Expiratory Technique versus Tracheal Suction to Obtain Good-Quality Sputum from Patients with Suspected Lower Respiratory Tract Infection: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Selection of Participants
2.3. Randomization and Masking
2.4. Interventions
2.5. Outcome Measures
2.6. Statistical Analysis
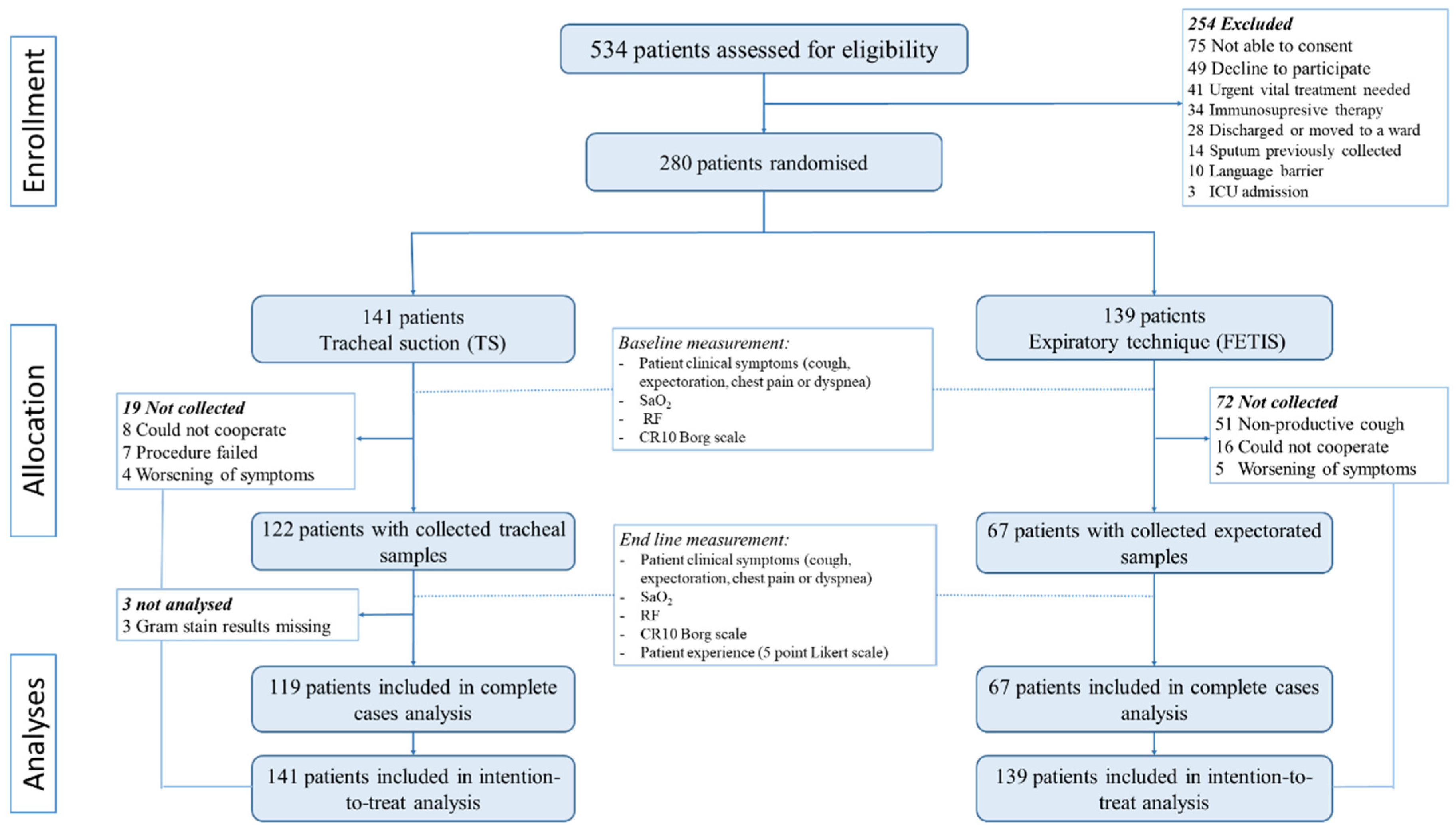
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef] [PubMed]
- Eccles, S.; Pincus, C.; Higgins, B.; Woodhead, M. Diagnosis and management of community and hospital acquired pneumonia in adults: Summary of NICE guidance. BMJ 2014, 349, g6722. [Google Scholar] [CrossRef] [PubMed]
- Del Rio-Pertuz, G.; Gutiérrez, J.F.; Triana, A.J.; Molinares, J.L.; Robledo-Solano, A.B.; Meza, J.L.; Ariza-Bolívar, O.M.; Acosta-Reyes, J.; Garavito, A.; Viasus, D.; et al. Usefulness of sputum gram stain for etiologic diagnosis in community-acquired pneumonia: A systematic review and meta-analysis. BMC Infect. Dis. 2019, 19, 403. [Google Scholar] [CrossRef]
- Ogawa, H.; Kitsios, G.D.; Iwata, M.; Terasawa, T. Sputum Gram Stain for Bacterial Pathogen Diagnosis in Community-acquired Pneumonia: A Systematic Review and Bayesian Meta-analysis of Diagnostic Accuracy and Yield. Clin. Infect. Dis. 2020, 71, 499–513. [Google Scholar] [CrossRef]
- Roson, B.; Carratala, J.; Verdaguer, R.; Dorca, J.; Manresa, F.; Gudiol, F. Prospective study of the usefulness of sputum Gram stain in the initial approach to community-acquired pneumonia requiring hospitalization. Clin. Infect. Dis. 2000, 31, 869–874. [Google Scholar] [CrossRef]
- Garcia-Vazquez, E.; Marcos, M.A.; Mensa, J.; de Roux, A.; Puig, J.; Font, C.; Francisco, G.; Torres, A. Assessment of the usefulness of sputum culture for diagnosis of community-acquired pneumonia using the PORT predictive scoring system. Arch. Intern. Med. 2004, 164, 1807–1811. [Google Scholar] [CrossRef]
- Ewig, S.; Schlochtermeier, M.; Goke, N.; Niederman, M.S. Applying sputum as a diagnostic tool in pneumonia: Limited yield, minimal impact on treatment decisions. Chest 2002, 121, 1486–1492. [Google Scholar] [CrossRef]
- Lewis, L.K.; Williams, M.T.; Olds, T.S. The active cycle of breathing technique: A systematic review and meta-analysis. Respir. Med. 2012, 106, 155–172. [Google Scholar] [CrossRef]
- Andrews, J.; Sathe, N.A.; Krishnaswami, S.; McPheeters, M.L. Nonpharmacologic airway clearance techniques in hospitalized patients: A systematic review. Respir. Care 2013, 58, 2160–2186. [Google Scholar] [CrossRef]
