Radioembolization-Induced Changes in Hepatic [18F]FDG Metabolism in Non-Tumorous Liver Parenchyma
Abstract
1. Introduction
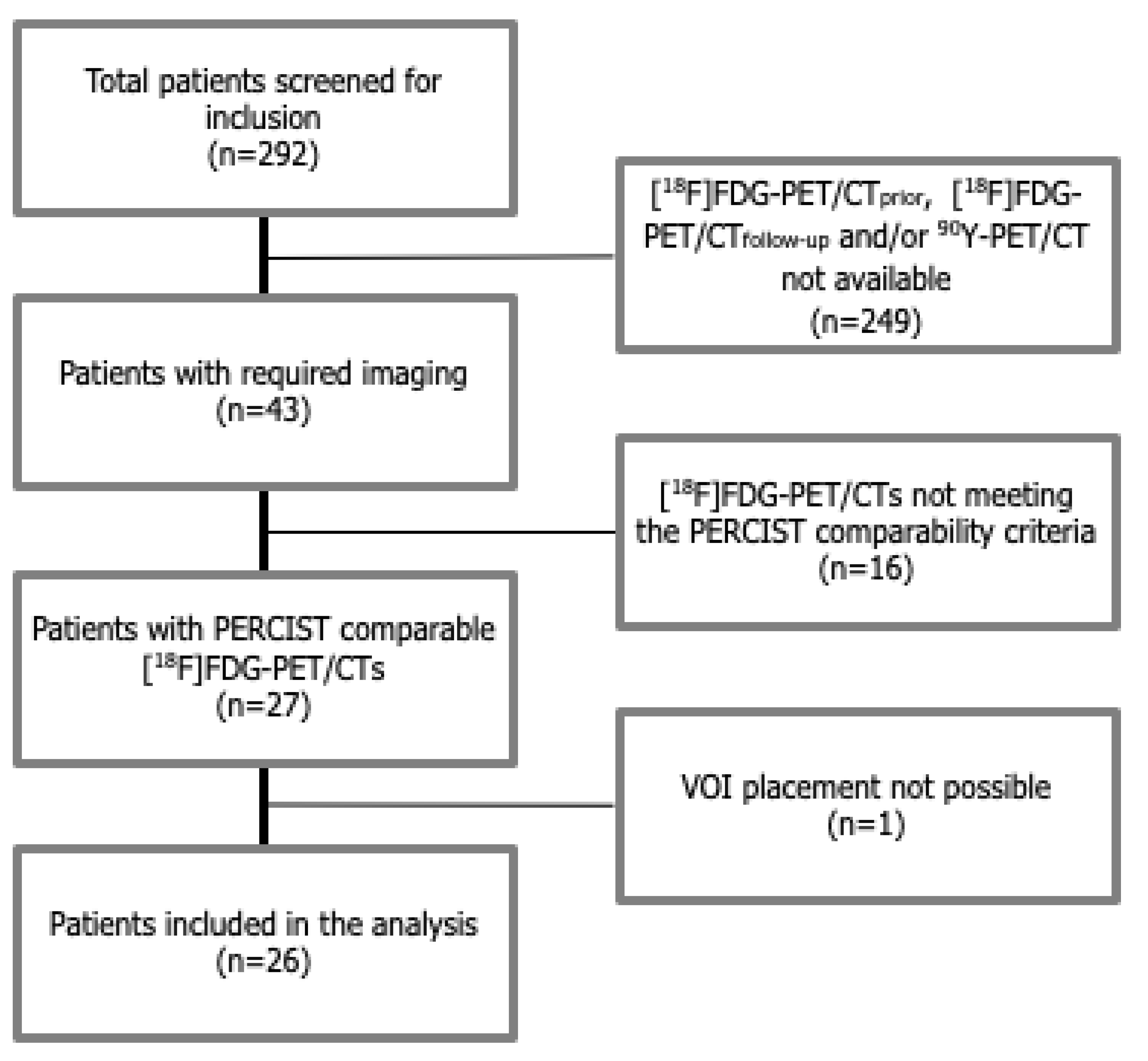
2. Methods
2.1. Study Population
2.2. Radioembolization Treatment
2.3. [18F]FDG-PET/CT Imaging Protocols
2.4. 90Y-PET/CT Imaging Protocol
2.5. Healthy Liver Parenchyma Analysis
2.6. Biochemical Changes
2.7. Statistical Analysis
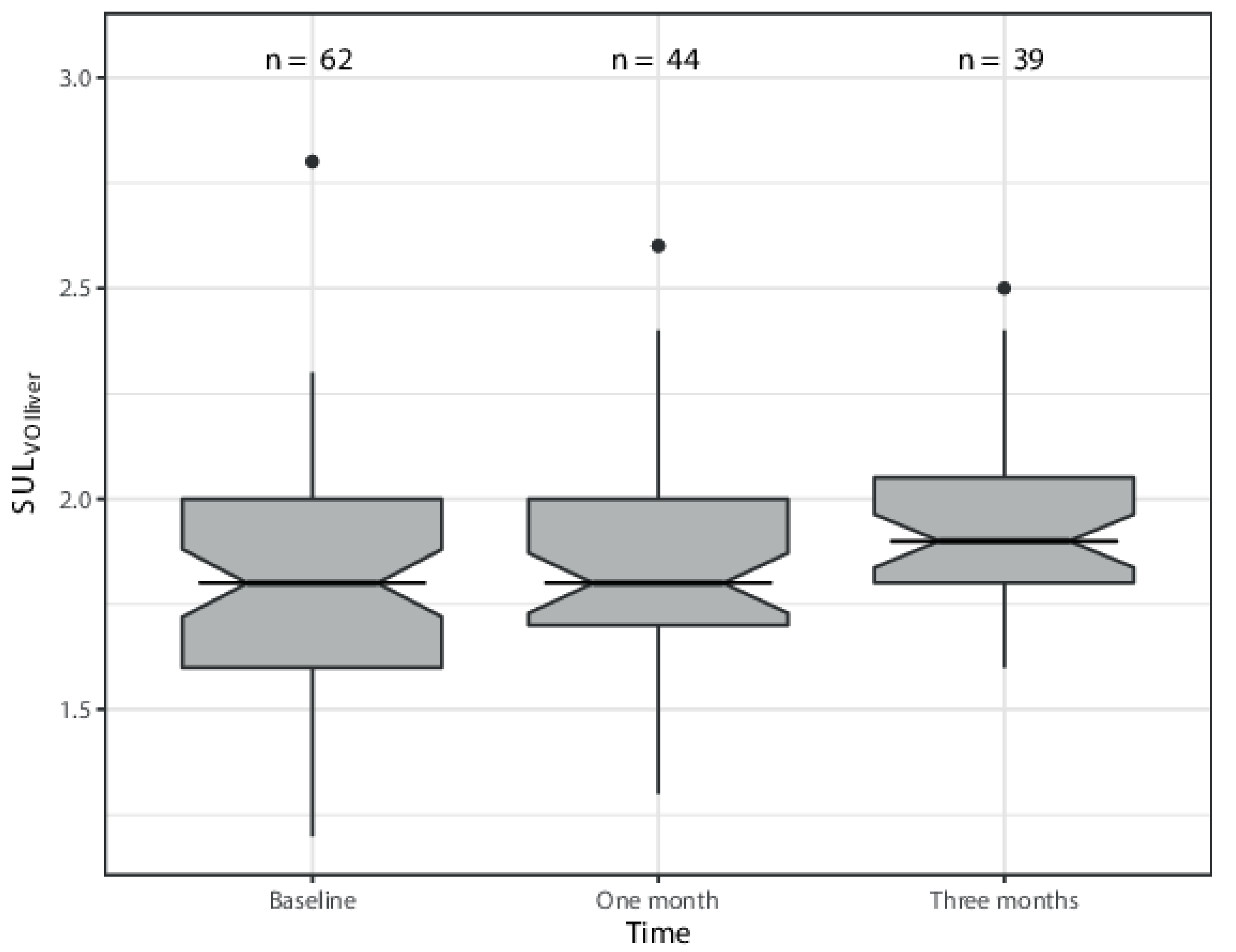
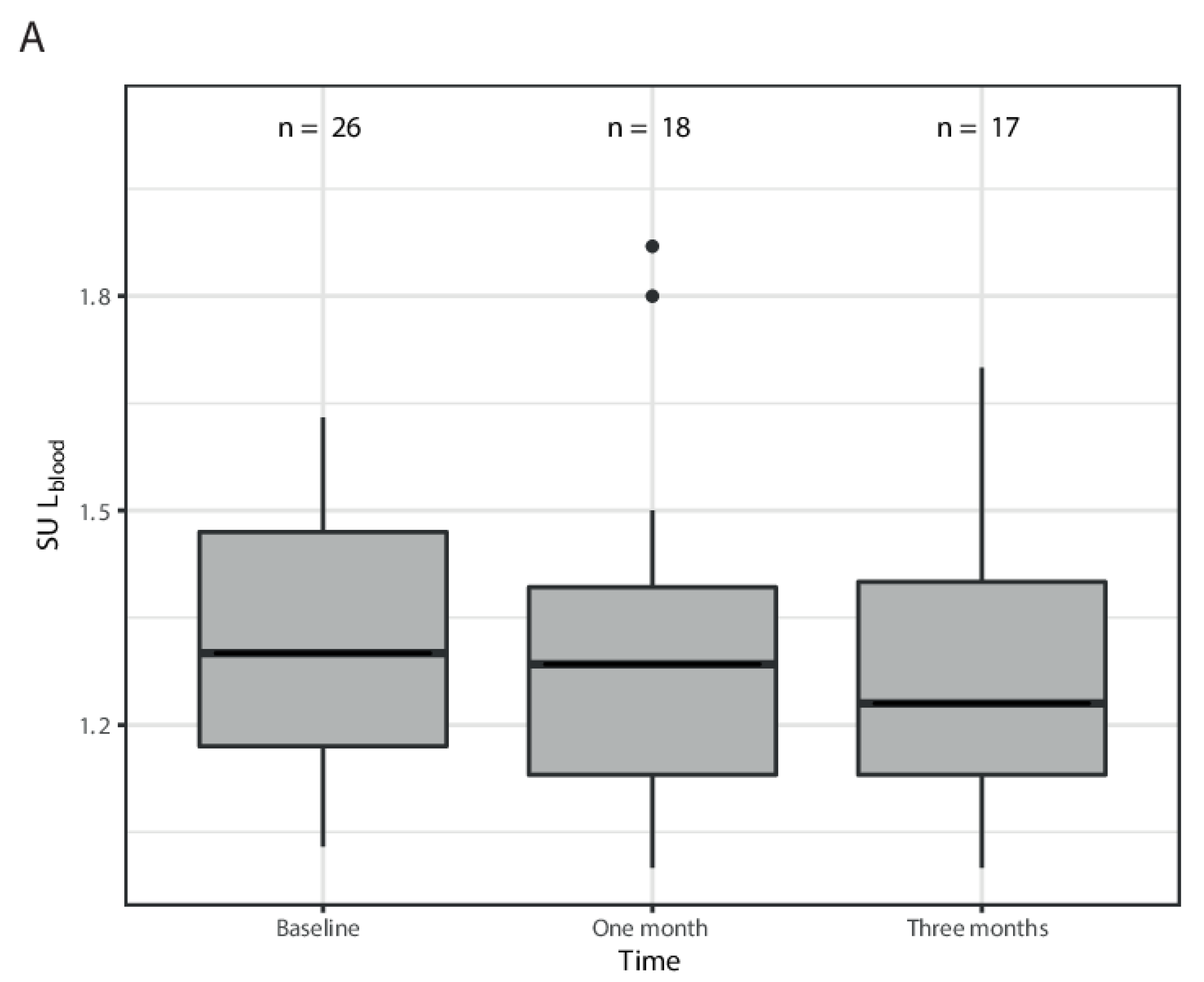
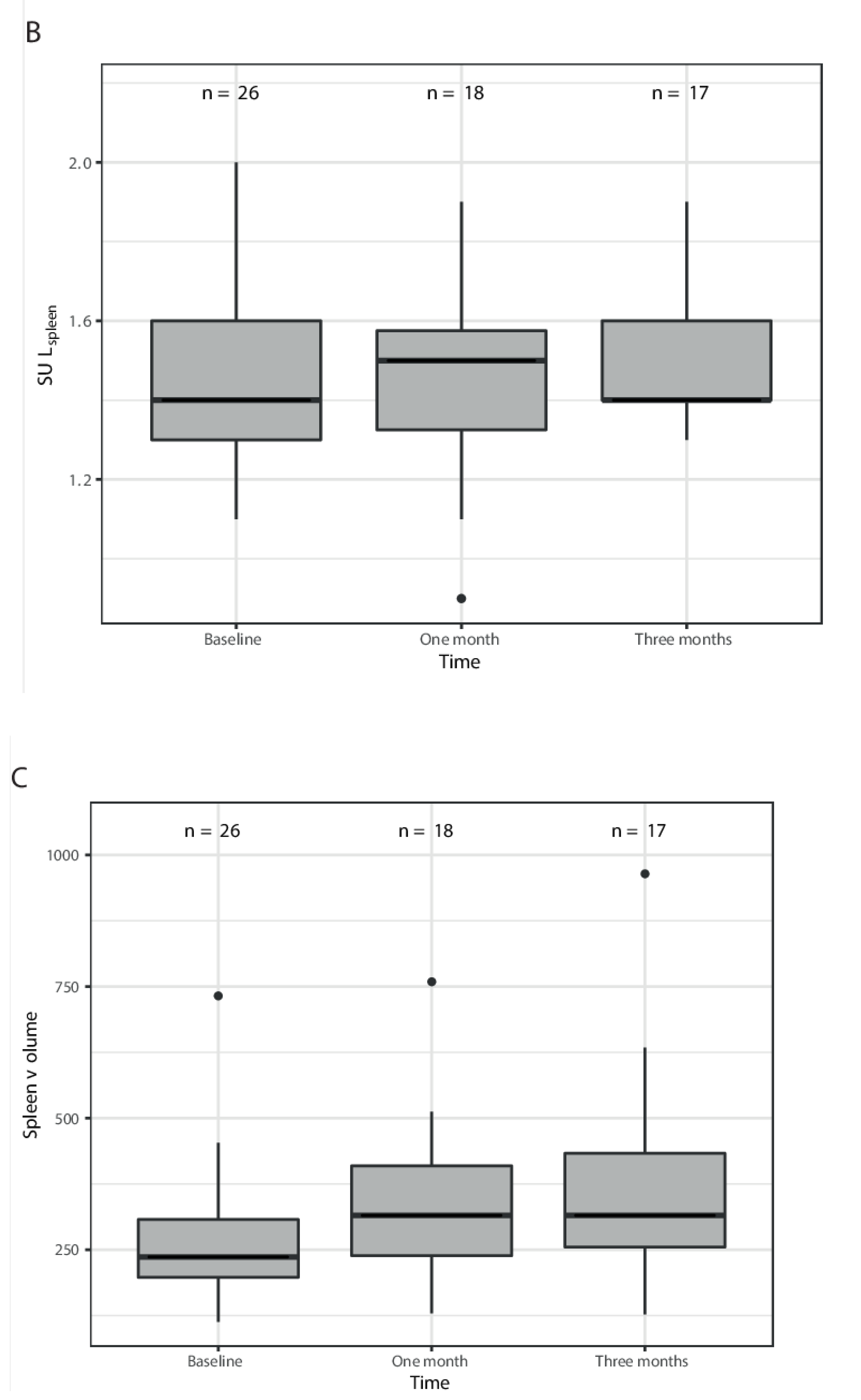
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50 (Suppl. 1), 122s–150s. [Google Scholar] [CrossRef] [PubMed]
- Young, H.; Baum, R.; Cremerius, U.; Herholz, K.; Hoekstra, O.; Lammertsma, A.A.; Pruim, J.; Price, P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur. J. Cancer. 1999, 35, 1773–1782. [Google Scholar] [CrossRef]
- Skougaard, K.; Nielsen, D.; Jensen, B.V.; Hendel, H.W. Comparison of EORTC criteria and PERCIST for PET/CT response evaluation of patients with metastatic colorectal cancer treated with irinotecan and cetuximab. J. Nucl. Med. 2013, 54, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Paquet, N.; Albert, A.; Foidart, J.; Hustinx, R. Within-patient variability of (18)F-FDG: Standardized uptake values in normal tissues. J. Nucl. Med. 2004, 45, 784–788. [Google Scholar]
- Kanstrup, I.L.; Klausen, T.L.; Bojsen-Moller, J.; Magnusson, P.; Zerahn, B. Variability and reproducibility of hepatic FDG uptake measured as SUV as well as tissue-to-blood background ratio using positron emission tomography in healthy humans. Clin. Physiol. Funct. Imaging 2009, 29, 108–113. [Google Scholar] [CrossRef]
