Switching to a Bictegravir Single Tablet Regimen in Elderly People Living with HIV-1: Data Analysis from the BICTEL Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
2.3. Ethical Aspects
3. Results
3.1. Demographic Features
3.1.1. Overall Population
3.1.2. Over 55 Years Old Population
3.2. Safety
3.3. Immunovirological Profile
3.4. Metabolic Profile
3.4.1. Lipidic Profile, Body Weight and BMI
3.4.2. Renal Function
3.4.3. Hepatic Profile
3.5. Neurological Assessment
3.6. Patients’ Self Reported Adherence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sabin, C.A.; Lundgren, J.D. The natural history of HIV infection. Curr. Opin. HIV AIDS 2013, 8, 311–317. [Google Scholar] [CrossRef]
- Holmes, K.K.; Bertozzi, S.; Bloom, B.R.; Jha, P. Global Mortality and Morbidity of HIV/AIDS. In Major Infectious Diseases, 3rd ed.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2017; Chapter 2. [Google Scholar] [CrossRef] [Green Version]
- Erlandson, K.M.; Karris, M.Y. HIV and Aging: Reconsidering the Approach to Management of Comorbidities. Infect. Dis. Clin. North Am. 2019, 33, 769–786. [Google Scholar] [CrossRef] [PubMed]
- European AIDS Clinical Society. Guidelines Version 10.1. 2020. Available online: http://eacsociety.org (accessed on 8 August 2021).
- Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. 2021. Available online: https://aidsinfo.nih.gov/guidelines (accessed on 8 August 2021).
- Gilead Sciences. Biktarvy® (Bictegravir, Emtricitabine, and Tenofo- Vir Alafenamide): US Prescribing Information. 2018. Available online: https://www.accessdata.fda.gov/ (accessed on 8 August 2021).
- Gilead Sciences. Biktarvy 50 mg/200 mg/25 mg Film-Coated Tab- Lets: EU Summary of Product Characteristics. 2018. Available online: http://www.ema.europa.eu/ (accessed on 8 August 2021).
- Markham, A. Bictegravir: First Global Approval. Drugs 2018, 78, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Daar, E.S.; DeJesus, E.; Ruane, P.; Crofoot, G.; Oguchi, G.; Creticos, C.; Rockstroh, J.K.; Molina, J.M.; Koenig, E.; Liu, Y.P.; et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, phase 3, non-inferiority trial. Lancet HIV 2018, 5, e347–e356. [Google Scholar] [PubMed]
- Kityo, C.; Hagins, D.; Koenig, E. Switching to bictegravir/ emtricitabine/tenofovir alafenamide in women. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Boston, MA, USA, 4–7 March 2018. [Google Scholar]
