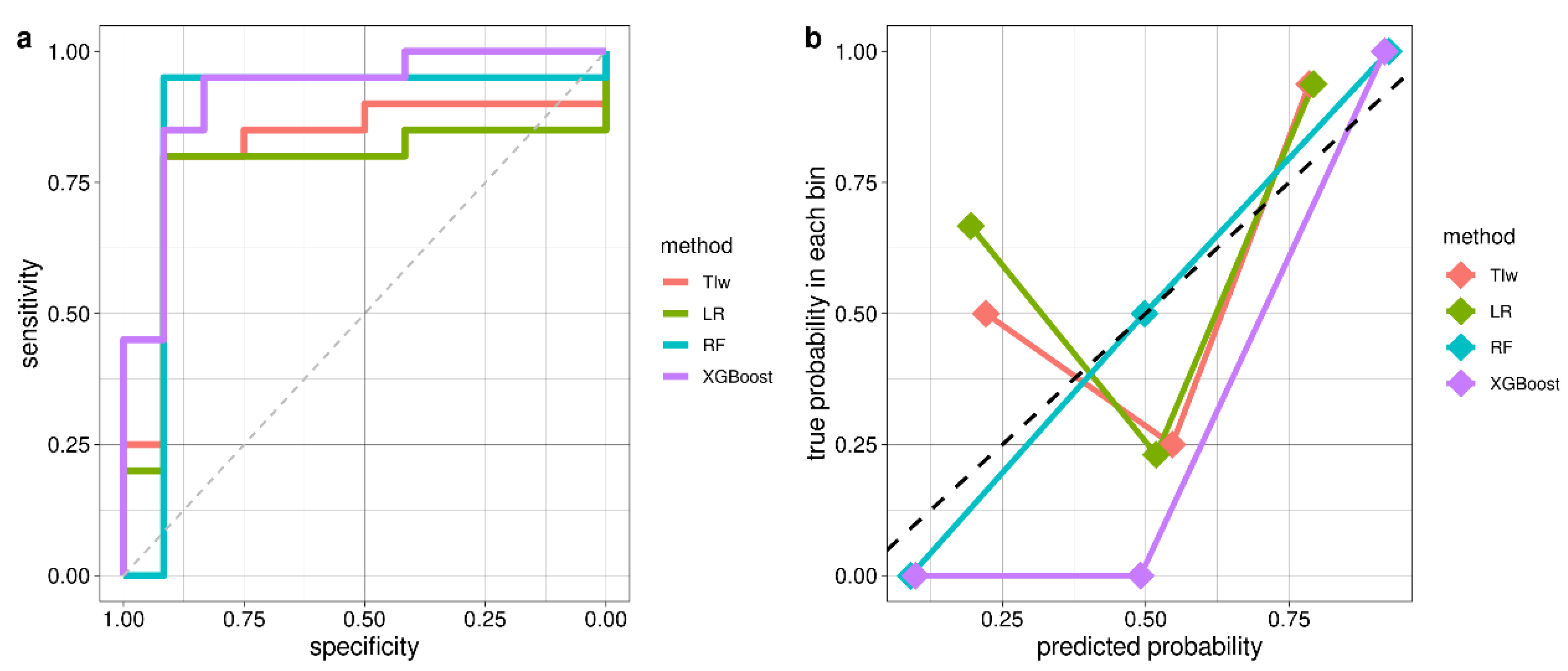
| Model | Accuracy (95% conf. int.) | AUC | Sensitivity | Specificity | No. of Features |
|---|---|---|---|---|---|
| TIw | 0.81 (0.64–0.93) | 0.82 | 0.80 | 0.83 | 1 |
| LR | 0.84 (0.67–0.95) | 0.77 | 0.80 | 0.92 | 2 |
| RF | 0.94 (0.79–0.99) | 0.87 | 0.95 | 0.92 | 5 |
| XGBoost | 0.91 (0.75–0.98) | 0.92 | 0.95 | 0.83 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salsone, M.; Quattrone, A.; Vescio, B.; Ferini-Strambi, L.; Quattrone, A. A Machine Learning Approach for Detecting Idiopathic REM Sleep Behavior Disorder. Diagnostics 2022, 12, 2689. https://doi.org/10.3390/diagnostics12112689
Salsone M, Quattrone A, Vescio B, Ferini-Strambi L, Quattrone A. A Machine Learning Approach for Detecting Idiopathic REM Sleep Behavior Disorder. Diagnostics. 2022; 12(11):2689. https://doi.org/10.3390/diagnostics12112689
Chicago/Turabian StyleSalsone, Maria, Andrea Quattrone, Basilio Vescio, Luigi Ferini-Strambi, and Aldo Quattrone. 2022. "A Machine Learning Approach for Detecting Idiopathic REM Sleep Behavior Disorder" Diagnostics 12, no. 11: 2689. https://doi.org/10.3390/diagnostics12112689
APA StyleSalsone, M., Quattrone, A., Vescio, B., Ferini-Strambi, L., & Quattrone, A. (2022). A Machine Learning Approach for Detecting Idiopathic REM Sleep Behavior Disorder. Diagnostics, 12(11), 2689. https://doi.org/10.3390/diagnostics12112689






