Could Chronic Rhinosinusitis Increase the Risk of Ulcerative Colitis? A Nationwide Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Data Availability
2.2. Study Design and Population
2.3. Statistical Analyses
3. Results
3.1. Effect of Chronic Rhinosinusitis on Inflammatory Bowel Diseases
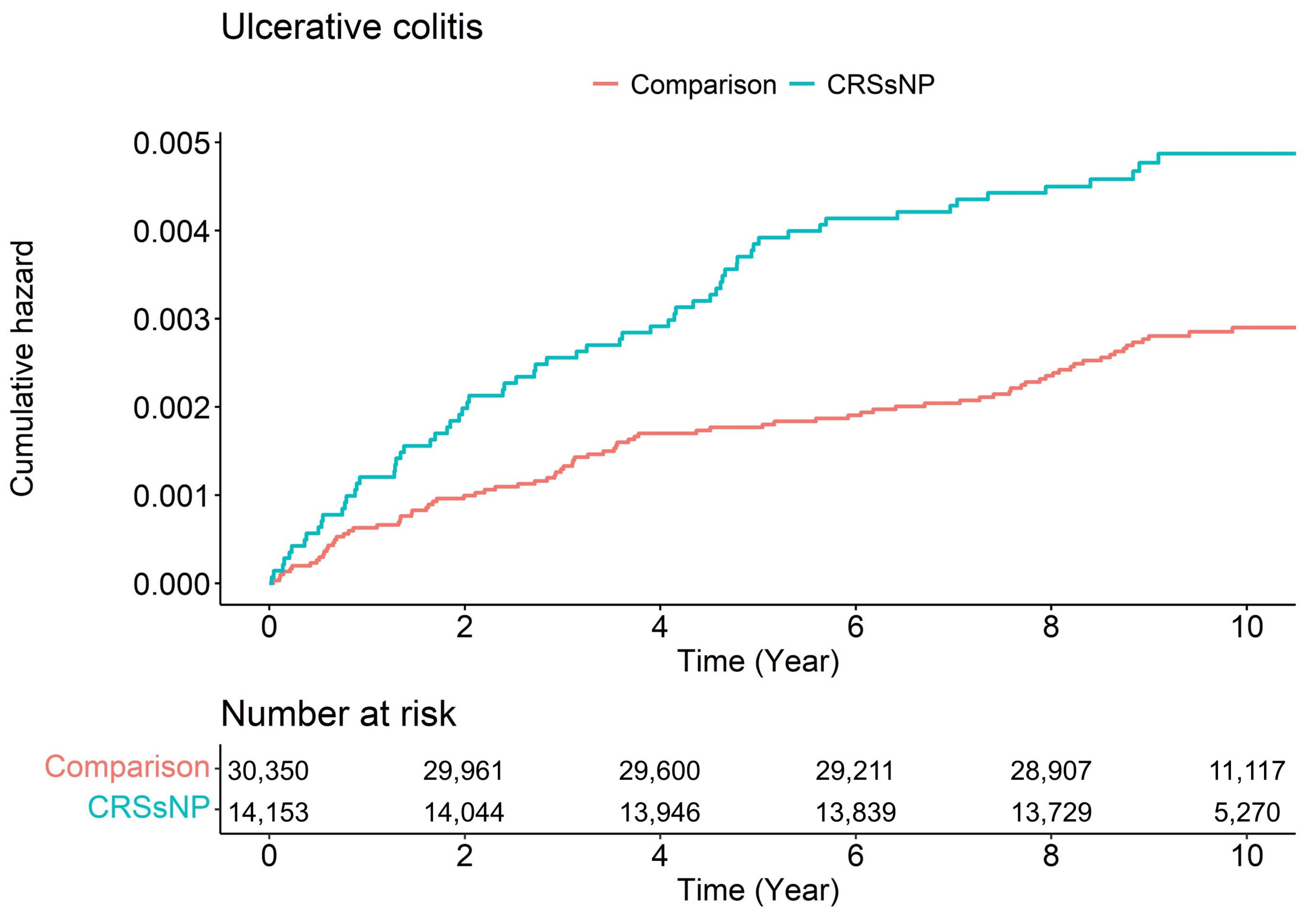
3.2. Chronic Rhinosinusitis with Nasal Polyp and Ulcerative Colitis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J. Executive summary of EPOS 2020 including integrated care pathways. Rhinology 2020, 58, 82–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Jo, A.; Lim, H.S.; Kim, J.Y.; Eun, K.M.; Oh, J.; Kim, J.K.; Cho, S.H.; Kim, D.W. Enhanced Type 2 Immune Reactions by Increased IL-22/IL-22Ra1 Signaling in chronic rhinosinusitis with Nasal Polyps. Allergy Asthma Immunol. Res. 2020, 12, 980–993. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Lee, B.C.; Park, K.J.; Son, G.M. Effect of obstructive sleep apnea on immunity in cases of chronic rhinosinusitis with nasal polyps. Clin. Exp. Otorhinolaryngol. 2021, 14, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Van Bruaene, N.; Pérez-Novo, C.A.; Basinski, T.M.; Van Zele, T.; Holtappels, G.; De Ruyck, N.; Schmidt-Weber, C.; Akdis, C.; Van Cauwenberge, P.; Bachert, C.; et al. T-cell regulation in chronic paranasal sinus disease. J. Allergy Clin. Immunol. 2008, 121, 1435–1441. [Google Scholar] [CrossRef]
- Polzehl, D.; Moeller, P.; Riechelmann, H.; Perner, S. Distinct features of chronic rhinosinusitis with and without nasal polyps. Allergy 2006, 61, 1275–1279. [Google Scholar] [CrossRef]
- Tieu, D.D.; Kern, R.C.; Schleimer, R.P. Alterations in epithelial barrier function and host defense responses in chronic rhinosinusitis. J. Allergy Clin. Immunol. 2009, 124, 37–42. [Google Scholar] [CrossRef]
- Schleimer, R.P. Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Annu. Rev. Pathol. 2017, 12, 331–357. [Google Scholar] [CrossRef]
- Aniwan, S.; Park, S.H.; Loftus, E.V. Epidemiology, natural history, and risk stratification of Crohn’s disease. Gastroenterol. Clin. N. Am. 2017, 46, 463–480. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Shouval, D.S.; Rufo, P.A. The role of environmental factors in the pathogenesis of inflammatory bowel diseases: A review. JAMA Pediatr. 2017, 171, 999–1005. [Google Scholar] [CrossRef]
- Card, T.R.; Langan, S.M.; Chu, T.P. Extra-gastrointestinal manifestations of inflammatory bowel disease may be less common than previously reported. Dig. Dis. Sci. 2016, 61, 2619–2626. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Wajda, A.; Blanchard, J.F. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: A population-based study. Gastroenterology 2005, 129, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A. The epithelial barrier hypothesis proposes a comprehensive understanding of the origins of allergic and other chronic noncommunicable diseases. J. Allergy Clin. Immunol. 2022, 149, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Chandra, R.K.; Pollak, J.; Kato, A.; Conley, D.B.; Peters, A.T.; Grammer, L.C.; Avila, P.C.; Kern, R.C.; Stewart, W.F.; et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 2013, 131, 1350–1360. [Google Scholar] [CrossRef]
