Nanoscale Contrast Agents for Ultrasound Imaging of Musculoskeletal System
Abstract
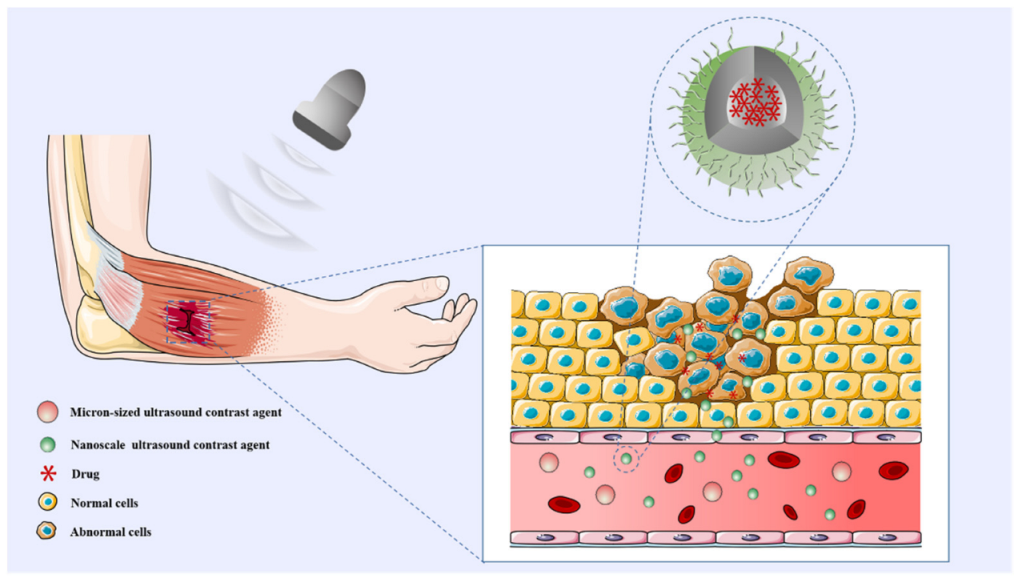
1. Introduction
2. Distinctive Features of nUCAs and Feasibility of Molecular Imaging in MSKUS
3. Applications and Insights of nUCAs of the Musculoskeletal System
3.1. Skeletal Muscle Disorders
3.2. Arthritic Diseases
3.3. Bone Regeneration and Repair
3.4. Other Musculoskeletal Disorders to Be Explored for Application
4. Summary and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Díaz-Gómez, J.L.; Mayo, P.H.; Koenig, S.J. Point-of-Care Ultrasonography. N. Engl. J. Med. 2021, 385, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Short Version). Ultraschall Med. 2018, 39, 154–180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huang, H.; Xu, Q.; Pan, Q.; Guo, J. Quantitative assessment of changes in skeletal muscle injury by computer-aided analysis based on two-dimensional ultrasonography combined with contrast-enhanced ultrasonography and estimated by a modified semi-quantitative scoring system: An experimental study in a contusion model. Int. J. Exp. Pathol. 2022, 103, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, J.; Xu, Q.; Sheng, J.; Diao, Z.; Liu, S. Quantitative evaluation of striated muscle injury by multiscale blob features method. J. Med. Ultrason. 2016, 43, 337–345. [Google Scholar] [CrossRef]
- Habibi, N.; Quevedo, D.F.; Gregory, J.V.; Lahann, J. Emerging methods in therapeutics using multifunctional nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1625. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Nolsøe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) in the Liver—Update 2020—WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultraschall Med. 2020, 41, 562–585. [Google Scholar] [CrossRef]
- Alheshibri, M.; Craig, V.S.J. Armoured nanobubbles; ultrasound contrast agents under pressure. J. Colloid Interface Sci. 2019, 537, 123–131. [Google Scholar] [CrossRef]
- Exner, A.A.; Kolios, M.C. Bursting Microbubbles: How Nanobubble Contrast Agents Can Enable the Future of Medical Ultrasound Molecular Imaging and Image-Guided Therapy. Curr. Opin. Colloid Interface Sci. 2021, 54, 101463. [Google Scholar] [CrossRef]
- de Leon, A.; Perera, R.; Hernandez, C.; Cooley, M.; Jung, O.; Jeganathan, S.; Abenojar, E.; Fishbein, G.; Sojahrood, A.J.; Emerson, C.C.; et al. Contrast enhanced ultrasound imaging by nature-inspired ultrastable echogenic nanobubbles. Nanoscale 2019, 11, 15647–15658. [Google Scholar] [CrossRef]
- Alphandéry, E. Ultrasound and nanomaterial: An efficient pair to fight cancer. J. Nanobiotechnol. 2022, 20, 139. [Google Scholar] [CrossRef]
- Siani, P.; Frigerio, G.; Donadoni, E.; Di Valentin, C. Molecular dynamics simulations of cRGD-conjugated PEGylated TiO(2) nanoparticles for targeted photodynamic therapy. J. Colloid Interface Sci. 2022, 627, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, M.; Hempel, C.; Andresen, T.L.; Urquhart, A.J. Biopharmaceutical nanoclusters: Towards the self-delivery of protein and peptide therapeutics. J. Control. Release 2022, 347, 282–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Hou, Y.; Zhu, M.; Deng, B.; Zhao, M.; Zhu, X.; Sun, Y.; Chen, D.; Jiang, C.; Wang, L.; et al. Death Pathways of Cancer Cells Modulated by Surface Molecule Density on Gold Nanorods. Adv. Sci. 2021, 8, e2102666. