Electrical Impedance Tomography (EIT) in a Patient Suffering from Post-COVID Syndrome with Dyspnea: A Case Report
Abstract
:1. Background
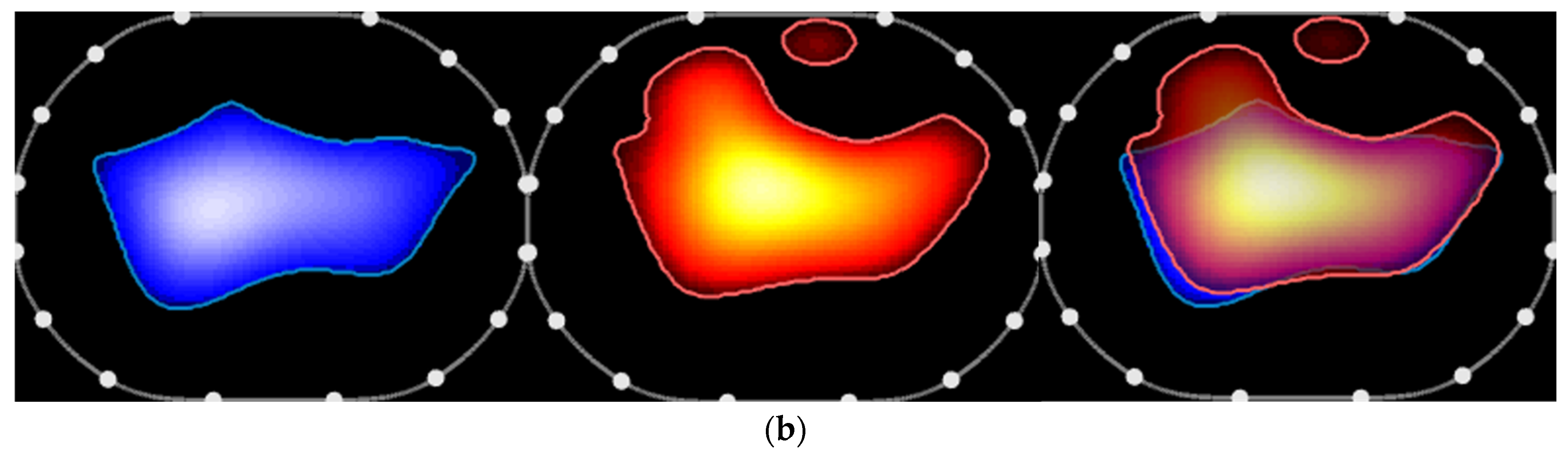
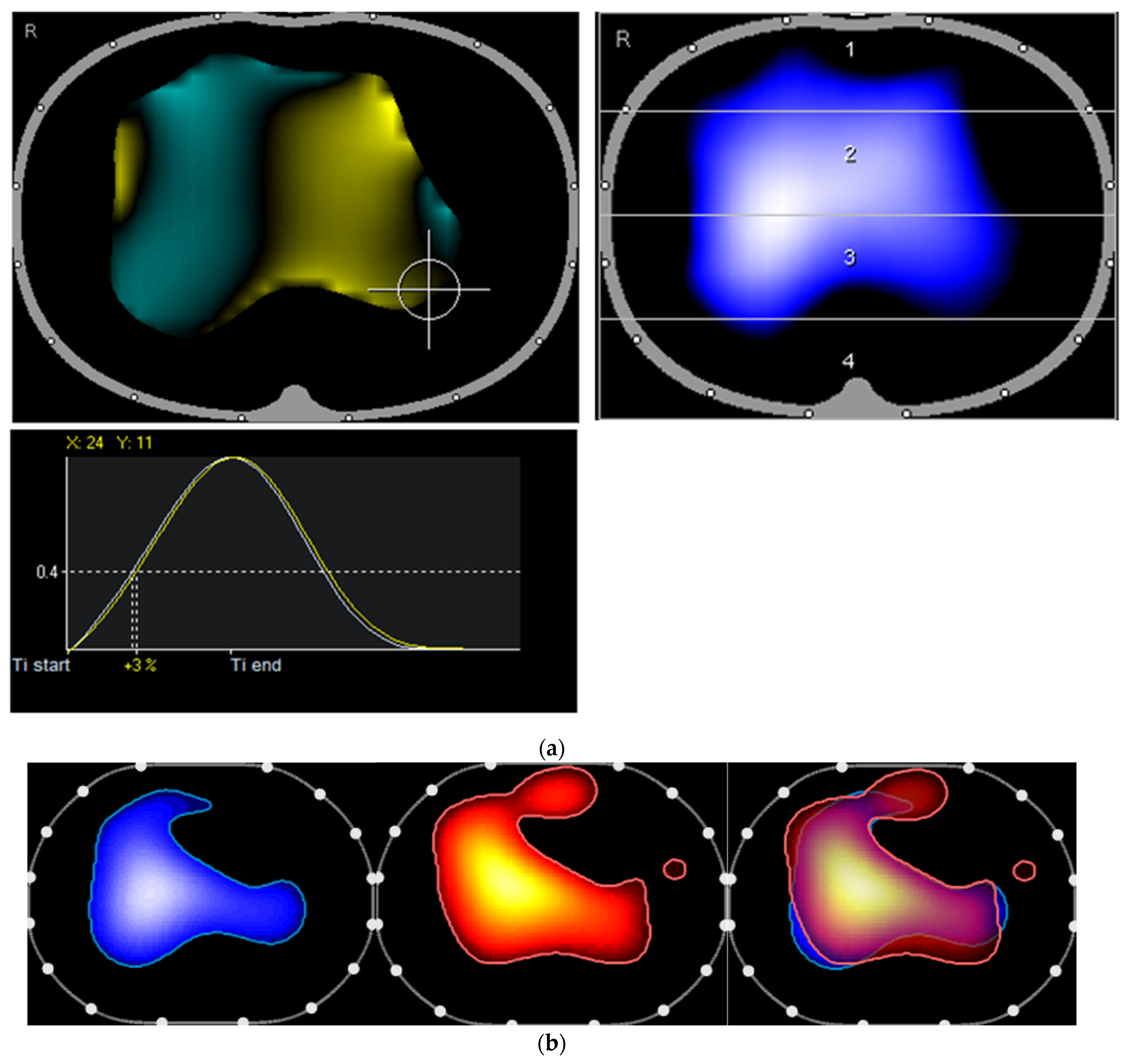
2. The Case and the Method
3. Discussion
4. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nasserie, T.; Hittle, M.; Goodman, S.N. Assessment of the Frequency and Variety of Persistent Symptoms Among Patients With COVID-19: A Systematic Review. JAMA Netw. Open 2021, 4, e2111417. [Google Scholar] [CrossRef] [PubMed]
- Groff, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P.; et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw. Open 2021, 4, e2128568. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Stallmach, A.; Kesselmeier, M.; Bauer, M.; Gramlich, J.; Finke, K.; Fischer, A.; Fleischmann-Struzek, C.; Heutelbeck, A.; Katzer, K.; Mutschke, S.; et al. Comparison of fatigue, cognitive dysfunction and psychological disorders in post-COVID patients and patients after sepsis: Is there a specific constellation? Infection 2022, 50, 661–669. [Google Scholar] [CrossRef]
- Solomon, J.J.; Heyman, B.; Ko, J.P.; Condos, R.; Lynch, D.A. CT of Post-Acute Lung Complications of COVID-19. Radiology 2021, 301, E383–E395. [Google Scholar] [CrossRef] [PubMed]
- Guler, S.A.; Ebner, L.; Aubry-Beigelman, C.; Bridevaux, P.-O.; Brutsche, M.; Clarenbach, C.; Garzoni, C.; Geiser, T.K.; Lenoir, A.; Mancinetti, M.; et al. Pulmonary function and radiological features 4 months after COVID-19: First results from the national prospective observational Swiss COVID-19 lung study. Eur. Respir. J. 2021, 57, 2003690. [Google Scholar] [CrossRef] [PubMed]
- Sonnweber, T.; Sahanic, S.; Pizzini, A.; Luger, A.; Schwabl, C.; Sonnweber, B.; Kurz, K.; Koppelstätter, S.; Haschka, D.; Petzer, V.; et al. Cardiopulmonary recovery after COVID-19: An observational prospective multicentre trial. Eur. Respir. J. 2021, 57, 2003481. [Google Scholar] [CrossRef]
- Komici, K.; Bianco, A.; Perrotta, F.; Iacono, A.D.; Bencivenga, L.; D’Agnano, V.; Rocca, A.; Bianco, A.; Rengo, G.; Guerra, G. Clinical Characteristics, Exercise Capacity and Pulmonary Function in Post-COVID-19 Competitive Athletes. J. Clin. Med. 2021, 10, 3053. [Google Scholar] [CrossRef]