- Boktor, R.R.; Walker, G.; Stacey, R.; Gledhill, S.; Pitman, A.G. Reference range for intrapatient variability in blood-pool and liver SUV for 18F-FDG PET. J. Nucl. Med. 2013, 54, 677–682. [Google Scholar] [CrossRef]
- Chiaravalloti, A.; Danieli, R.; Abbatiello, P.; Di Pietro, B.; Travascio, L.; Cantonetti, M.; Guazzaroni, M.; Orlacchio, A.; Simonetti, G.; Schillaci, O. Factors affecting intrapatient liver and mediastinal blood pool (1)(8)F-FDG standardized uptake value changes during ABVD chemotherapy in Hodgkin’s lymphoma. Eur. J. Nucl. Med. Mol. Imaging. 2014, 41, 1123–1132. [Google Scholar] [CrossRef]
- Ceriani, L.; Suriano, S.; Ruberto, T.; Zucca, E.; Giovanella, L. 18F-FDG uptake changes in liver and mediastinum during chemotherapy in patients with diffuse large B-cell lymphoma. Clin. Nucl. Med. 2012, 37, 949–952. [Google Scholar] [CrossRef]
- Rubello, D.; Gordien, P.; Morliere, C.; Guyot, M.; Bordenave, L.; Colletti, P.M.; Hindié, E. Variability of Hepatic 18F-FDG Uptake at Interim PET in Patients With Hodgkin Lymphoma. Clin. Nucl. Med. 2015, 40, e405–e410. [Google Scholar] [CrossRef]
- Fencl, P.; Belohlavek, O.; Harustiak, T.; Zemanova, M. The analysis of factors affecting the threshold on repeated 18F-FDG-PET/CT investigations measured by the PERCIST protocol in patients with esophageal carcinoma. Nucl. Med. Commun. 2012, 33, 1188–1194. [Google Scholar] [CrossRef]
- Barrington, S.F.; Qian, W.; Somer, E.J.; Franceschetto, A.; Bagni, B.; Brun, E.; Almquist, H.; Loft, A.; Højgaard, L.; Federico, M.; et al. Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Bagni, O.; Filippi, L.; Schillaci, O. The role of (18)F-FDG positron emission tomography in the follow-up of liver tumors treated with (90)Yttrium radioembolization. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 220–232. [Google Scholar] [PubMed]