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ma, M.; Wang, J.; Wang, F.; Chern, S.X.; Zhao, E.R.; Jhunjhunwala, A.; Darmadi, S.; Chen, H.; Jokerst, J.V. Exosome-like silica nanoparticles: A novel ultrasound contrast agent for stem cell imaging. Nanoscale 2017, 9, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Kubelick, K.P.; Snider, E.J.; Ethier, C.R.; Emelianov, S. Development of a stem cell tracking platform for ophthalmic applications using ultrasound and photoacoustic imaging. Theranostics 2019, 9, 3812–3824. [Google Scholar] [CrossRef] [PubMed]
- Selmani, A.; Kovačević, D.; Bohinc, K. Nanoparticles: From synthesis to applications and beyond. Adv. Colloid Interface Sci. 2022, 303, 102640. [Google Scholar] [CrossRef]
- Fan, C.H.; Ho, Y.J.; Lin, C.W.; Wu, N.; Chiang, P.H.; Yeh, C.K. State-of-the-art of ultrasound-triggered drug delivery from ultrasound-responsive drug carriers. Expert Opin. Drug Deliv. 2022, 19, 997–1009. [Google Scholar] [CrossRef]
- Kauscher, U.; Holme, M.N.; Björnmalm, M.; Stevens, M.M. Physical stimuli-responsive vesicles in drug delivery: Beyond liposomes and polymersomes. Adv. Drug Deliv. Rev. 2019, 138, 259–275. [Google Scholar] [CrossRef]
- Ho, Y.J.; Chiang, Y.J.; Kang, S.T.; Fan, C.H.; Yeh, C.K. Camptothecin-loaded fusogenic nanodroplets as ultrasound theranostic agent in stem cell-mediated drug-delivery system. J. Control. Release 2018, 278, 100–109. [Google Scholar] [CrossRef]
- Cheng, B.; Ahn, H.H.; Nam, H.; Jiang, Z.; Gao, F.J.; Minn, I.; Pomper, M.G. A Unique Core-Shell Structured, Glycol Chitosan-Based Nanoparticle Achieves Cancer-Selective Gene Delivery with Reduced Off-Target Effects. Pharmaceutics 2022, 14, 373. [Google Scholar] [CrossRef]
- Zlitni, A.; Gambhir, S.S. Molecular imaging agents for ultrasound. Curr. Opin. Chem. Biol. 2018, 45, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Joenathan, A.; Patwa, A.; Snyder, B.D.; Grinstaff, M.W. Tantalum Oxide Nanoparticles for the Quantitative Contrast-Enhanced Computed Tomography of Ex Vivo Human Cartilage: Assessment of Biochemical Composition and Biomechanics. ACS Nano 2021, 15, 19175–19184. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Luo, S.; Li, Y.; Lu, L.; Deng, C.; Cheng, Y.; Yin, F. Intra-articular tracking of adipose-derived stem cells by chitosan-conjugated iron oxide nanoparticles in a rat osteoarthritis model. RSC Adv. 2019, 9, 12010–12019. [Google Scholar] [CrossRef] [PubMed]
- Nejadnik, H.; Tseng, J.; Daldrup-Link, H. Magnetic resonance imaging of stem cell-macrophage interactions with ferumoxytol and ferumoxytol-derived nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1552. [Google Scholar] [CrossRef] [PubMed]
- Hotfiel, T.; Heiss, R.; Swoboda, B.; Kellermann, M.; Gelse, K.; Grim, C.; Strobel, D.; Wildner, D. Contrast-Enhanced Ultrasound as a New Investigative Tool in Diagnostic Imaging of Muscle Injuries—A Pilot Study Evaluating Conventional Ultrasound, CEUS, and Findings in MRI. Clin. J. Sport Med. 2018, 28, 332–338. [Google Scholar] [CrossRef]
- Negishi, Y.; Ishii, Y.; Shiono, H.; Akiyama, S.; Sekine, S.; Kojima, T.; Mayama, S.; Kikuchi, T.; Hamano, N.; Endo-Takahashi, Y.; et al. Bubble liposomes and ultrasound exposure improve localized morpholino oligomer delivery into the skeletal muscles of dystrophic mdx mice. Mol. Pharm. 2014, 11, 1053–1061. [Google Scholar] [CrossRef]