- Molina, J.M.; Ward, D.; Brar, I.; Mills, A.; Stellbrink, H.J.; López-Cortés, L.; Ruane, P.; Podzamczer, D.; Brinson, C.; Custodio, J.; et al. Switching to fixed-dose bicte- gravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, mul- ticentre, active-controlled, phase 3, non-inferiority trial. Lancet HIV 2018, 5, e357–e365. [Google Scholar] [PubMed]
- Pepperrell, T.; Hill, A.; Moorhouse, M.; Clayden, P.; McCann, K.; Sokhela, S.; Serenata, C.; Venter, W.D.F. Phase 3 trials of new antiretrovirals are not representative of the global HIV epidemic. J. Virus Erad. 2020, 30, 70–73. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Braun, L.T.; Ndumele, C.E.; Smith SCJr Sperling, L.S.; Virani, S.S.; Blumenthal, R.S. Use of risk assessment tools to guide decision-making in the primary prevention of atherosclerotic cardiovascular disease: A special report from the American Heart Association and American College of Cardiology. J. Am. Coll. Cardiol. 2019, 73, 3153–3167. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, G.; Zona, S.; Silva, A.R.; Menozzi, M.; Dolci, G.; Milic, J.; Carli, F.; Mussini, C. The dynamic association between Frailty, CD4 and CD4/CD8 ratio in people aging with HIV. PLoS ONE 2019, 14, e0212283. [Google Scholar] [CrossRef] [PubMed]
- Demontès, M.; Eymard Duvernay, S.; Allavena, C.; Jovelin, T.; Reynes, J.; Hentzien, M.; Ravaux, I.; Delobel, P.; Bregigeon, S.; Rey, D.; et al. Dat’AIDS Study Group. Multimorbidity in Elderly Persons According to the Year of Diagnosis of Human Immunodeficiency Virus Infection: A Cross-sectional Dat’AIDS Cohort Study. Clin. Infect. Dis. 2020, 71, 2880–2888. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, J.A.; Cooley, S.A.; Strain, J.F.; Boerwinkle, A.; Paul, R.; Presti, R.M.; Ances, B.M. Altered neuropsychological performance and reduced brain volumetrics in people living with HIV on integrase strand transfer inhibitors. AIDS 2019, 33, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Wohl, D.; Clarke, A.; Maggiolo, F.; Garner, W.; Laouri, M.; Martin, H.; Quirk, E. Patient-Reported Symptoms Over 48 Weeks Among Participants in Randomized, Double-Blind, Phase III Non-inferiority Trials of Adults with HIV on Co-formulated Bictegravir, Emtricitabine, and Tenofovir Alafenamide versus Co-formulated Abacavir, Dolutegravir, and Lamivudine. Patient 2018, 11, 561–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeks, E.D. Bictegravir/Emtricitabine/Tenofovir