Peritoneal Seeding Is More Common in Gastric Cancer Patients with FGFR2 Amplification or High Tumor Mutation Burden
Abstract
1. Introduction
2. Materials and Methods
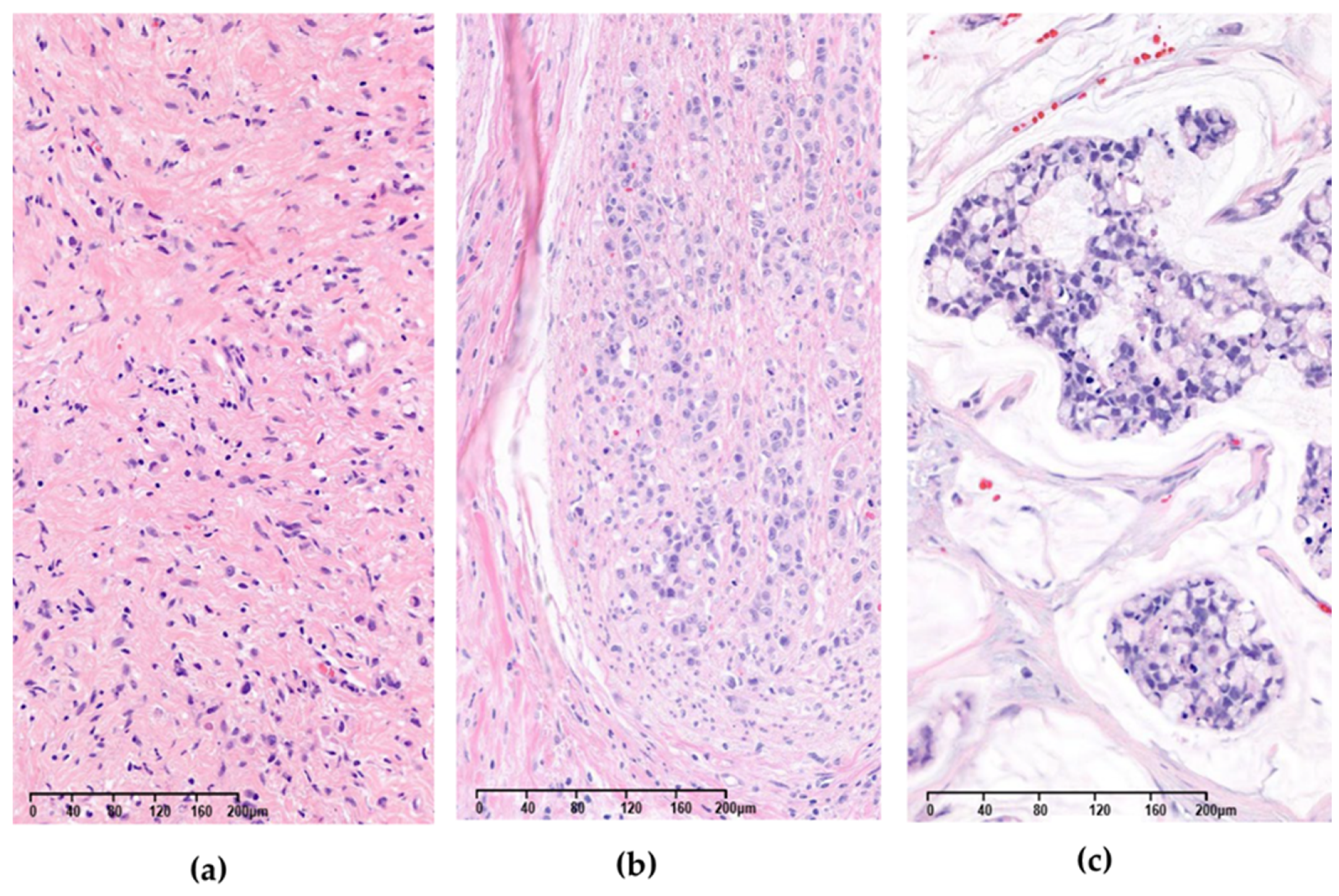
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IARC. The Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 25 November 2021).
