Visible Lymph Affluents in the D3 Volume: An MDCTA Pictorial Essay
Abstract
1. Introduction
1.1. Literature Review
1.2. Aim
2. Material and Methods
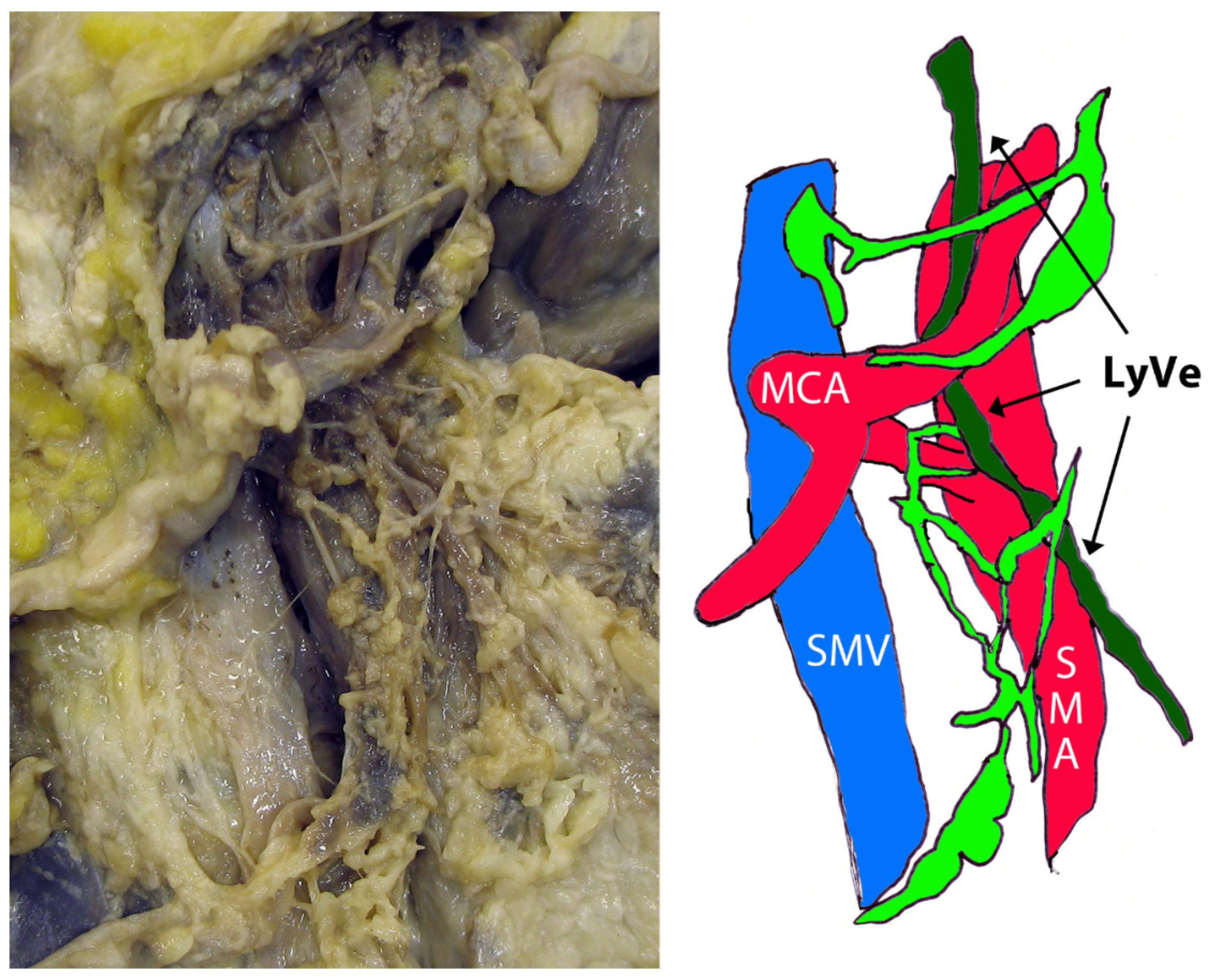
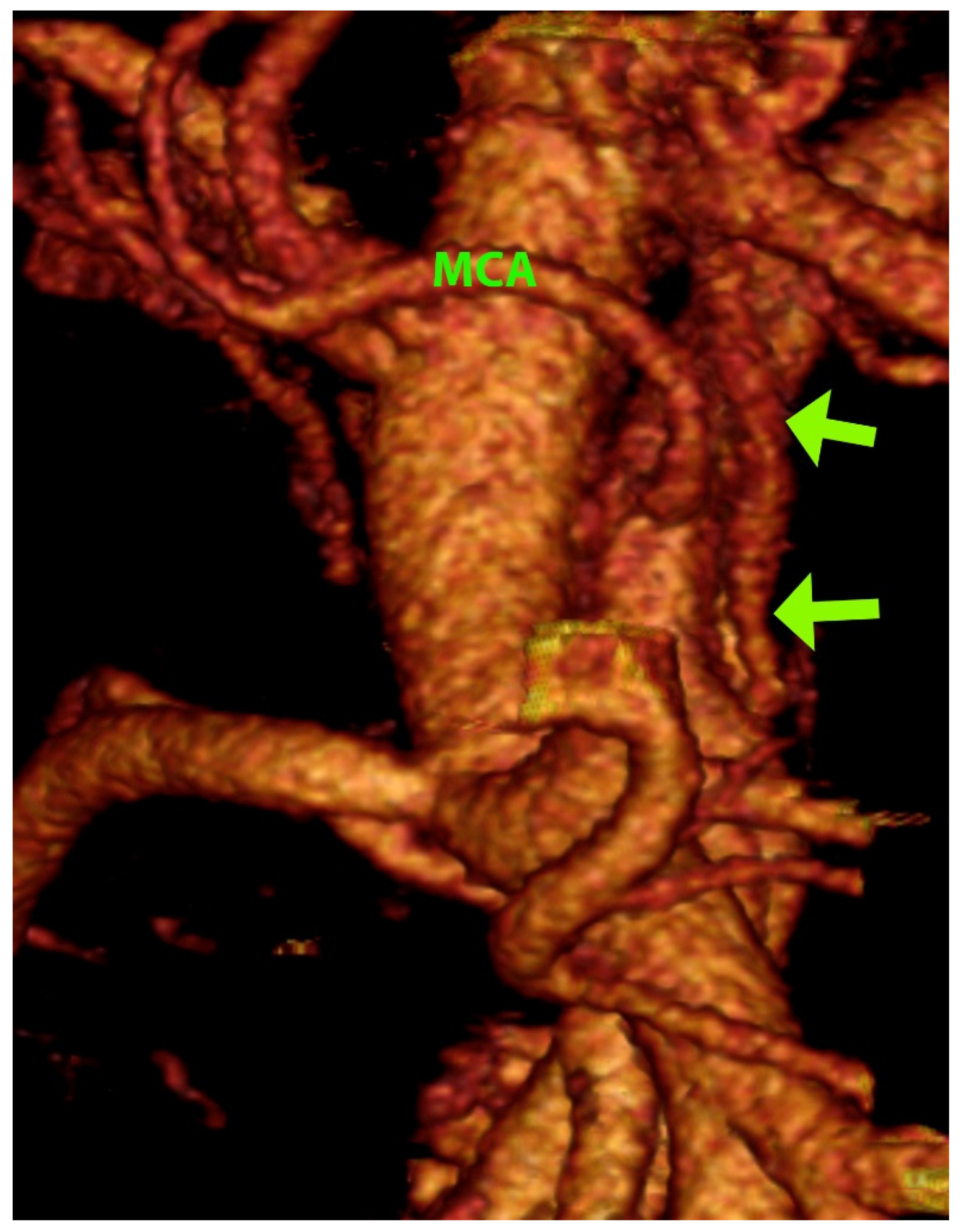
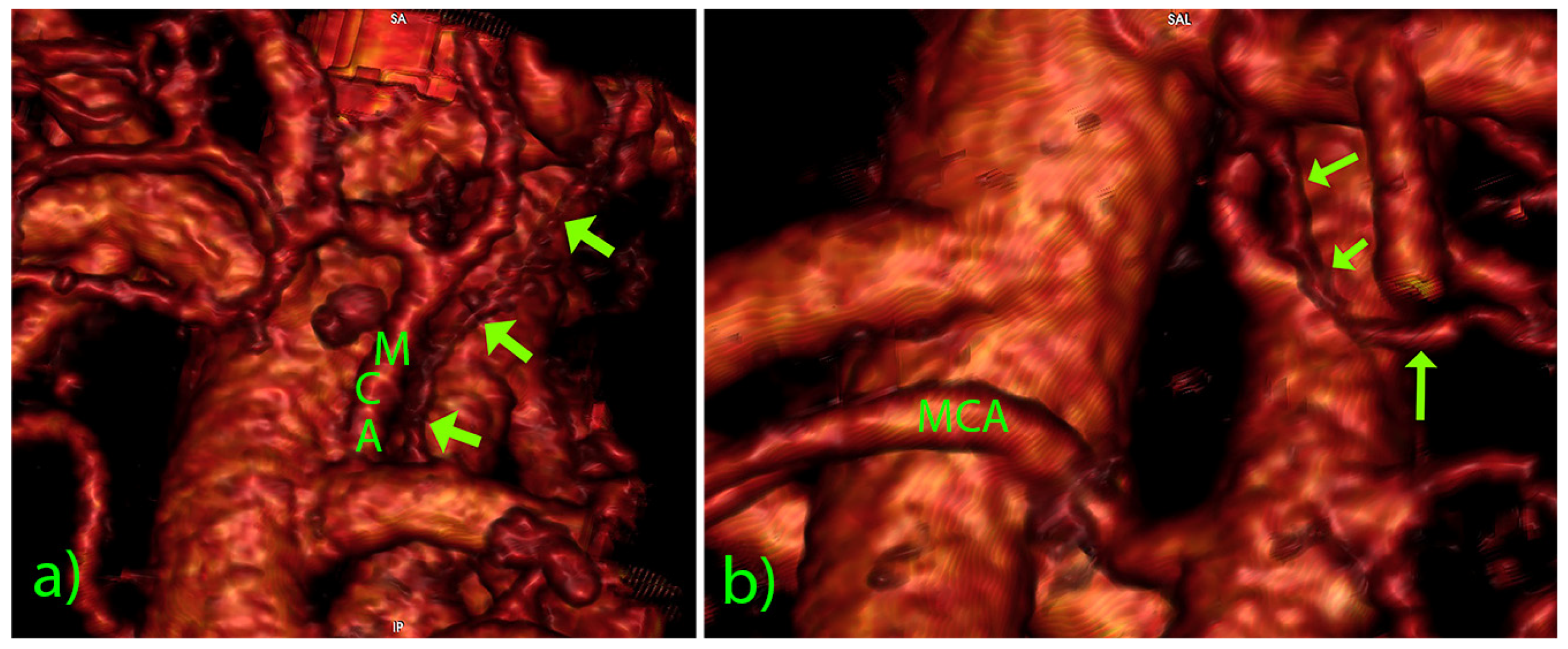
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skandalakis, J.E.; Skandalakis, L.J.; Skandalakis, P.N. Anatomy of the lymphatics. Surg. Oncol. Clin. N. Am. 2007, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.K.; Khaja, M.S.; Downing, T.; Kokabi, N.; Saad, W.E.; Majdalany, B.S. Lymphatic Anatomy and Physiology. Semin. Interv. Radiol. 2020, 37, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Sung, H.K.; Koh, G.Y. Lymphatic development in mouse small intestine. Dev. Dyn. 2007, 236, 2020–2025. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Ota, M.; Suwa, Y.; Ishibe, A.; Masui, H.; Nagahori, K. Real-Time Indocyanine Green Fluorescence Imaging-Guided Complete Mesocolic Excision in Laparoscopic Flexural Colon Cancer Surgery. Dis. Colon Rectum 2016, 59, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Stelzner, S.; Hohenberger, W.; Weber, K.; West, N.P.; Witzigmann, H.; Wedel, T. Anatomy of the transverse colon revisited with respect to complete mesocolic excision and possible pathways of aberrant lymphatic tumor spread. Int. J. Colorectal. Dis. 2016, 31, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Luzon, J.A.; Thorsen, Y.; Nogueira, L.P.; Andersen, S.N.; Edwin, B.; Haugen, H.J.; Ignjatovic, D.; Stimec, B.V. Reconstructing topography and extent of injury to the superior mesenteric artery plexus in right colectomy with extended D3 mesenterectomy: A composite multimodal 3-dimensional analysis. Surg. Endosc. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Nesgaard, J.M.; Stimec, B.V.; Soulie, P.; Edwin, B.; Bakka, A.; Ignjatovic, D. Defining minimal clearances for adequate lymphatic resection relevant to right colectomy for cancer: A post-mortem study. Surg. Endosc. 2018, 32, 3806–3812. [Google Scholar] [CrossRef] [PubMed]
- Culligan, K.; Sehgal, R.; Mulligan, D.; Dunne, C.; Walsh, S.; Quondamatteo, F.; Dockery, P.; Coffey, J.C. A detailed appraisal of mesocolic lymphangiology—An immunohistochemical and stereological analysis. J. Anat. 2014, 225, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Borisov, A.V. Recent data on morphology of the lymph vessels of the mesentery of the small intestine in man. Arkh. Anat. Gistol. Embriol. 1958, 35, 75–81. [Google Scholar] [PubMed]
- Erden, A.; Fitoz, S.; Yagmurlu, B.; Erden, I. Abdominal confluence of lymph trunks: Detectability and morphology on heavily T2-weighted images. AJR Am. J. Roentgenol. 2005, 184, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Loukas, M.; Wartmann, C.T.; Louis, R.G., Jr.; Tubbs, R.S.; Salter, E.G.; Gupta, A.A.; Curry, B. Cisterna chyli: A detailed anatomic investigation. Clin. Anat. 2007, 20, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Niggemann, P.; Förg, A.; Grosskurth, D.; Beyer, H.-K. Postural effect on the size of the cisterna chyli. Lymphat. Res. Biol. 2010, 8, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Agustsdottir, E.E.S.; Stimec, B.V.; Stroemmen, T.T.; Sheikh, A.E.; Elaiyarajah, I.; Lindstroem, J.C.; Ignjatovic, D. Preventing chylous ascites after right hemicolectomy with D3 extended mesenterectomy. Langenbecks Arch. Surg. 2020, 405, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Cesmebasi, A.; Malefant, J.; Patel, S.D.; Du Plessis, M.; Renna, S.; Tubbs, R.S.; Loukas, M. The surgical anatomy of the lymphatic system of the pancreas. Clin. Anat. 2015, 28, 527–537. [Google Scholar] [CrossRef]