Alafenamide: A Review in HIV-1 Infection. Drugs 2018, 78, 1817–1828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggiolo, F.; Rizzardini, G.; Molina, J.M.; Pulido, F.; De Wit, S.; Vandekerckhove, L.; Berenguer, J.; D’Antoni, M.L.; Blair, C.; Chuck, S.K.; et al. Bictegravir/Emtricitabine/Tenofovir Alafenamide in Virologically Suppressed People with HIV Aged ≥ 65 Years: Week 48 Results of a Phase 3b, Open-Label Trial. Infect. Dis. Ther. 2021, 10, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Sax, P.E.; Erlandson, K.M.; Lake, J.E.; Mccomsey, G.A.; Orkin, C.; Esser, S.; Brown, T.T.; Rockstroh, J.K.; Wei, X.; Carter, C.C.; et al. Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin. Infect. Dis. 2020, 71, 1379–1389. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, R.N.; Kania, A.T.; Michienzi, S.M.; Patel, M.; Badowski, M.E. Weight Gain in Incarcerated Individuals Living With HIV After Switching to Integrase Strand Inhibitor-Based Therapy. J. Int. Assoc. Provid. AIDS Care 2021, 20, 2325958221996860. [Google Scholar] [CrossRef] [PubMed]
| Overall n = 147 | Over 55 Years n = 93 | |||
|---|---|---|---|---|
| m or # | IQR or % | m or # | IQR or % | |
| Age (Years) | 57 | 49–61 | 60 | 57–64 |
| Gender (Male) | 104 | 70.7% | 69 | 74.2% |
| Smoking (Yes) | 98 | 66.7% | 59 | 63.4% |
| Time from HIV-1 diagnosis (Years) | 16 | 10–22 | 19 | 13–25 |
| HIV-RNA < 50 cp/mL before switch (Months) | 37 | 37–48 | 37 | 37–48 |
| History of AIDS diagnosis (Yes) | 46 | 31.3% | 34 | 36.6% |
| HBV co-infection (Yes) | 12 | 8.2% | 8 | 8.6% |
| Former HCV infection (Yes) | 16 | 10.9% | 13 | 14% |
| HIV-1-related non-AIDS comorbidities | ||||
| >=1 | 87 | 59.2% | 64 | 68.8% |
| How many | 1 | 0–1 | 1 | 0–2 |
| Osteopenia | 35 | 23.8% | 24 | 25.8% |
| Ostoporosis | 25 | 17% | 22 | 23.7% |
| Type 2 Diabetes | 12 | 8.2% | 10 | 10.8% |
| Hypertension | 31 | 21.1% | 23 | 24.7% |
| Cardiovascular disease | 12 | 8.2% | 11 | 11.8% |
| ASCVD risk score (%) | 39 | 20–50 | 39 | 19–50 |
| Other than ART co-medications | ||||
| >=1 | 57 | 38.8% | 43 | 46.2% |
| >=2 | 32 | 21.8% | 28 | 30.% |
| Pre-switch ART regimen | ||||
| INSTI | 23 | 15.6% | 16 | 17.2% |
| NNRTI | 20 | 13.6% | 10 | 10.8% |
| PI | 97 | 66% | 63 | 67.7% |
| PI + INSTI | 7 | 4.8% | 4 | 4.3% |
| TDF-based backbone | 94 | 63.9% | 62 | 66.7% |
| TAF-based backbone | 33 | 22.4% | 19 | 20.4% |
| ABC-based backcone | 5 | 3.4% | 2 | 2.2% |