- Chandra, R.K.; Lin, D.; Tan, B.; Tudor, R.S.; Conley, D.B.; Peters, A.T.; Grammer, L.C.; Schleimer, R.P.; Kern, R.C. Chronic rhinosinusitis in the setting of other chronic inflammatory diseases. Am. J. Otolaryngol. 2011, 32, 388–391. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, C.L.; Kao, C.H. Adults with inflammatory bowel disease are at a greater risk of developing chronic rhinosinusitis: A nationwide population-based study. Clin. Otolaryngol. 2021, 46, 196–205. [Google Scholar] [CrossRef]
- Book, D.T.; Smith, T.L.; McNamar, J.P.; Saeian, K.; Binion, D.G.; Toohill, R.J. Chronic sinonasal disease in patients with inflammatory bowel disease. Am. J. Rhinol. 2003, 17, 87–90. [Google Scholar] [CrossRef]
- Yang, P.C.; Liu, T.; Wang, B.Q.; Zhang, T.Y.; An, Z.Y.; Zheng, P.Y.; Tian, D.F. Rhinosinusitis derived Staphylococcal enterotoxin B possibly associates with pathogenesis of ulcerative colitis. BMC Gastroenterol. 2005, 5, 28. [Google Scholar] [CrossRef]
- Hoggard, M.; Wagner Mackenzie, B.; Jain, R.; Taylor, M.W.; Biswas, K.; Douglas, R.G. Chronic rhinosinusitis and the evolving understanding of microbial ecology in chronic inflammatory mucosal disease. Clin. Microbiol. Rev. 2017, 30, 321–348. [Google Scholar] [CrossRef]
- Månsson, A.; Bogefors, J.; Cervin, A.; Uddman, R.; Cardell, L.O. NOD-like receptors in the human upper airways: A potential role in nasal polyposis. Allergy 2011, 66, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Tsuchiya, K.; Fukushima, K.; Horita, N.; Hibiya, S.; Kitagaki, K.; Negi, M.; Itoh, E.; Akashi, T.; Eishi, Y.; et al. Reduced human α-defensin 6 in noninflamed jejunal tissue of patients with Crohn’s disease. Inflamm. Bowel Dis. 2016, 22, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Niyonsaba, F.; Kiatsurayanon, C.; Ogawa, H. The role of human β-defensins in allergic diseases. Clin. Exp. Allergy 2016, 46, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Sugita, K.; Kabashima, K. Tight junctions in the development of asthma, chronic rhinosinusitis, atopic dermatitis, eosinophilic esophagitis, and inflammatory bowel diseases. J. Leukoc. Biol. 2020, 107, 749–762. [Google Scholar] [CrossRef]
- Rai, V.; Traboulsi, C.; Silfen, A.; Ackerman, M.T.; Erondu, A.I.; Karpin, J.E.; Gulotta, G.; Rubin, D.T. Identification of risk factors for coexisting sinusitis and inflammatory bowel disease. Crohns Colitis 360 2021, 3, otab054. [Google Scholar] [CrossRef]