- Jung, E.; Noh, J.; Kang, C.; Yoo, D.; Song, C.; Lee, D. Ultrasound imaging and on-demand therapy of peripheral arterial diseases using H(2)O(2)-Activated bubble generating anti-inflammatory polymer particles. Biomaterials 2018, 179, 175–185. [Google Scholar] [CrossRef]
- Kim, G.W.; Kang, C.; Oh, Y.B.; Ko, M.H.; Seo, J.H.; Lee, D. Ultrasonographic Imaging and Anti-inflammatory Therapy of Muscle and Tendon Injuries Using Polymer Nanoparticles. Theranostics 2017, 7, 2463–2476. [Google Scholar] [CrossRef]
- Kim, G.W.; Song, N.H.; Park, M.R.; Kim, T.E.; Kim, D.S.; Oh, Y.B.; Lee, D.W. Diagnosis and Simultaneous Treatment of Musculoskeletal Injury Using H(2)O(2)-Triggered Echogenic Antioxidant Polymer Nanoparticles in a Rat Model of Contusion Injury. Nanomaterials 2021, 11, 2571. [Google Scholar] [CrossRef]
- Lee, D.; Bae, S.; Ke, Q.; Lee, J.; Song, B.; Karumanchi, S.A.; Khang, G.; Choi, H.S.; Kang, P.M. Hydrogen peroxide-responsive copolyoxalate nanoparticles for detection and therapy of ischemia-reperfusion injury. J. Control. Release 2013, 172, 1102–1110. [Google Scholar] [CrossRef]
- Tang, Q.; Cui, J.; Tian, Z.; Sun, J.; Wang, Z.; Chang, S.; Zhu, S. Oxygen and indocyanine green loaded phase-transition nanoparticle-mediated photo-sonodynamic cytotoxic effects on rheumatoid arthritis fibroblast-like synoviocytes. Int. J. Nanomed. 2017, 12, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; He, Y.; Wu, H.; Zhou, M.; Xu, Z.; Xiong, R.; Yan, F.; Liu, H. Near-infrared fluorescence imaging-guided focused ultrasound-mediated therapy against Rheumatoid Arthritis by MTX-ICG-loaded iRGD-modified echogenic liposomes. Theranostics 2020, 10, 10092–10105. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; He, Y.; Zhou, M.; Xin, H.; Pan, M.; Fiaz, M.; Liu, H.; Yan, F. Ultrasound imaging tracking of mesenchymal stem cells intracellularly labeled with biosynthetic gas vesicles for treatment of rheumatoid arthritis. Theranostics 2022, 12, 2370–2382. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ji, Y.; Hu, X.; Cui, C.; Liu, H.; Tang, Y.; Qi, B.; Niu, Y.; Hu, X.; Yu, A.; et al. Cationic poly-l-lysine-encapsulated melanin nanoparticles as efficient photoacoustic agents targeting to glycosaminoglycans for the early diagnosis of articular cartilage degeneration in osteoarthritis. Nanoscale 2018, 10, 13471–13484. [Google Scholar] [CrossRef] [PubMed]
- Theis, K.A.; Murphy, L.B.; Guglielmo, D.; Boring, M.A.; Okoro, C.A.; Duca, L.M.; Helmick, C.G. Prevalence of Arthritis and Arthritis-Attributable Activity Limitation—United States, 2016–2018. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1401–1407. [Google Scholar] [CrossRef]
- Buch, M.H.; Eyre, S.; McGonagle, D. Persistent inflammatory and non-inflammatory mechanisms in refractory rheumatoid arthritis. Nat. Rev. Rheumatol. 2021, 17, 17–33. [Google Scholar] [CrossRef]
- Silvagni, E.; Zandonella Callegher, S.; Mauric, E.; Chiricolo, S.; Schreiber, N.; Tullio, A.; Zabotti, A.; Scirè, C.A.; Dejaco, C.; Sakellariou, G. Musculoskeletal ultrasound for treating rheumatoid arthritis to target-a systematic literature review. Rheumatology, 2022; online ahead of print. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, R.; Luo, Y.; Liu, S.; Tang, T.; Yang, F.; Zhu, L.; He, X.; Yang, M.; Jiang, Y. Multimodal VEGF-Targeted Contrast-Enhanced Ultrasound and Photoacoustic Imaging of Rats with Inflammatory Arthritis: Using Dye-VEGF-Antibody-Loaded Microbubbles. Ultrasound Med. Biol. 2020, 46, 2400–2411. [Google Scholar] [CrossRef]