- Koizumi, W.; Narahara, H.; Hara, T.; Takagane, A.; Akiya, T.; Takagi, M.; Miyashita, K.; Nishizaki, T.; Kobayashi, O.; Takiyama, W.; et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol. 2008, 9, 215–221. [Google Scholar] [CrossRef]
- Wu, X.; Li, Z.; Li, Z.; Jia, Y.; Shan, F.; Ji, X.; Bu, Z.; Zhang, L.; Wu, A.; Ji, J. Hyperthermic intraperitoneal chemotherapy plus simultaneous versus staged cytoreductive surgery for gastric cancer with occult peritoneal metastasis. J. Surg. Oncol. 2015, 111, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.S.; Smalley, S.R.; Benedetti, J.; Hundahl, S.A.; Estes, N.C.; Stemmermann, G.N.; Haller, D.G.; Ajani, J.A.; Gunderson, L.L.; Jessup, J.M.; et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N. Engl. J. Med. 2001, 345, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Rothenbacher, D.; Arndt, V. Epidemiology of stomach cancer. Methods Mol. Biol. 2009, 472, 467–477. [Google Scholar]
- Sarela, A.I.; Miner, T.J.; Karpeh, M.S.; Coit, D.G.; Jaques, D.P.; Brennan, M.F. Clinical outcomes with laparoscopic stage M1, unresected gastric adenocarcinoma. Ann. Surg. 2006, 243, 189–195. [Google Scholar] [CrossRef]
- Kanda, M.; Kodera, Y. Molecular mechanisms of peritoneal dissemination in gastric cancer. World J. Gastroenterol. 2016, 22, 6829–6840. [Google Scholar] [CrossRef] [PubMed]
- Miyake, S.; Kitajima, Y.; Nakamura, J.; Kai, K.; Yanagihara, K.; Tanaka, T.; Hiraki, M.; Miyazaki, K.; Noshiro, H. HIF-1α is a crucial factor in the development of peritoneal dissemination via natural metastatic routes in scirrhous gastric cancer. Int. J. Oncol. 2013, 43, 1431–1440. [Google Scholar] [CrossRef]
- Shimotsuma, M.; Shields, J.W.; Simpson-Morgan, M.W.; Sakuyama, A.; Shirasu, M.; Hagiwara, A.; Takahashi, T. Morpho-physiological function and role of omental milky spots as omentum-associated lymphoid tissue (OALT) in the peritoneal cavity. Lymphology 1993, 26, 90–101. [Google Scholar]
- Feingold, P.L.; Kwong, M.L.; Sabesan, A.; Sorber, R.; Rudloff, U. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for gastric cancer and other less common disease histologies: Is it time? J. Gastrointest. Oncol. 2016, 7, 87–98. [Google Scholar] [PubMed]
- Turner, N.; Grose, R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Yashiro, M.; Matsuoka, T. Fibroblast growth factor receptor signaling as therapeutic targets in gastric cancer. World J. Gastroenterol. 2016, 22, 2415–2423. [Google Scholar] [CrossRef]
- Krook, M.A.; Reeser, J.W.; Ernst, G.; Barker, H.; Wilberding, M.; Li, G.; Chen, H.Z.; Roychowdhury, S. Fibroblast growth factor receptors in cancer: Genetic alterations, diagnostics, therapeutic targets and mechanisms of resistance. Br. J. Cancer 2021, 124, 880–892. [Google Scholar] [CrossRef]
- Matsumoto, K.; Arao, T.; Hamaguchi, T.; Shimada, Y.; Kato, K.; Oda, I.; Taniguchi, H.; Koizumi, F.; Yanagihara, K.; Sasaki, H.; et al. FGFR2 gene amplification and clinicopathological features in gastric cancer. Br. J. Cancer 2012, 106, 727–732. [Google Scholar] [CrossRef]