- Fernandez-Ros, N.; Iñarrairaegui, M.; Paramo, A.J.; Berasain, C.; Avila, M.; Chopitea, A.; Varo, N.; Sarobe, P.; Bilbao, I.J.; Dominguez, I.; et al. Radioembolization of hepatocellular carcinoma activates liver regeneration, induces inflammation and endothelial stress and activates coagulation. Liver Int. 2015, 35, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Chew, V.; Lee, Y.H.; Pan, L.; Nasir, N.J.; Lim, C.J.; Chua, C.; Lai, L.; Hazirah, S.N.; Lim, T.K.H.; Goh, B.K.; et al. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut 2019, 68, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Nalesnik, M.A.; Federle, M.; Buck, D.; Fontes, P.; Carr, B.I. Hepatobiliary effects of 90yttrium microsphere therapy for unresectable hepatocellular carcinoma. Hum. Pathol. 2009, 40, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Shai, S.E.; Lin, Y.H.; Lai, Y.L.; Tang, H.W.; Hsieh, Y.W.; Hung, S.C. Phantom simulation of liver metastasis on a positron emission tomography with computed tomography scan after neoadjuvant chemoradiotherapy for distal esophageal cancer: A case report. J. Med. Case Rep. 2020, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Kaalep, A.; Sera, T.; Oyen, W.; Krause, B.J.; Chiti, A.; Liu, Y.; Boellaard, R. EANM/EARL FDG-PET/CT accreditation—summary results from the first 200 accredited imaging systems. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 412–422. [Google Scholar] [CrossRef]
- Viner, M.; Mercier, G.; Hao, F.; Malladi, A.; Subramaniam, R.M. Liver SULmean at FDG PET/CT: Interreader agreement and impact of placement of volume of interest. Radiology 2013, 267, 596–601. [Google Scholar] [CrossRef]