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef]
- Fischer, C.; Haug, T.; Weber, M.A.; Kauczor, H.U.; Bruckner, T.; Schmidmaier, G. Contrast-Enhanced Ultrasound (CEUS) Identifies Perfusion Differences Between Tibial Fracture Unions and Non-Unions. Ultraschall Med. 2020, 41, 44–51. [Google Scholar] [CrossRef]
- Burger, M.G.; Grosso, A.; Briquez, P.S.; Born, G.M.E.; Lunger, A.; Schrenk, F.; Todorov, A.; Sacchi, V.; Hubbell, J.A.; Schaefer, D.J.; et al. Robust coupling of angiogenesis and osteogenesis by VEGF-decorated matrices for bone regeneration. Acta Biomater. 2022, 149, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Kim, B.S.; Seo, S.H.; Kim, M.; Kang, Y.G.; Shin, J.W.; Cho, K.H.; Shin, M.C.; Yoon, C.; Min, K.A. Synergistic Effect of Growth Factor Releasing Polymeric Nanoparticles and Ultrasound Stimulation on Osteogenic Differentiation. Pharmaceutics 2021, 13, 457. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.; Bloise, N.; Fiorilli, S.; Novajra, G.; Vallet-Regí, M.; Bruni, G.; Torres-Pardo, A.; González-Calbet, J.M.; Visai, L.; Vitale-Brovarone, C. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 2017, 55, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, S.; Zeng, W.; Yu, J.; Chen, Y.; Yu, B. Controlled in vivo Bone Formation and Vascularization Using Ultrasound-Triggered Release of Recombinant Vascular Endothelial Growth Factor From Poly(D,L-lactic-co-glycolicacid) Microbubbles. Front. Pharmacol. 2019, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Nomikou, N.; Feichtinger, G.A.; Saha, S.; Nuernberger, S.; Heimel, P.; Redl, H.; McHale, A.P. Ultrasound-responsive gene-activated matrices for osteogenic gene therapy using matrix-assisted sonoporation. J. Tissue Eng. Regen. Med. 2018, 12, e250–e260. [Google Scholar] [CrossRef]
- Yu, Y.; Tan, L.; Li, Z.; Liu, X.; Zheng, Y.; Feng, X.; Liang, Y.; Cui, Z.; Zhu, S.; Wu, S. Single-Atom Catalysis for Efficient Sonodynamic Therapy of Methicillin-Resistant Staphylococcus aureus-Infected Osteomyelitis. ACS Nano 2021, 15, 10628–10639. [Google Scholar] [CrossRef]
- Kunz, P.; Kiesl, S.; Groß, S.; Kauczor, H.U.; Schmidmaier, G.; Fischer, C. Intra-observer and Device-Dependent Inter-observer Reliability of Contrast-Enhanced Ultrasound for Muscle Perfusion Quantification. Ultrasound Med. Biol 2020, 46, 275–285. [Google Scholar] [CrossRef]
- Huang, S.; Xiang, X.; Qiu, L.; Wang, L.; Zhu, B.; Guo, R.; Tang, X. Transfection of TGF-β shRNA by Using Ultrasound-targeted Microbubble Destruction to Inhibit the Early Adhesion Repair of Rats Wounded Achilles Tendon In vitro and In vivo. Curr. Gene Ther. 2020, 20, 71–81. [Google Scholar] [CrossRef]
- Fischer, C.; Gross, S.; Zeifang, F.; Schmidmaier, G.; Weber, M.A.; Kunz, P. Contrast-Enhanced Ultrasound Determines Supraspinatus Muscle Atrophy After Cuff Repair and Correlates to Functional Shoulder Outcome. Am. J. Sports Med. 2018, 46, 2735–2742. [Google Scholar] [CrossRef]
- Ntoulia, A.; Barnewolt, C.E.; Doria, A.S.; Ho-Fung, V.M.; Lorenz, N.; Mentzel, H.J.; Back, S.J. Contrast-enhanced ultrasound for musculoskeletal indications in children. Pediatr. Radiol. 2021, 51, 2303–2323. [Google Scholar] [CrossRef]