- Jang, J.H.; Shin, K.H.; Park, J.G. Mutations in fibroblast growth factor receptor 2 and fibroblast growth factor receptor 3 genes associated with human gastric and colorectal cancers. Cancer Res. 2001, 61, 3541–3543. [Google Scholar]
- Hur, J.Y.; Chao, J.; Kim, K.; Kim, S.T.; Kim, K.M.; Klempner, S.J.; Lee, J. High-level FGFR2 amplification is associated with poor prognosis and Lower response to chemotherapy in gastric cancers. Pathol. Res. Pract. 2020, 216, 152878. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.A.; Lee, H.; Kim, D.G.; Kim, H.; Ha, S.Y.; Choi, Y.L.; Jang, K.T.; Kim, K.M. PD-L1 Expression Is Significantly Associated with Tumor Mutation Burden and Microsatellite Instability Score. Cancers 2021, 13, 4659. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Kim, C.; Kim, J.H.; Kwon, W.S.; Lee, W.S.; Kim, J.M.; Park, J.Y.; Kim, H.S.; Park, K.H.; Kim, T.S.; et al. Genetic alterations and their clinical implications in gastric cancer peritoneal carcinomatosis revealed by whole-exome sequencing of malignant ascites. Oncotarget 2016, 7, 8055–8066. [Google Scholar] [CrossRef] [PubMed]
- Kurashige, J.; Hasegawa, T.; Niida, A.; Sugimachi, K.; Deng, N.; Mima, K.; Uchi, R.; Sawada, G.; Takahashi, Y.; Eguchi, H.; et al. Integrated Molecular Profiling of Human Gastric Cancer Identifies DDR2 as a Potential Regulator of Peritoneal Dissemination. Sci. Rep. 2016, 6, 22371. [Google Scholar] [CrossRef]
- De Luca, A.; Esposito Abate, R.; Rachiglio, A.M.; Maiello, M.R.; Esposito, C.; Schettino, C.; Izzo, F.; Nasti, G.; Normanno, N. FGFR Fusions in Cancer: From Diagnostic Approaches to Therapeutic Intervention. Int. J. Mol. Sci. 2020, 21, 6856. [Google Scholar] [CrossRef]
- AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017, 7, 818–831. [CrossRef]
- Xie, L.; Su, X.; Zhang, L.; Yin, X.; Tang, L.; Zhang, X.; Xu, Y.; Gao, Z.; Liu, K.; Zhou, M.; et al. FGFR2 gene amplification in gastric cancer predicts sensitivity to the selective FGFR inhibitor AZD4547. Clin. Cancer Res. 2013, 19, 2572–2583. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Lee, J.; Hong, M.; Kim, S.T.; Park, S.H.; Choi, M.G.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; Kim, S.; et al. FGFR2 in gastric cancer: Protein overexpression predicts gene amplification and high H-index predicts poor survival. Mod. Pathol. 2016, 29, 1095–1103. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.; Kang, S.Y.; Heo, Y.J.; Park, S.H.; Kim, S.T.; Kang, W.K.; Lee, J.; Kim, K.M. Tumor Mutational Burden Determined by Panel Sequencing Predicts Survival After Immunotherapy in Patients with Advanced Gastric Cancer. Front. Oncol. 2020, 10, 314. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, Q.; Wang, H.; Zhuo, W.; Ding, Y.; Lu, J.; Wu, G.; Xu, N.; Teng, L. Predicting Peritoneal Dissemination of Gastric Cancer in the Era of Precision Medicine: Molecular Characterization and Biomarkers. Cancers 2020, 12, 2236. [Google Scholar] [CrossRef] [PubMed]