- Overend, T.J.; Anderson, C.M.; Brooks, D.; Cicutto, L.; Keim, M.; McAuslan, D.; Nonoyama, M. Updating the evidence base for suctioning adult patients: A systematic review. Can. Respir. J. 2009, 16, e6–e17. [Google Scholar] [CrossRef] [PubMed]
- Geckler, R.W.; Gremillion, D.H.; McAllister, C.K.; Ellenbogen, C. Microscopic and bacteriological comparison of paired sputa and transtracheal aspirates. J. Clin. Microbiol. 1977, 6, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, J.; Saksena, R.; Jain, M.; Gaind, R. Can sputum gram stain be used to predict lower respiratory tract infection and guide empiric antimicrobial treatment: Experience from a tertiary care hospital. J. Microbiol. Methods 2019, 166, 105731. [Google Scholar] [CrossRef] [PubMed]
- Nagendra, S.; Bourbeau, P.; Brecher, S.; Dunne, M.; LaRocco, M.; Doern, G. Sampling variability in the microbiological evaluation of expectorated sputa and endotracheal aspirates. J. Clin. Microbiol. 2001, 39, 2344–2347. [Google Scholar] [CrossRef]
- Tetenta, S.; Metersky, M.L. Tracheal aspirate Gram stain has limited sensitivity and specificity for detecting Staphylococcus aureus. Respirology 2011, 16, 86–89. [Google Scholar] [CrossRef]
- Arroyo-Novoa, C.M.; Figueroa-Ramos, M.I.; Puntillo, K.A.; Stanik-Hutt, J.; Thompson, C.L.; White, C.; Wild, L.R. Pain related to tracheal suctioning in awake acutely and critically ill adults: A descriptive study. Intensive Crit. Care Nurs. 2008, 24, 20–27. [Google Scholar] [CrossRef]
- Van de Leur, J.P.; Zwaveling, J.H.; Loef, B.G.; Van der Schans, C.P. Endotracheal suctioning versus minimally invasive airway suctioning in intubated patients: A prospective randomised controlled trial. Intensive Care Med. 2003, 29, 426–432. [Google Scholar] [CrossRef]
- Chanez, P.; Holz, O.; Ind, P.; Djukanović, R.; Maestrelli, P.; Sterk, P. Sputum induction. Eur. Respir. J. 2002, 20, 3s–8s. [Google Scholar]
- Bandyopadhyay, T.; Gerardi, D.A.; Metersky, M.L. A comparison of induced and expectorated sputum for the microbiological diagnosis of community acquired pneumonia. Respiration 2000, 67, 173–176. [Google Scholar] [CrossRef]
- Butov, D.; Feshchenko, Y.; Myasoedov, V.; Kuzhko, M.; Gumeniuk, M.; Gumeniuk, G.; Tkachenko, A.; Nataliya, N.; Borysova, O.; Butova, T. Effectiveness of inhaled hypertonic saline application for sputum induction to improve Mycobacterium tuberculosis identification in patients with pulmonary tuberculosis. Wien. Med. Wochenschr. 2022, 172, 261–267. [Google Scholar] [CrossRef]