- Beltrán, C.J.; Núñez, L.E.; Díaz-Jiménez, D.; Farfan, N.; Candia, E.; Heine, C.; López, F.; González, M.J.; Quera, R.; Hermoso, M.A. Characterization of the novel ST2/IL-33 system in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2010, 16, 1097–1107. [Google Scholar] [CrossRef]
- De Salvo, C.; Wang, X.M.; Pastorelli, L.; Mattioli, B.; Omenetti, S.; Buela, K.A.; Chowdhry, S.; Garg, R.R.; Goodman, W.A.; Rodriguez-Palacios, A.; et al. IL-33 Drives eosinophil infiltration and pathogenic type 2 Helper T-cell immune responses leading to chronic experimental ileitis. Am. J. Pathol. 2016, 186, 885–898. [Google Scholar] [CrossRef]
- Sehmi, R. Role of interleukin 33 in chronic rhinosinusitis. Thorax 2017, 72, 591–593. [Google Scholar] [CrossRef]
- Kim, D.K.; Jin, H.R.; Eun, K.M.; Mo, J.H.; Cho, S.H.; Oh, S.; Cho, D.; Kim, D.W. The role of interleukin-33 in chronic rhinosinusitis. Thorax 2017, 72, 635–645. [Google Scholar] [CrossRef]
- Baba, S.; Kondo, K.; Kanaya, K.; Suzukawa, K.; Ushio, M.; Urata, S.; Asakage, T.; Kakigi, A.; Suzukawa, M.; Ohta, K.; et al. Expression of IL-33 and its receptor ST2 in chronic rhinosinusitis with nasal polyps. Laryngoscope 2014, 124, E115–E122. [Google Scholar] [CrossRef]
- Waddell, A.; Vallance, J.E.; Moore, P.D.; Hummel, A.T.; Wu, D.; Shanmukhappa, S.K.; Fei, L.; Washington, M.K.; Minar, P.; Coburn, L.A.; et al. IL-33 Signaling protects from murine oxazolone colitis by supporting intestinal epithelial function. Inflamm. Bowel Dis. 2015, 21, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.H.; Che, X.; Kwak, M.S.; Kim, S.; Kim, J.H.; Ma, H.W.; Kim, D.H.; Kim, T.I.; Kim, W.H.; Kim, S.W.; et al. Interleukin-33 regulates intestinal inflammation by modulating macrophages in inflammatory bowel disease. Sci. Rep. 2017, 7, 851. [Google Scholar] [CrossRef]
- Williams, M.A.; O’Callaghan, A.; Corr, S.C. IL-33 and IL-18 in inflammatory bowel disease etiology and microbial interactions. Front. Immunol. 2019, 10, 1091. [Google Scholar] [CrossRef]
- Kobori, A.; Yagi, Y.; Imaeda, H.; Ban, H.; Bamba, S.; Tsujikawa, T.; Saito, Y.; Fujiyama, Y.; Andoh, A. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J. Gastroenterol. 2010, 45, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Sponheim, J.; Pollheimer, J.; Olsen, T.; Balogh, J.; Hammarström, C.; Loos, T.; Kasprzycka, M.; Sørensen, D.R.; Nilsen, H.R.; Küchler, A.M.; et al. Inflammatory bowel disease-associated interleukin-33 is preferentially expressed in ulceration-associated myofibroblasts. Am. J. Pathol. 2010, 177, 2804–2815. [Google Scholar] [CrossRef] [PubMed]
- Sedhom, M.A.; Pichery, M.; Murdoch, J.R.; Foligné, B.; Ortega, N.; Normand, S.; Mertz, K.; Sanmugalingam, D.; Brault, L.; Grandjean, T.; et al. Neutralisation of the interleukin-33/ST2 pathway ameliorates experimental colitis through enhancement of mucosal healing in mice. Gut 2013, 62, 1714–1723. [Google Scholar] [CrossRef]