- D’Angelica, M.; Gonen, M.; Brennan, M.F.; Turnbull, A.D.; Bains, M.; Karpeh, M.S. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann. Surg. 2004, 240, 808–816. [Google Scholar] [CrossRef]
- Lee, J.H.; Son, S.Y.; Lee, C.M.; Ahn, S.H.; Park, D.J.; Kim, H.H. Factors predicting peritoneal recurrence in advanced gastric cancer: Implication for adjuvant intraperitoneal chemotherapy. Gastric Cancer 2014, 17, 529–536. [Google Scholar] [CrossRef]
- Mizrak Kaya, D.; Nogueras-González, G.M.; Harada, K.; Amlashi, F.G.; Roy-Chowdhuri, S.; Estrella, J.S.; Das, P.; Lee, J.H.; Weston, B.; Bhutani, M.S.; et al. Risk of peritoneal metastases in patients who had negative peritoneal staging and received therapy for localized gastric adenocarcinoma. J. Surg. Oncol. 2018, 117, 678–684. [Google Scholar] [CrossRef]
- Helsten, T.; Elkin, S.; Arthur, E.; Tomson, B.N.; Carter, J.; Kurzrock, R. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clin. Cancer Res. 2016, 22, 259–267. [Google Scholar] [CrossRef]
- Poon, D.; Tan, M.H.; Khor, D. Stage 4 pancreatic adenocarcinoma harbouring an FGFR2-TACC2 fusion mutation with complete response to erdafitinib a pan-fibroblastic growth factor receptor inhibitor. BMJ Case Rep. 2021, 14, e244271. [Google Scholar] [CrossRef]
- Mazzaferro, V.; El-Rayes, B.F.; Droz Dit Busset, M.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer 2019, 120, 165–171. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Chang, G.; Liu, X.; Wang, S.; Zhang, Z.; Wu, Z.; Zhang, X.; Li, J. Prognostic Value of FGFR Gene Amplification in Patients with Different Types of Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e105524. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, J.H.; Jang, H.J. Pathologic and prognostic impacts of FGFR2 amplification in gastric cancer: A meta-analysis and systemic review. J. Cancer 2019, 10, 2560–2567. [Google Scholar] [CrossRef] [PubMed]
| FGFR2 Amplification | Total | |||
|---|---|---|---|---|
| Positive | Negative | |||
| Peritoneal Seeding | Positive | 18 (P/D: 15) | 157 (P/D: 123) | 175 (P/D: 138) |
| Negative | 8 (P/D: 7) | 177 (P/D: 123) | 185 (P/D: 130) | |
| Total | 26 (P/D: 22) | 334 (P/D: 246) | 360 (P/D: 268) | |
| ID | SEX | AGE | FGFR2 Amplification | Peritoneal Seeding | FGFR2 Copy Number | Other FGFR Alterations | Differentiation | PD-L1 CPS | ERBB2 Amplification | HIGH TMB | MSI | EBV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | F | 42 | Y | Y | 27.6 | FGFR2-WDR11 | PD | Neg | N | N | N | Neg |
| 34 | F | 52 | Y | Y | 57 | none | PD | Pos | N | N | N | Neg |
| 49 | M | 53 | Y | Y | 19.6 | FGFR2-TACC2 | PD | Pos | N | N | N | Neg |
| 74 | M | 45 | Y | Y | 254 | none | PD | Neg | N | N | N | Neg |
| 78 | M | 52 | Y | N | 20.8 | none | MD | Pos | Y | N | N | Neg |
| 109 | F | 33 | Y | Y | 6.2 | none | PD | ND | N | N | N | ND |