- van Schaik, C.J.; Boer, L.L.; Draaisma, J.M.T.; van der Vleuten, C.J.M.; Janssen, J.J.; Fütterer, J.J.; Schultze Kool, L.J.; Klein, W.M. The lymphatic system throughout history: From hieroglyphic translations to state of the art radiological techniques. Clin. Anat. 2022, 35, 701–710. [Google Scholar] [CrossRef]
- Sun, X.; Shen, W.; Chen, X.; Wen, T.; Duan, Y.; Wang, R. Primary intestinal lymphangiectasia: Multiple detector computed tomography findings after direct lymphangiography. J. Med. Imaging Radiat. Oncol. 2017, 61, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Ignjatovic, D. Safe D3 Right Hemicolectomy for Cancer through Multidetector Computed Tomography (MDCT) Angio. Available online: https://clinicaltrials.gov/ct2/show/NCT01351714 (accessed on 18 September 2022).
- Japanese Society for Cancer of the Colon and Rectum, Lymph Node Groups. Japanese Classification of Colorectal Carcinoma, 2nd ed.; Sugihara, K., Ed.; Kanehara & Co., Ltd.: Tokyo, Japan, 2009; pp. 8–10. (In English)
- Kubik, S. Visceral lymphatic system. In Atlas of Lymphography; Viamonte, M., Jr., Rüttimann, A., Eds.; Georg Thieme Verlag: Stuttgart, Germany, 1980; pp. 91–106. ISBN 3-13-547801-7. [Google Scholar]
- Rouvière, H. Ganglions viscéraux de l’abdomen. In Anatomie des Lymphatiques de L’homme; Masson: Paris, France, 1981; pp. 303–322. ISBN 2-225-69388-9. [Google Scholar]
- Servelle, M.; Nogués, C. Anatomy of the lymphatics of the small intestine. In The Chyliferous Vessels; Expansion Scientifique Française: Paris, France, 1981; pp. 1–9. ISBN 2-7046-1090-8. [Google Scholar]