- Lam, M.G.; Banerjee, A.; Louie, J.D.; Sze, D.Y. Splenomegaly-associated thrombocytopenia after hepatic yttrium-90 radioembolization. Cardiovasc. Intervent. Radiol. 2014, 37, 1009–1017. [Google Scholar] [CrossRef]
- van den Hoven, A.F.; Rosenbaum, C.E.; Elias, S.G.; de Jong, H.W.; Koopman, M.; Verkooijen, H.M.; Alavi, A.; van den Bosch, M.A.; Lam, M.G. Insights into the Dose-Response Relationship of Radioembolization with Resin 90Y-Microspheres: A Prospective Cohort Study in Patients with Colorectal Cancer Liver Metastases. J. Nucl. Med. 2016, 57, 1014–1019. [Google Scholar] [CrossRef]
- Grant, M.J.; Didier, R.A.; Stevens, J.S.; Beyder, D.D.; Hunter, J.G.; Thomas, C.R.; Coakley, F.V. Radiation-induced liver disease as a mimic of liver metastases at serial PET/CT during neoadjuvant chemoradiation of distal esophageal cancer. Abdom. Imaging 2014, 39, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.B.; Balachandran, A.; Bruzzi, J.F.; Johnson, V.; Macapinlac, H.A. Munden RF. PET/CT and hepatic radiation injury in esophageal cancer patients. Cancer Imaging 2007, 7, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.J.; Anthony, M.P.; Lan Khong, P. Hepatic radiation injury in distal esophageal carcinoma: A case report. Clin. Nucl. Med. 2012, 37, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Takagi, Y.; Takemasa, K.; Mitsui, Y.; Tsuyuki, A.; Shigematsu, N.; Kubo, A. Dose-related fluorodeoxyglucose uptake in acute radiation-induced hepatitis. Eur. J. Gastroenterol. Hepatol. 2008, 20, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Bienert, M.; McCook, B.; Carr, B.I.; Geller, D.A.; Sheetz, M.; Tutor, C.; Amesur, N.; Avril, N. 90Y microsphere treatment of unresectable liver metastases: Changes in 18F-FDG uptake and tumour size on PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 778–787. [Google Scholar] [CrossRef]