- De Marchi, A.; Pozza, S.; Charrier, L.; Cannone, F.; Cavallo, F.; Linari, A.; Piana, R.; Geniò, I.; Balocco, P.; Massè, A. Small Subcutaneous Soft Tissue Tumors (<5 cm) Can Be Sarcomas and Contrast-Enhanced Ultrasound (CEUS) Is Useful to Identify Potentially Malignant Masses. Int. J. Environ. Res. Public Health 2020, 17, 8868. [Google Scholar] [CrossRef] [PubMed]
- Helbert, A.; Von Wronski, M.; Colevret, D.; Botteron, C.; Padilla, F.; Bettinger, T.; Tardy, I.; Hyvelin, J.M. Ultrasound Molecular Imaging With BR55, a Predictive Tool of Antiangiogenic Treatment Efficacy in a Chemo-Induced Mammary Tumor Model. Investig. Radiol. 2020, 55, 657–665. [Google Scholar] [CrossRef]
- Thakur, S.S.; Chen, Y.S.; Houston, Z.H.; Fletcher, N.; Barnett, N.L.; Thurecht, K.J.; Rupenthal, I.D.; Parekh, H.S. Ultrasound-responsive nanobubbles for enhanced intravitreal drug migration: An ex vivo evaluation. Eur. J. Pharm. Biopharm. 2019, 136, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Beaumont, N.; Ma, C.; Rojas, J.; Vu, T.; Harlacher, M.; O’Connell, G.; Gessner, R.C.; Kilian, H.; Kasatkina, L.; et al. Three-dimensional Deep-tissue Functional and Molecular Imaging by Integrated Photoacoustic, Ultrasound, and Angiographic Tomography (PAUSAT). IEEE Trans. Med. Imaging 2022, 41, 2704–2714. [Google Scholar] [CrossRef] [PubMed]
| Year | Modality | Diameter | Disease | Application | Ref |
|---|---|---|---|---|---|
| 2014 | US | 400–500 nm | Duchenne muscular dystrophy | Imaging and gene delivery | [26] |
| 2018 | US | ~360 nm | Muscle of peripheral artery disease | Imaging and drug delivery | [27] |
| 2017 | US | ~400 nm | Musculoskeletal contusion injury | Imaging and anti-inflammatory therapy | [28] |
| 2021 | US+FOI | ~330 nm | Musculoskeletal contusion injury | Imaging, diagnosis and simultaneous treatment | [29] |
| 2013 | BLI | ~450 nm | Muscle ischemia reperfusion injury | Imaging and drug delivery | [30] |
| 2017 | US+PAI | ~278 nm | Rheumatoid arthritis | Imaging, photodynamic therapy and sonodynamic therapy | [31] |
| 2020 | FOI | ~113 nm | Rheumatoid arthritis | Imaging and drug delivery | [32] |
| 2022 | US | ~218 nm | Rheumatoid arthritis | Imaging and stem cell tracking | [33] |
| 2018 | PAI | ~39 nm | Osteoarthritis | Imaging | [34] |
| 2019 | MRI | 17 nm | Osteoarthritis | Imaging | [23] |
| 2021 | CT | 3–6nm | Osteoarthritis | Imaging | [22] |
| 2019 | MRI | ~6 nm | Degenerative bone diseases | Imaging and stem cell tracking | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Zhao, M.; Li, W.; Zhao, J. Nanoscale Contrast Agents for Ultrasound Imaging of Musculoskeletal System. Diagnostics 2022, 12, 2582. https://doi.org/10.3390/diagnostics12112582
Tang X, Zhao M, Li W, Zhao J. Nanoscale Contrast Agents for Ultrasound Imaging of Musculoskeletal System. Diagnostics. 2022; 12(11):2582. https://doi.org/10.3390/diagnostics12112582
Chicago/Turabian StyleTang, Xiaoyi, Mengxin Zhao, Wei Li, and Jiaqi Zhao. 2022. "Nanoscale Contrast Agents for Ultrasound Imaging of Musculoskeletal System" Diagnostics 12, no. 11: 2582. https://doi.org/10.3390/diagnostics12112582
APA StyleTang, X., Zhao, M., Li, W., & Zhao, J. (2022). Nanoscale Contrast Agents for Ultrasound Imaging of Musculoskeletal System. Diagnostics, 12(11), 2582. https://doi.org/10.3390/diagnostics12112582