- Fanous, M.Y.; Phillips, A.J.; Windsor, J.A. Mesenteric lymph: The bridge to future management of critical illness. JOP 2007, 8, 374–399. [Google Scholar] [PubMed]
- Ji, R.M.; Jiang, E.P.; Shen, X.J.; Xiong, S.H.; Lin, N.; Liu, F.; Li, Y.Q.; Liu, Y.C.; Ma, L.Y. The anatomic study of chyle leakage due to operation on abdominal region. Zhonghua Wai Ke Za Zhi 2004, 42, 857–860. [Google Scholar] [PubMed]
- Usovich, A.K.; Makhmudov, Z.A.; Borziak, E.I. Is there an intestinal lymphatic trunk in man? Arkh. Anat. Gistol. Embriol. 1981, 80, 31–34. [Google Scholar]
- Christiansen, A.; Detmar, M. Lymphangiogenesis and cancer. Genes Cancer 2011, 2, 1146–1158. [Google Scholar] [CrossRef]

| Voltage 120 kV |
| Tube current 440–747 mA |
| Single collimation 0.6–3.0 mm |
| scan-pitch ratio 1.375:1; reconstruction interval 0.452 mm. |
| Case | Sex | Age | Caliber (mm) | Length (mm) | Course (Related to the SMA) | Level (Related to the MCA) | CT Slice (mm) | Serpiginous |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 60 | 1.60 | 26.36 | in front, longitudinal | From ICA, left to MCA | 3 | No |
| 2 | F | 63 | 1.68 | 28.68 | in front, longitudinal | From MCA, left to MCA | 3 | No |
| 3 | M | 74 | 2.20 | 52.69 | in front, longitudinal | From MCA, left to MCA | 0.9 | Yes |
| 4 | M | 64 | 3.10 | 33.09 | to the left, longitudinal | From MCA/ICA midpoint, left to MCA | 0.67 | No |
| 5 | M | 68 | 2.93 | 24.85 | in front, longitudinal | From GTH, right to MCA | 3 | Yes |
| 6 | M | 75 | 1.20 | 21.21 | in front, transverse | Above MCA, left to MCA | 0.9 | No |
| 7 | M | 75 | 1.91 | 30.23 | in front, transverse | Above MCA, left to MCA | 2 | Yes |