- Pizzichini, M.; Leigh, R.; Djukanović, R.; Sterk, P. Safety of sputum induction. Eur. Respir. J. 2002, 20, 9s–18s. [Google Scholar]
- Herrero-Cortina, B.; Alcaraz, V.; Vilaró, J.; Torres, A.; Polverino, E. Impact of hypertonic saline solutions on sputum expectoration and their safety profile in patients with bronchiectasis: A randomized crossover trial. J. Aerosol Med. Pulm. Drug Deliv. 2018, 31, 281–289. [Google Scholar] [CrossRef] [PubMed]
- McCauley, L.M.; Webb, B.J.; Sorensen, J.; Dean, N.C. Use of Tracheal Aspirate Culture in Newly Intubated Patients with Community-Onset Pneumonia. Ann. Am. Thorac. Soc. 2016, 13, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.M.; Binnicker, M.J.; Campbell, S.; Carroll, K.C.; Chapin, K.C.; Gilligan, P.H.; Gonzalez, M.D.; Jerris, R.C.; Kehl, S.C.; Patel, R. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin. Infect. Dis. 2018, 67, e1–e94. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef] [PubMed]
- Cartuliares, M.B.; Skjøt-Arkil, H.; Rosenvinge, F.S.; Mogensen, C.B.; Skovsted, T.A.; Pedersen, A.K. Effectiveness of expiratory technique and induced sputum in obtaining good quality sputum from patients acutely hospitalized with suspected lower respiratory tract infection: A statistical analysis plan for a randomized controlled trial. Trials 2021, 22, 675. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Murray, P.R.; Washington, J.A. Microscopic and baceriologic analysis of expectorated sputum. Mayo Clin. Proc. 1975, 50, 339–344. [Google Scholar]
- Möller, S.; Bliddal, M.; Rubin, K.H. Methodical considerations on adjusting for Charlson Comorbidity Index in epidemiological studies. Eur. J. Epidemiol. 2021, 36, 1123–1128. [Google Scholar] [CrossRef]
- Anevlavis, S.; Petroglou, N.; Tzavaras, A.; Maltezos, E.; Pneumatikos, I.; Froudarakis, M.; Anevlavis, E.; Bouros, D. A prospective study of the diagnostic utility of sputum Gram stain in pneumonia. J. Infect. 2009, 59, 83–89. [Google Scholar] [CrossRef]
- Datta, S.; Shah, L.; Gilman, R.H.; Evans, C.A. Comparison of sputum collection methods for tuberculosis diagnosis: A systematic review and pairwise and network meta-analysis. Lancet Glob. Health 2017, 5, e760–e771. [Google Scholar] [CrossRef]
- Chuard, C.; Fracheboud, D.; Regamey, C. Effect of sputum induction by hypertonic saline on specimen quality. Diagn. Microbiol. Infect. Dis. 2001, 39, 211–214. [Google Scholar] [CrossRef]