- Bilbao, J.I.; de Martino, A.; de Luis, E.; Díaz-Dorronsoro, L.; Alonso-Burgos, A.; de la Cuesta, M.A.; Sangro, B.; García de Jalón, J.A. Biocompatibility, inflammatory response, and recannalization characteristics of nonradioactive resin microspheres: Histological findings. Cardiovasc. Intervent. Radiol. 2009, 32, 727–736. [Google Scholar] [CrossRef]
- Sangro, B.; Gil-Alzugaray, B.; Rodriguez, J.; Sola, I.; Martinez-Cuesta, A.; Viudez, A.; Chopitea, A.; Iñarrairaegui, M.; Arbizu, J.; Bilbao, J.I. Liver disease induced by radioembolization of liver tumors: Description and possible risk factors. Cancer 2008, 112, 1538–1546. [Google Scholar] [CrossRef]
- Pasciak, A.S.; Abiola, G.; Liddell, R.P.; Crookston, N.; Besharati, S.; Donahue, D.; Thompson, R.E.; Frey, E.; Anders, R.A.; Dreher, M.R. The number of microspheres in Y90 radioembolization directly affects normal tissue radiation exposure. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 816–827. [Google Scholar] [CrossRef]
- Higashi, K.; Clavo, A.C.; Wahl, R.L. In vitro assessment of 2-fluoro-2-deoxy-D-glucose, L-methionine and thymidine as agents to monitor the early response of a human adenocarcinoma cell line to radiotherapy. J. Nucl. Med. 1993, 34, 773–779. [Google Scholar]
- Kim, H.; Baek, S.E.; Moon, J.; Roh, Y.H.; Lee, N.; Cho, A. Increased hepatic FDG uptake on PET/CT in hepatic sinusoidal obstructive syndrome. Oncotarget 2016, 7, 69024–69031. [Google Scholar] [CrossRef]