| 8 | F | 46 | 1.93 | 24.16 | in front, transverse | Above MCA, left to MCA | 0.9 | No |
| 9 | M | 72 | 0.92 | 18.17 | in front, transverse | Above MCA, left to MCA | 0.9 | No |
| 10 | M | 60 | 1.39 | 3.663 | in front, longitudinal | From MCA, left to MCA | 2 | No |
| 11 | M | 51 | 1.20 | 2.985 | in front, longitudinal | From MCA, left to MCA | 0.9 | No |
| 12 | M | 77 | 1.96 | 8.134 | in front, longitudinal | From ICA, left to MCA | 1 | Yes |
| 13 | F | 74 | 1.81 | 3.636 | in front, longitudinal | From MCA, above MCA | 1 | No |
| 14 | M | 55 | 2.75 | 3.274 | in front, longitudinal | From MCA, above MCA | 1 | Yes |
| 15 | F | 66 | 1.57 | 2.812 | in front, longitudinal | From RCA, left to MCA | 1 | No |
| 16 | M | 61 | 1.60 | 5.879 | in front, longitudinal | From ICA, left to MCA | 1 | Yes |
| 17 | M | 66 | 1.07 | 2.079 | in front, oblique | Above MCA, left to MCA | 1 | No |
| 18 | F | 64 | 1.82 | 10.128 | to the left, longitudinal | From MCA, left to MCA | 0.9 | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stimec, B.V.; Ignjatovic, D. Visible Lymph Affluents in the D3 Volume: An MDCTA Pictorial Essay. Diagnostics 2022, 12, 2441. https://doi.org/10.3390/diagnostics12102441
Stimec BV, Ignjatovic D. Visible Lymph Affluents in the D3 Volume: An MDCTA Pictorial Essay. Diagnostics. 2022; 12(10):2441. https://doi.org/10.3390/diagnostics12102441
Chicago/Turabian StyleStimec, Bojan V., and Dejan Ignjatovic. 2022. "Visible Lymph Affluents in the D3 Volume: An MDCTA Pictorial Essay" Diagnostics 12, no. 10: 2441. https://doi.org/10.3390/diagnostics12102441
APA StyleStimec, B. V., & Ignjatovic, D. (2022). Visible Lymph Affluents in the D3 Volume: An MDCTA Pictorial Essay. Diagnostics, 12(10), 2441. https://doi.org/10.3390/diagnostics12102441