- Miller, R.F.; Kocjan, G.; Buckland, J.; Holton, J.; Malin, A.; Semple, S.J. Sputum induction for the diagnosis of pulmonary disease in HIV positive patients. J. Infect. 1991, 23, 5–15. [Google Scholar] [CrossRef]
| TS (n = 141) | FETIS (n = 139) | Total (n = 280) | |
|---|---|---|---|
| Hospital Sønderjylland | |||
| ED in Aabenraa | 116 (82%) | 112 (81%) | 228 (81%) |
| ED in Sønderborg | 25 (18%) | 27 (19%) | 52 (18%) |
| Sex (male) | 79 (56%) | 82 (59%) | 161 (58%) |
| Age, mean years | 72.9 (12.3) | 71.5 (12.7) | 72.2 (12.5) |
| Nursing home resident | 8 (6%) | 4 (3%) | 12 (4%) |
| Smoking status | |||
| Non-smokers | 38 (27%) | 32 (23%) | 70 (25%) |
| Ex-smokers | 76 (54%) | 83 (60%) | 159 (57%) |
| Current smokers | 26 (18%) | 24 (17%) | 50 (18%) |
| Length of hospital stay †, days | 5.0 (2.1; 8.0) | 4.0 (1.9; 6.9) | 4.1 (2.0; 7.1) |
| SYMPTOMS | |||
| Cough | 86 (61%) | 81 (58%) | 167 (60%) |
| Expectoration | 84 (60%) | 77 (55%) | 161 (58%) |
| Chest tightness | 45 (32%) | 49 (35%) | 94 (34%) |
| Dyspnoea | 96 (68%) | 92 (66%) | 188 (67%) |
| SEVERITY ASSESSMENT † | |||
| CURB-65 * | |||
| Mild 0–1 | 62 (50%) | 67 (60%) | 129 (53%) |
| Moderate 2 | 43 (34%) | 42 (35%) | 85 (35%) |
| Severe 3–5 | 20 (16%) | 11 (9%) | 31 (13%) |
| Triage ** | |||
| Triage level 1 | 8 (6%) | 10 (7%) | 18 (7%) |
| Triage level 2–3 | 99 (71%) | 105 (76%) | 204 (73%) |
| Triage level 4–5 | 33 (24%) | 24 (17%) | 57 (20%) |
| Suspicion of pneumonia | 99 (70%) | 100 (72%) | 199 (71%) |
| SARS-CoV-2 positive | 24 (17%) | 16 (12%) | 40 (14%) |
| COMORBIDITIES † | |||
| Any comorbidity | 128 (91%) | 119 (86%) | 247 (88%) |
| Respiratory diseases | 66 (52%) | 64 (55%) | 130 (54%) |
| COPD *** | 50 (36%) | 53 (38%) | 103 (37%) |
| Cardiovascular Diseases | 85 (68%) | 78 (68%) | 163 (68%) |
| Neurological diseases | 24 (19%) | 25 (22%) | 49 (20%) |
| DM **** | 29 (21%) | 32 (23%) | 61 (22%) |
| Cancer | 30 (21%) | 23 (17%) | 53 (19%) |
| Other diseases | 60 (43%) | 61 (44%) | 121 (43%) |
| VITAL PARAMETERS | |||
| Oxygen saturation, % | 95.0 (93.0; 97.0) | 96.0 (93.0; 98.0) | 95.0 (93.0; 97.0) |
| Respiratory rate/min | 21.0 (18.0; 24.0) | 21.0 (18.0; 24.0) | 21.0 (18.0; 24.0) |
| Heart rate/min | 91.6 (21.6) | 90.1 (17.3) | 90.9 (19.6) |
| Systolic Blood pressure, mmHg | 130.9 (20.8) | 134.3 (22.6) | 132.6 (21.8) |
| Diastolic blood pressure, mmHg | 71.9 (14.5) | 74.7 (16.4) | 73.3 (15.5) |