| Dual therapy | 11 | 7.5% | 8 | 8.6% |
| Reason to switch | ||||
| Adherence | 3 | 2% | 3 | 3.2% |
| Adverse events | 1 | 0.7% | 1 | 1.1% |
| Pro-active | 14 | 9.5% | 6 | 6.5% |
| Simplification | 95 | 64.6% | 62 | 66.7% |
| Toxicity | 34 | 23.1% | 21 | 22.6% |
| Overall | Baseline n = 147 | Week 48 n = 147 | Change from Baseline Absolute Percentage | p-Value | |||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | ||
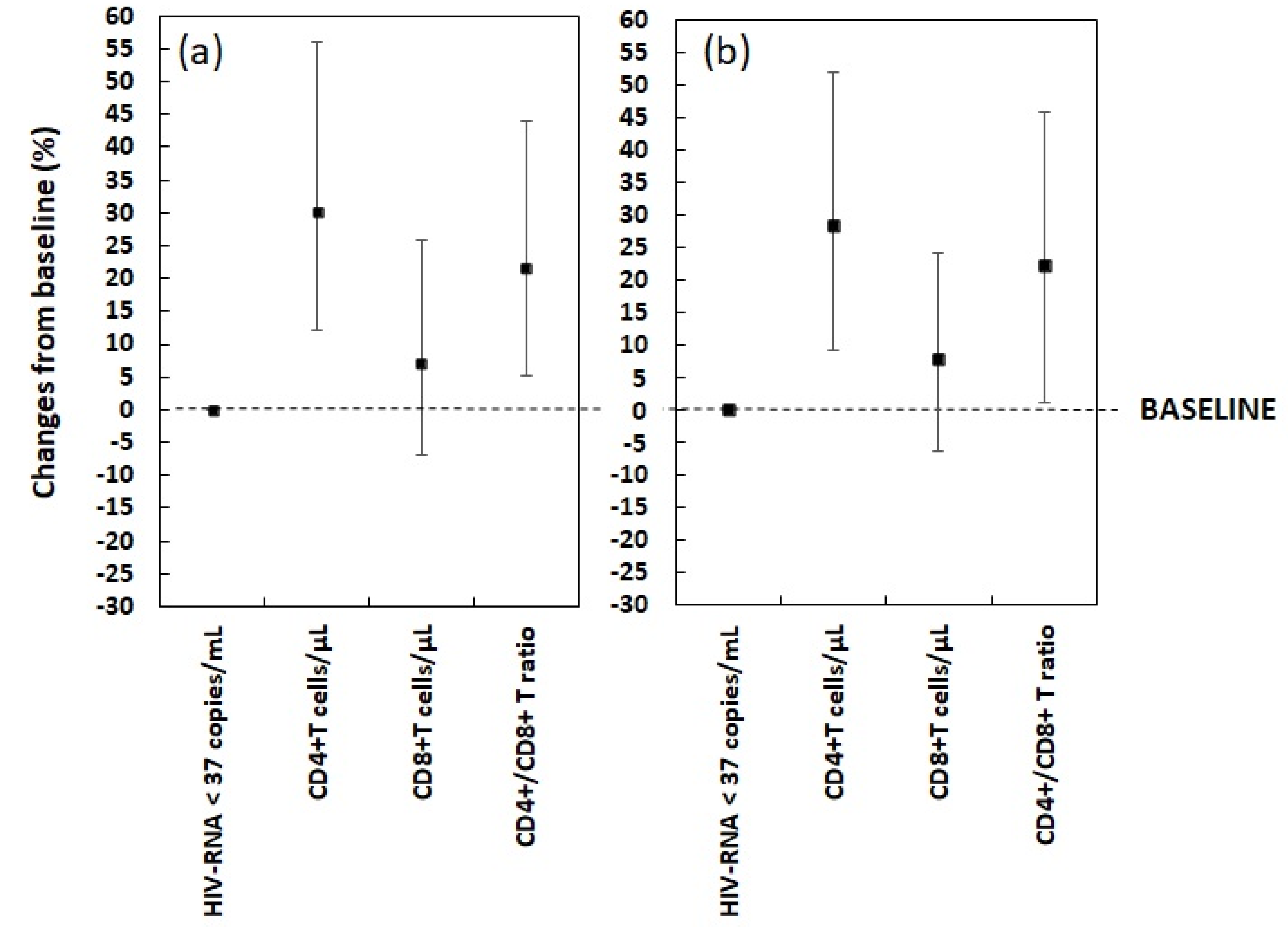
| CD4+ T cells count (cells/μL) | 584 | 454–746 | 767 | 590–1033 | 184 | 73–286 | 30.1 | 12.2–56.2 | <0.001 |
| CD8+ T cells count (cells/μL) | 750 | 580–1002 | 850 | 651–1032 | 60 | −50–150 | 7.1 | −6.8–25.7 | <0.001 |
| CD4+/CD8+ T cells ratio | 0.7 | 0.6–0.83 | 0.9 | 0.8–1 | 0.15 | 0.04–0.3 | 21.5 | 5.3–43.9 | <0.001 |
| Total Cholesterol (mg/dL) | 190 | 168–212 | 178 | 155–202 | −14 | −24–3 | −6.9 | −13.2–1.5 | <0.001 |
| LDL (mg/dL) | 108 | 88–133 | 100 | 84–131 | −7 | −17–7 | −6.8 | −15.6–8 | 0.007 |
| HDL (mg/dL) | 49 | 41–60 | 50 | 44–62 | 2 | −5–8 | 4.4 | −8.9–20.2 | 0.033 |
| Total Cholesterol/HDL ratio | 3.77 | 3.12–4.71 | 3.40 | 2.91–4.19 | −0.29 | −0.92–0.19 | −8 | −22.7–6 | <0.001 |
| AST (mg/dL) | 21 | 17–24 | 20 | 16–23 | 0 | −4–2 | −2.2 | −18.2–14.3 | 0.129 |
| ALT (mg/dL) | 21 | 16–26 | 20 | 16–25 | −1 | −4–2 | −5 | −18.8–12.5 | 0.143 |
| Body weight (Kg) | 77 | 69–83 | 79 | 71–85 | 1 | 0–3 | 1.8 | 0–3.9 | 0.014 |
| Body Mass Index | 22 | 20–24 | 22 | 21–24 | 1 | 0–1.3 | 4.2 | 0–7 | <0.001 |
| Creatinine (mg/dL) | 0.93 | 0.84–1.04 | 1 | 0.84–1.12 | 0.01 | −0.01–0.08 | 0.8 | −0.9–7.8 | 0.014 |
| eGFR-CKD-EPI (mL/min/1.73 m2) | 86 | 76–96 | 86 | 72–95 | 0 | −5–5 | 0 | −5.6–5.8 | 0.784 |
| Level of Adherence (%) | 95 | 95–99 | 99 | 99–99 | 4 | 0–4 | 4.2 | 0–4.2 | <0.001 |
| Over 55 Years | Baseline n = 93 | Week 48 n = 93 | Change from Baseline Absolute Percentage | p-Value | |||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | ||
| CD4+ T cells count (cells/μL) | 585 | 462–728 | 762 | 589–956 | 176 | 52–264 | 28.3 | 9.1–51.9 | <0.001 |
| CD8+ T cells count (cells/μL) | 750 | 595–1000 | 850 | 677–1000 | 56 | −54–140 | 7.9 | −6.3–24.3 | 0.010 |
| CD4+/CD8+ T cells ratio | 0.7 | 0.6–0.82 | 0.9 | 0.8–1 | 0.14 | 0.01–0.3 | 22.2 | 1.1–46 | <0.001 |
| Total Cholesterol (mg/dL) | 191 | 169–214 | 180 | 156–203 | −14 | −24–0 | −6.9 | −13–0 | <0.001 |