| 122 | M | 28 | Y | N | 4.3 | FGFR2-BTBD | PD | Pos | N | N | N | Neg |
| 143 | F | 50 | Y | Y | 19.1 | none | PD | Pos | N | N | N | Neg |
| 145 | F | 48 | Y | Y | 15.3 | none | MD | ND | N | N | N | ND |
| 146 | M | 76 | Y | Y | 52 | none | MD | Pos | Y | Y | N | ND |
| 155 | F | 70 | Y | Y | 32.8 | none | PD | Neg | N | N | N | Neg |
| 163 | M | 67 | Y | N | 17.8 | FGFR2-TACC2 | PD | Pos | N | N | N | Neg |
| 173 | M | 54 | Y | Y | 93.5 | none | PD | ND | N | N | N | Neg |
| 205 | F | 48 | Y | Y | 9.1 | none | PD | Neg | N | N | N | Neg |
| 208 | M | 50 | Y | Y | 274 | FGFR2-C10orf90 | PD | ND | N | N | N | ND |
| 221 | M | 54 | Y | Y | 6.2 | none | MD | Neg | N | N | N | ND |
| 222 | F | 58 | Y | Y | 185.9 | FGFR2-INPP5F | PD | Neg | N | N | N | ND |
| 242 | M | 60 | Y | N | 64.1 | FGFR2-PPAPDC1A | PD | Pos | N | N | N | Neg |
| 251 | M | 61 | Y | N | 66 | none | PD | ND | N | N | N | ND |
| 253 | F | 51 | Y | N | 9.2 | FGFR2-TACC2 | PD | Neg | N | N | N | Neg |
| 276 | F | 50 | Y | Y | 3.7 | none | PD | Pos | Y | N | N | ND |
| 305 | M | 67 | Y | Y | 6.7 | none | PD | Pos | N | N | N | Neg |
| 338 | F | 77 | Y | Y | 11.6 | none | PD | Pos | N | N | N | Neg |
| 348 | M | 57 | Y | Y | 4.1 | none | PD | ND | N | N | N | Neg |
| 357 | F | 37 | Y | N | 105.5 | none | PD | Pos | N | N | N | Neg |
| 359 | M | 66 | Y | N | 4.5 | none | PD | Pos | N | N | N | Neg |
| 14 | M | 74 | N | Y | NA | FGFR3 amplification, FGFR3-FAM175B | PD | Pos | Y | N | N | Neg |
| 211 | F | 52 | N | Y | NA | FGFR1 amplification | PD | Pos | N | N | N | ND |
| 233 | M | 59 | N | N | NA | FGFR1 amplification, FGFR1-RTN4 fusion | PD | ND | N | N | N | ND |
| 254 | M | 76 | N | Y | NA | FGFR2 | WD | Pos | N | N | N | Neg |
| 267 | M | 77 | N | N | NA | FGFR1 amplification | WD | ND | Y | Y | N | Neg |
| 339 | M | 81 | N | N | NA | FGFR4 Amplification | PD | Pos | N | N | N | Neg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Park, S.; Kang, S.Y.; Ahn, S.; Kim, K.-M. Peritoneal Seeding Is More Common in Gastric Cancer Patients with FGFR2 Amplification or High Tumor Mutation Burden. Diagnostics 2022, 12, 2355. https://doi.org/10.3390/diagnostics12102355
Kim H, Park S, Kang SY, Ahn S, Kim K-M. Peritoneal Seeding Is More Common in Gastric Cancer Patients with FGFR2 Amplification or High Tumor Mutation Burden. Diagnostics. 2022; 12(10):2355. https://doi.org/10.3390/diagnostics12102355
Chicago/Turabian StyleKim, Hyunjin, Sujin Park, So Young Kang, Soomin Ahn, and Kyoung-Mee Kim. 2022. "Peritoneal Seeding Is More Common in Gastric Cancer Patients with FGFR2 Amplification or High Tumor Mutation Burden" Diagnostics 12, no. 10: 2355. https://doi.org/10.3390/diagnostics12102355
APA StyleKim, H., Park, S., Kang, S. Y., Ahn, S., & Kim, K.-M. (2022). Peritoneal Seeding Is More Common in Gastric Cancer Patients with FGFR2 Amplification or High Tumor Mutation Burden. Diagnostics, 12(10), 2355. https://doi.org/10.3390/diagnostics12102355