| Fever > 38 °C | 42 (30%) | 45 (32%) | 87 (31%) |
| Altered mental status | 13 (10%) | 9 (7%) | 22 (8%) |
| BLOOD TESTS | |||
| C-reactive protein, mg/L | 74.0 (20.0; 168.0) | 46.0 (16.0; 116.0) | 54.0 (19.0; 149.0) |
| Leucocytes, 109/L | 10.8 (8.0; 14.5) | 10.4 (7.5; 14.1) | 10.7 (7.9; 14.2) |
| Neutrophilocytes, 109/L | 8.4 (5.9; 11.8) | 7.9 (5.2; 11.0) | 8.2 (5.5; 11.3) |
| ANTIBIOTIC TREATMENT | |||
| Within one month | 47 (33%) | 48 (35%) | 95 (34%) |
| Prior sputum collection | 58 (41%) | 56 (40%) | 114 (41%) |
| Inhaled medications | 33 (23%) | 38 (27%) | 71 (25%) |
| Primary Outcome | Unadjusted OR (95% CI) | p-Value |
| Quality of sputum samples | 1.83 (1.05; 3.19) | 0.035 |
| Secondary Outcome | Unadjusted IRR (95% CI) | p-Value |
| Adverse effects | 1.21 (0.94; 1.66) | 0.136 |
| Patient experience of sputum collection procedure | N/A | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cartuliares, M.B.; Rosenvinge, F.S.; Mogensen, C.B.; Skovsted, T.A.; Andersen, S.L.; Pedersen, A.K.; Skjøt-Arkil, H. Expiratory Technique versus Tracheal Suction to Obtain Good-Quality Sputum from Patients with Suspected Lower Respiratory Tract Infection: A Randomized Controlled Trial. Diagnostics 2022, 12, 2504. https://doi.org/10.3390/diagnostics12102504
Cartuliares MB, Rosenvinge FS, Mogensen CB, Skovsted TA, Andersen SL, Pedersen AK, Skjøt-Arkil H. Expiratory Technique versus Tracheal Suction to Obtain Good-Quality Sputum from Patients with Suspected Lower Respiratory Tract Infection: A Randomized Controlled Trial. Diagnostics. 2022; 12(10):2504. https://doi.org/10.3390/diagnostics12102504
Chicago/Turabian StyleCartuliares, Mariana B., Flemming S. Rosenvinge, Christian B. Mogensen, Thor A. Skovsted, Steen L. Andersen, Andreas K. Pedersen, and Helene Skjøt-Arkil. 2022. "Expiratory Technique versus Tracheal Suction to Obtain Good-Quality Sputum from Patients with Suspected Lower Respiratory Tract Infection: A Randomized Controlled Trial" Diagnostics 12, no. 10: 2504. https://doi.org/10.3390/diagnostics12102504
APA StyleCartuliares, M. B., Rosenvinge, F. S., Mogensen, C. B., Skovsted, T. A., Andersen, S. L., Pedersen, A. K., & Skjøt-Arkil, H. (2022). Expiratory Technique versus Tracheal Suction to Obtain Good-Quality Sputum from Patients with Suspected Lower Respiratory Tract Infection: A Randomized Controlled Trial. Diagnostics, 12(10), 2504. https://doi.org/10.3390/diagnostics12102504