- Delemarre, T.; Holtappels, G.; De Ruyck, N.; Zhang, N.; Nauwynck, H.; Bachert, C.; Gevaert, E. Type 2 inflammation in chronic rhinosinusitis without nasal polyps: Another relevant endotype. J. Allergy Clin. Immunol. 2020, 146, 337–343.e6. [Google Scholar] [CrossRef]
- Stevens, W.W.; Peters, A.T.; Tan, B.K.; Klingler, A.I.; Poposki, J.A.; Hulse, K.E.; Leslie, C.G.; Kevin, C.W.; Stephanie, S.S.; David, B.C.; et al. Associations between inflammatory endotypes and clinical presentations in chronic rhinosinusitis. J. Allergy Clin. Immunol. Pract. 2019, 7, 2812–2820.e3. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort profile: The National Health Insurance Service-national sample cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef]
- Seong, S.C.; Kim, Y.Y.; Park, S.K.; Khang, Y.H.; Kim, H.C.; Park, J.H.; Kang, H.J.; Do, C.H.; Song, J.S.; Lee, E.J.; et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 2017, 7, e016640. [Google Scholar] [CrossRef]



| Variables | Comparison (n = 30,350) | Chronic Rhinosinusitis (n = 15,175) | p-Value |
|---|---|---|---|
| Sex | 1.000 | ||
| Male | 12,216 (40.3%) | 6108 (40.3%) | |
| Female | 18,134 (59.7%) | 9067 (59.7%) | |
| Age (years) | 1.000 | ||
| <45 | 18,514 (61.0%) | 9257 (61.0%) | |
| 45–64 | 9322 (30.7%) | 4661 (30.7%) | |
| >64 | 2514 (8.3%) | 1257 (8.3%) | |
| Residence | 1.000 | ||
| Seoul (largest city) | 8106 (26.7%) | 4053 (26.7%) | |
| Other metropolitan | 8004 (26.4%) | 4002 (26.4%) | |
| Other areas | 14,240 (46.9%) | 7120 (46.9%) | |
| Income level | 1.000 | ||
| Low (0–30%) | 5620 (18.5%) | 2810 (18.5%) | |
| Middle (30–70%) | 11,294 (37.2%) | 5647 (37.2%) | |
| High (70–100%) | 13,436 (44.3%) | 6718 (44.3%) | |
| CCI | 1.000 | ||
| 0 | 18,964 (62.5%) | 9482 (62.5%) | |
| 1 | 6876 (22.7%) | 3438 (22.7%) | |
| ≥2 | 4510 (14.9%) | 2255 (14.9%) |
| Variables | N | Case | Person-Years | Incidence Rate | Crude HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|---|---|
| Crohn’s disease | ||||||
| Comparison | 30,350 | 64 | 297,761.1 | 0.21 | 1.00 (ref) | 1.00 (ref) |
| CRS | 15,175 | 32 | 143,933.7 | 0.22 | 1.01 (0.66–1.54) | 1.01 (0.66–1.54) |
| Ulcerative colitis | ||||||
| Comparison | 30,350 | 85 | 297,675.4 | 0.29 | 1.00 (ref) | 1.00 (ref) |
| CRS | 15,175 | 73 | 143,654.1 | 0.51 | 1.74 (1.27–2.38) *** | 1.72 (1.26–2.36) *** |
| The Number of CD Events | The Number of UC Events | |
|---|---|---|
| Event | 96 | 158 |
| Comparison | 64 | 85 |
| CRS | 32 | 73 |
| Total censored (No event) | 45,429 | 45,367 |
| Comparison | 30,286 | 30,265 |
| CRS | 15,143 | 15,102 |
| Termination of study | 43,229 | 43,169 |
| Comparison | 28,582 | 28,562 |
| CRS | 14,647 | 14,607 |
| Loss to follow up/Drop-out | 2200 | 2198 |