| LDL (mg/dL) | 110 | 88–134 | 103 | 84–134 | −7 | −17.5–7.5 | −6.7 | −15.5–7.1 | 0.043 |
| HDL (mg/dL) | 46 | 40–57 | 50 | 43–61 | 3 | −5–8 | 6.8 | −9.1–22.4 | 0.027 |
| Total Cholesterol/HDL ratio | 3.85 | 3.37–4.74 | 3.48 | 2.93–4.24 | −0.38 | −1.01–0.17 | −8.6 | −24.8–5.5 | <0.001 |
| AST (mg/dL) | 21 | 18–24 | 20 | 17–23 | −1 | −4–2 | −5.8 | −18.4–14.5 | 0.149 |
| ALT (mg/dL) | 21 | 16–25 | 20 | 16–25 | −1 | −4–2 | −5.9 | −16–9.5 | 0.139 |
| Body weight (Kg) | 77 | 69–86 | 78 | 71–88 | 1 | 0–3 | 1.9 | 0–3.5 | 0.063 |
| Body Mass Index | 22 | 21–24 | 22 | 21–23.25 | 0 | 0–1 | 0 | 0–5.6 | 0.025 |
| Creatinine (mg/dL) | 0.9 | 0.81–1.01 | 0.99 | 0.82–1.11 | 0 | −0.02–0.08 | 0 | −2–8.2 | 0.073 |
| eGFR-CKD-EPI (mL/min/1.73 m2) | 83 | 74–91 | 82 | 72–91 | 0 | −5–4 | 0 | −6.3–4.9 | 0.737 |
| Level of Adherence (%) | 95 | 95−99 | 99 | 99–99 | 4 | 0–4 | 4.2 | 0–4.2 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzaro, A.; Cacciola, E.G.; Borrazzo, C.; Innocenti, G.P.; Cavallari, E.N.; Mezzaroma, I.; Falciano, M.; Fimiani, C.; Mastroianni, C.M.; Ceccarelli, G.; et al. Switching to a Bictegravir Single Tablet Regimen in Elderly People Living with HIV-1: Data Analysis from the BICTEL Cohort. Diagnostics 2022, 12, 76. https://doi.org/10.3390/diagnostics12010076
Lazzaro A, Cacciola EG, Borrazzo C, Innocenti GP, Cavallari EN, Mezzaroma I, Falciano M, Fimiani C, Mastroianni CM, Ceccarelli G, et al. Switching to a Bictegravir Single Tablet Regimen in Elderly People Living with HIV-1: Data Analysis from the BICTEL Cohort. Diagnostics. 2022; 12(1):76. https://doi.org/10.3390/diagnostics12010076
Chicago/Turabian StyleLazzaro, Alessandro, Elio Gentilini Cacciola, Cristian Borrazzo, Giuseppe Pietro Innocenti, Eugenio Nelson Cavallari, Ivano Mezzaroma, Mario Falciano, Caterina Fimiani, Claudio Maria Mastroianni, Giancarlo Ceccarelli, and et al. 2022. "Switching to a Bictegravir Single Tablet Regimen in Elderly People Living with HIV-1: Data Analysis from the BICTEL Cohort" Diagnostics 12, no. 1: 76. https://doi.org/10.3390/diagnostics12010076
APA StyleLazzaro, A., Cacciola, E. G., Borrazzo, C., Innocenti, G. P., Cavallari, E. N., Mezzaroma, I., Falciano, M., Fimiani, C., Mastroianni, C. M., Ceccarelli, G., & d’Ettorre, G. (2022). Switching to a Bictegravir Single Tablet Regimen in Elderly People Living with HIV-1: Data Analysis from the BICTEL Cohort. Diagnostics, 12(1), 76. https://doi.org/10.3390/diagnostics12010076