- Kuruva, M.; Mittal, B.R.; Abrar, M.L.; Kashyap, R.; Bhattacharya, A. Multivariate analysis of various factors affecting background liver and mediastinal standardized uptake values. Indian J. Nucl. Med. 2012, 27, 20–23. [Google Scholar] [PubMed]
- Yuan, H.; Tong, D.K.; Vardhanabhuti, V.; Khong, P.L. Factors that affect PERCIST-defined test-retest comparability: An exploration of feasibility in routine clinical practice. Clin. Nucl. Med. 2015, 40, 941–944. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hofheinz, F.; Apostolova, I.; Oehme, L.; Kotzerke, J.; van den Hoff, J. Test-Retest Variability in Lesion SUV and Lesion SUR in (18)F-FDG PET: An Analysis of Data from Two Prospective Multicenter Trials. J. Nucl. Med. 2017, 58, 1770–1775. [Google Scholar] [CrossRef]
- Hofheinz, F.; Maus, J.; Zschaeck, S.; Rogasch, J.; Schramm, G.; Oehme, L.; Apostolova, I.; Kotzerke, J.; Hoff, J.V.D. Interobserver variability of image-derived arterial blood SUV in whole-body FDG PET. EJNMMI Res. 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Torizuka, T.; Tamaki, N.; Inokuma, T.; Magata, Y.; Sasayama, S.; Yonekura, Y.; Tanaka, A.; Yamaoka, Y.; Yamamoto, K.; Konishi, J. In vivo assessment of glucose metabolism in hepatocellular carcinoma with FDG-PET. J Nucl Med. 1995, 36, 1811–1817. [Google Scholar]
- Deipolyi, A.R.; England, R.W.; Ridouani, F.; Riedl, C.C.; Kunin, H.S.; Boas, F.E.; Yarmohammadi, H.; Sofocleous, C.T. PET/CT Imaging Characteristics After Radioembolization of Hepatic Metastasis from Breast Cancer. Cardiovasc. Intervent. Radiol. 2020, 43, 488–494. [Google Scholar] [CrossRef]


| Total Number of Evaluable Patients | 26 |
|---|---|
| Male/Female | 16/10 |