| Comparison | 1704 | 1703 |
| CRS | 496 | 495 |
| Variables | N | Case | Person-Years | Incidence Rate | Crude HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|---|---|
| Crohn’s disease | ||||||
| Comparison | 30,350 | 64 | 297,761.1 | 0.21 | 1.00 (ref) | 1.00 (ref) |
| CRSsNP | 14,153 | 32 | 134,205.9 | 0.24 | 1.08 (0.71–1.66) | 1.08 (0.71–1.65) |
| CRSwNP | 1022 | 0 | 9727.8 | 0 | 0.00 (0–Inf) | 0.00 (0–Inf) |
| Ulcerative colitis | ||||||
| Comparison | 30,350 | 64 | 297,761.1 | 0.21 | 1.00 (ref) | 1.00 (ref) |
| CRSsNP | 14,153 | 67 | 133,960.2 | 0.50 | 1.71 (1.24–2.36) ** | 1.71 (1.24–2.35) ** |
| CRSwNP | 1022 | 6 | 9693.8 | 0.62 | 2.14 (0.93–4.89) | 1.97 (0.86–4.51) |
| Time (Year) | Number of Ulcerative Colitis | Adjusted HR (95% CI) | |
|---|---|---|---|
| Comparison | CRSsNP | ||
| 1 | 19 | 17 | 1.94 (1.01–3.73) * |
| 2 | 30 | 28 | 2.01 (1.20–3.37) ** |
| 3 | 39 | 36 | 1.99 (1.26–3.12) ** |
| 4 | 51 | 41 | 1.73 (1.14–2.60) ** |
| 5 | 53 | 54 | 2.18 (1.49–3.19) *** |
| 6 | 57 | 58 | 2.18 (1.51–3.14) *** |
| 7 | 61 | 60 | 2.10 (1.47–3.00) *** |
| 8 | 70 | 63 | 1.92 (1.36–2.70) *** |
| 9 | 83 | 66 | 1.72 (1.24–2.37) ** |
| 10 | 85 | 67 | 1.71 (1.24–2.35) ** |
| 11 | 85 | 67 | 1.71 (1.24–2.35) ** |
| Year | Chron’s Disease (Patients Aged over Twenty) | Ulcerative Colitis (Patients Aged over Twenty) |
|---|---|---|
| 2002 | 567 (0.08%) | 491 (0.07%) |
| 2003 | 477 (0.06%) | 642 (0.09%) |
| 2004 | 447 (0.06%) | 557 (0.07%) |
| 2005 | 376 (0.05%) | 564 (0.07%) |
| 2006 | 303 (0.04%) | 545 (0.07%) |
| 2007 | 220 (0.03%) | 546 (0.07%) |
| 2008 | 226 (0.03%) | 573 (0.08%) |
| 2009 | 189 (0.02%) | 576 (0.08%) |
| 2010 | 224 (0.03%) | 589 (0.08%) |
| 2011 | 283 (0.04%) | 647 (0.08%) |
| 2012 | 264 (0.03%) | 617 (0.08%) |
| 2013 | 288 (0.04%) | 642 (0.08%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, I.H.; Ha, S.-S.; Son, G.M.; Yang, H.G.; Kim, D.-K. Could Chronic Rhinosinusitis Increase the Risk of Ulcerative Colitis? A Nationwide Cohort Study. Diagnostics 2022, 12, 2344. https://doi.org/10.3390/diagnostics12102344
Lee IH, Ha S-S, Son GM, Yang HG, Kim D-K. Could Chronic Rhinosinusitis Increase the Risk of Ulcerative Colitis? A Nationwide Cohort Study. Diagnostics. 2022; 12(10):2344. https://doi.org/10.3390/diagnostics12102344
Chicago/Turabian StyleLee, Il Hwan, Seung-Su Ha, Gil Myeong Son, Hee Gyu Yang, and Dong-Kyu Kim. 2022. "Could Chronic Rhinosinusitis Increase the Risk of Ulcerative Colitis? A Nationwide Cohort Study" Diagnostics 12, no. 10: 2344. https://doi.org/10.3390/diagnostics12102344
APA StyleLee, I. H., Ha, S.-S., Son, G. M., Yang, H. G., & Kim, D.-K. (2022). Could Chronic Rhinosinusitis Increase the Risk of Ulcerative Colitis? A Nationwide Cohort Study. Diagnostics, 12(10), 2344. https://doi.org/10.3390/diagnostics12102344