- Joris, M.; Minguet, P.; Colson, C.; Joris, J.; Fadeur, M.; Minguet, G.; Guiot, J.; Misset, B.; Rousseau, A.-F. Cardiopulmonary Exercise Testing in Critically Ill Coronavirus Disease 2019 Survivors: Evidence of a Sustained Exercise Intolerance and Hypermetabolism. Crit. Care Explor. 2021, 3, e0491. [Google Scholar] [CrossRef]
- Churruca, M.; Martinez-Besteiro, E.; Counago, F.; Landete, P. COVID-19 pneumonia: A review of typical radiological characteristics. World J. Radiol. 2021, 13, 327–343. [Google Scholar] [CrossRef]
- Manolescu, D.; Timar, B.; Bratosin, F.; Rosca, O.; Citu, C.; Oancea, C. Predictors for COVID-19 Complete Remission with HRCT Pattern Evolution: A Monocentric, Prospective Study. Diagnostics 2022, 12, 1397. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, M.; Landini, N.; Sambataro, G.; Nardi, C.; Tofani, L.; Bruni, C.; Randone, S.B.; Blagojevic, J.; Melchiorre, D.; Hughes, M.; et al. The role of chest CT in deciphering interstitial lung involvement: Systemic sclerosis versus COVID-19. Rheumatology 2022, 61, 1600–1609. [Google Scholar] [CrossRef]
- Frerichs, I.; Hinz, J.; Herrmann, P.; Weisser, G.; Hahn, G.; Dudykevych, T.; Quintel, M.; Hellige, G. Detection of local lung air content by electrical impedance tomography compared with electron beam CT. J. Appl. Physiol. 2002, 93, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Victorino, J.A.; Borges, J.B.; Okamoto, V.N.; Matos, G.F.; Tucci, M.R.; Caramez, M.P.; Tanaka, H.; Sipmann, F.S.; Santos, D.C.B.; Barbas, C.S.V.; et al. Imbalances in regional lung ventilation: A validation study on electrical impedance tomography. Am. J. Respir. Crit. Care Med. 2004, 169, 791–800. [Google Scholar] [CrossRef]
- Kunst, P.W.; Noordegraaf, A.V.; Hoekstra, O.S.; Postmus, P.E.; De Vries, P.M. Ventilation and perfusion imaging by electrical impedance tomography: A comparison with radionuclide scanning. Physiol. Meas. 1998, 19, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Serrano, R.E.; de Lema, B.; Casas, O.; Feixas, T.; Calaf, N.; Camacho, V.; Carrió, I.; Casan, P.; Sanchis, J.; Riu, P.J. Use of electrical impedance tomography (EIT) for the assessment of unilateral pulmonary function. Physiol. Meas. 2002, 23, 211–220. [Google Scholar] [CrossRef]
- Marquis, F.; Coulombe, N.; Costa, R.; Gagnon, H.; Guardo, R.; Skrobik, Y. Electrical impedance tomography’s correlation to lung volume is not influenced by anthropometric parameters. J. Clin. Monit. Comput. 2006, 20, 201–207. [Google Scholar] [CrossRef]