| Median age in years (range) | 66 (34–79) |
| Median time from baseline F-18 FDG-PET/CT to Y-90-RE in days (range) | 24 (1–44) |
| Median weight in kg (range) | 82 (57–110) |
| Number of patients known with diabetes mellitus | 1 |
| Number of patients with use of anti-diabetic medication | 1 |
Primary tumor
| 25 1 |
Prior locoregional treatment
| 3 1 3 1 |
| Median injected activity in MBq (range) | 1484 (345–2164) |
Treatment
| 22 1 3 |
| Prior systemic treatment | 26 |
No. of systemic treatment lines
| 9 12 4 1 |
| Time After RE | Baseline | One Month | Three Months |
|---|---|---|---|
| Number of examinations | 26 | 18 | 17 |
| Median (range) | |||
| Serum glucose level in mmol/L | 6.4 (5.1–9.8) | 6.3 (4.2–10.3) | 6.6 (4.4–10.4) |
| Post-injection time in minutes | 64.5 (56–85) | 62.5 (58–78) | 65 (51–86) |
| Injected activity in MBq/kg | 2.03 (1.81–2.37) | 2.03 (1.66–2.50) | 1.94 (1.69–2.62) |
| SULmean segment 2-3 | 1.8 (1.5–2.2) | 1.9 (1.5–2.6) | 1.95 (1.6–2.4) |
| SULmean segment 4 | 1.7 (1.5–2.1) | 1.7 (1.6–2.1) | 1.8 (1.6–2.2) |
| SULmean right hemiliver | 1.9 (1.2–2.8) | 1.7 (1.3–2.6) | 1.9 (1.7–2.5) |
| SULmean spleen | 1.4 (1.1–2.0) | 1.5 (0.9–1.9) | 1.5 (1.3–1.9) |
| SULmean bloodpool | 1.3 (1.03–1.63) | 1.27 (1.00–1.87) | 1.23 (1.00–1.70) |
| Independent Variable | Mean Change in SULVOI Per Month Increase in Time (95%CI); p-Value | |
|---|---|---|
| SULVOI (patient-level) | Unadjusted | Adjusted (for administered activity Y90) |
| 0.05 (0.01–0.08); 0.016 | 0.03 (−0.04–0.1); 0.38 | |
| SULVOI,liver (VOI-level) | Unadjusted | Adjusted (for absorbed dose per VOI) |
| 0.05 (0.02–0.09), 0.00088 | 0.05 (0.02–0.08); 0.00084 | |
| CTCAE Grade | 1 | 2 | 3 |