- Sang, L.; Zhao, Z.; Yun, P.-J.; Frerichs, I.; Möller, K.; Fu, F.; Liu, X.; Zhong, N.; Li, Y. Qualitative and quantitative assessment of pendelluft: A simple method based on electrical impedance tomography. Ann. Transl. Med. 2020, 8, 1216. [Google Scholar] [CrossRef]
- Sang, L.; Zhao, Z.; Lin, Z.; Liu, X.; Zhong, N.; Li, Y. A narrative review of electrical impedance tomography in lung diseases with flow limitation and hyperinflation: Methodologies and applications. Ann. Transl. Med. 2020, 8, 1688. [Google Scholar] [CrossRef]
- Huntley, C.C.; Patel, K.; Bil Bushra, S.E.; Mobeen, F.; Armitage, M.N.; Pye, A.; Knight, C.B.; Mostafa, A.; Kershaw, M.; Mughal, A.Z.; et al. Pulmonary function test and computed tomography features during follow-up after SARS, MERS and COVID-19: A systematic review and meta-analysis. ERJ Open Res. 2022, 8, 00056-2022. [Google Scholar] [CrossRef]
- Albouaini, K.; Egred, M.; Alahmar, A.; Wright, D.J. Cardiopulmonary exercise testing and its application. Postgrad. Med. J. 2007, 83, 675–682. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katzer, K.; Gremme, Y.; Moshmosh Alsabbagh, M.; Stallmach, A.; Reuken, P.; Lewejohann, J.-C. Electrical Impedance Tomography (EIT) in a Patient Suffering from Post-COVID Syndrome with Dyspnea: A Case Report. Diagnostics 2022, 12, 2284. https://doi.org/10.3390/diagnostics12102284
Katzer K, Gremme Y, Moshmosh Alsabbagh M, Stallmach A, Reuken P, Lewejohann J-C. Electrical Impedance Tomography (EIT) in a Patient Suffering from Post-COVID Syndrome with Dyspnea: A Case Report. Diagnostics. 2022; 12(10):2284. https://doi.org/10.3390/diagnostics12102284
Chicago/Turabian StyleKatzer, Katrin, Yvonne Gremme, Majd Moshmosh Alsabbagh, Andreas Stallmach, Philipp Reuken, and Jan-Christoph Lewejohann. 2022. "Electrical Impedance Tomography (EIT) in a Patient Suffering from Post-COVID Syndrome with Dyspnea: A Case Report" Diagnostics 12, no. 10: 2284. https://doi.org/10.3390/diagnostics12102284
APA StyleKatzer, K., Gremme, Y., Moshmosh Alsabbagh, M., Stallmach, A., Reuken, P., & Lewejohann, J.-C. (2022). Electrical Impedance Tomography (EIT) in a Patient Suffering from Post-COVID Syndrome with Dyspnea: A Case Report. Diagnostics, 12(10), 2284. https://doi.org/10.3390/diagnostics12102284