|---|---|---|---|
| Alanine aminotransferase | 11 | ||
| Alkaline phosphatase | 6 | 3 | |
| Aspartate aminotransferase | 14 | 1 | |
| Bilirubin | 5 | 1 | 1 |
| Gamma-glutamyl transferase | 3 | 5 | 3 |
| Platelets | 9 | 2 | |
| APTT | 13 | 1 | 1 |
| Clinically Relevant Biochemical Toxicity Yes Versus No | ||
|---|---|---|
| Dependent variable | Odds ratio for clinically relevant biochemical toxicity for every month increase in SULVOI,liver (95% CI); p-value | |
| Unadjusted model | Adjusted model (for progressive disease) | |
| Clinically relevant biochemical toxicity (based on laboratory parameters) (no n = 14, yes n = 21) | 0.42 (0.0006–296.82); 0.69 | 0.48 (0.0006–333.29); 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braat, M.N.; van Roekel, C.; Lam, M.G.; Braat, A.J. Radioembolization-Induced Changes in Hepatic [18F]FDG Metabolism in Non-Tumorous Liver Parenchyma. Diagnostics 2022, 12, 2518. https://doi.org/10.3390/diagnostics12102518
Braat MN, van Roekel C, Lam MG, Braat AJ. Radioembolization-Induced Changes in Hepatic [18F]FDG Metabolism in Non-Tumorous Liver Parenchyma. Diagnostics. 2022; 12(10):2518. https://doi.org/10.3390/diagnostics12102518
Chicago/Turabian StyleBraat, Manon N., Caren van Roekel, Marnix G. Lam, and Arthur J. Braat. 2022. "Radioembolization-Induced Changes in Hepatic [18F]FDG Metabolism in Non-Tumorous Liver Parenchyma" Diagnostics 12, no. 10: 2518. https://doi.org/10.3390/diagnostics12102518
APA StyleBraat, M. N., van Roekel, C., Lam, M. G., & Braat, A. J. (2022). Radioembolization-Induced Changes in Hepatic [18F]FDG Metabolism in Non-Tumorous Liver Parenchyma. Diagnostics, 12(10), 2518. https://doi.org/10.3390/diagnostics12102518






