Electromyography of Extrinsic and Intrinsic Ear Muscles in Healthy Probands and Patients with Unilateral Postparalytic Facial Synkinesis
Abstract
:1. Introduction
2. Material and Methods
2.1. Cadaver Preparations
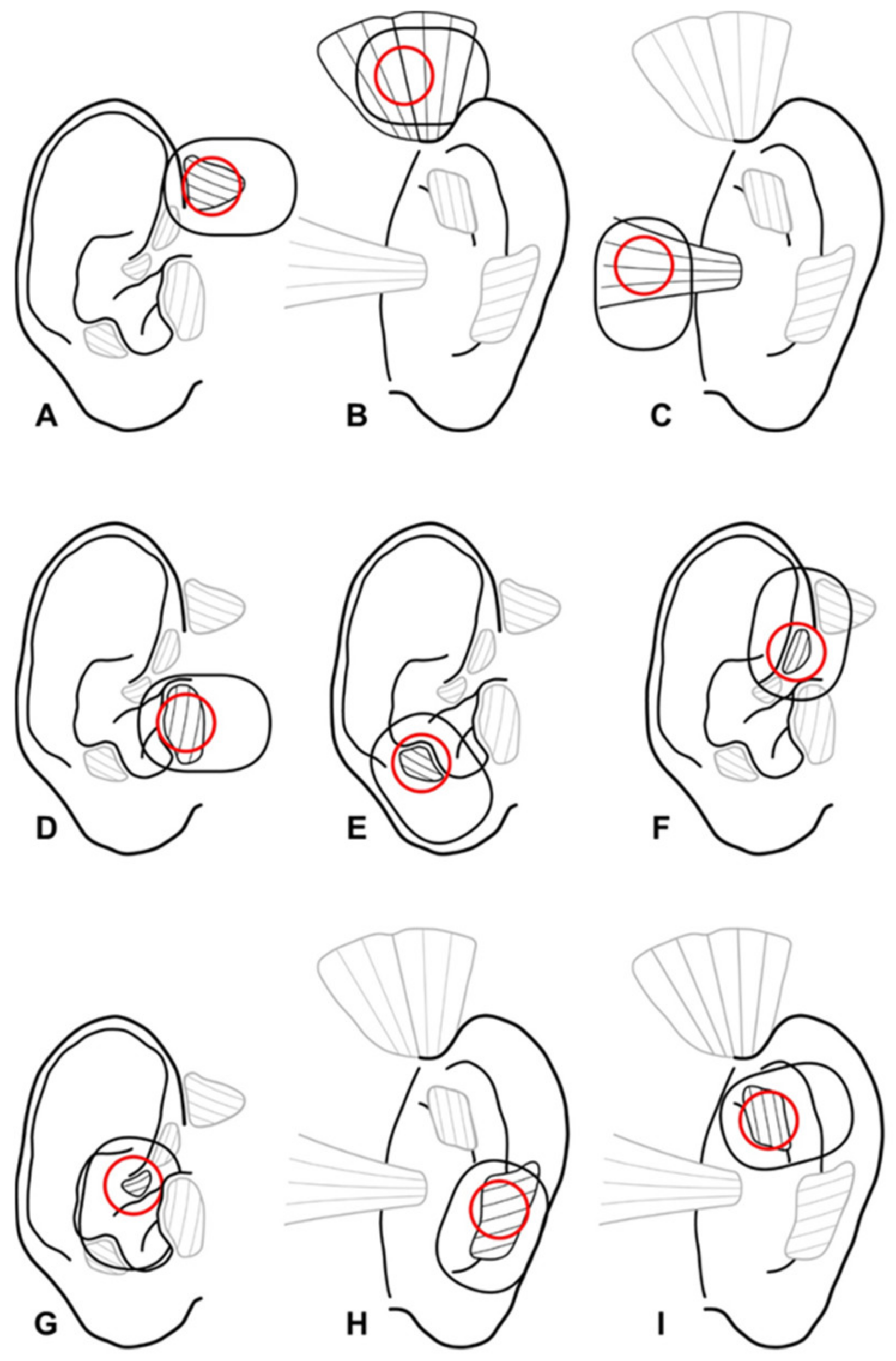
2.2. One-Channel Needle and Surface Electromyography Setting
2.3. Multi-Channel Surface Electromyography Setting
2.4. Statistical Analysis
3. Results
3.1. Cadaver Preparations of the Extrinsic and Intrinsic Ear Muscles
3.2. One-Channel Needle Electromyography of the Extrinsic and Intrinsic Ear Muscles in One Healthy Proband
3.3. One-Channel Surface Electromyography of the Extrinsic and Intrinsic Ear Muscles in Healthy Probands
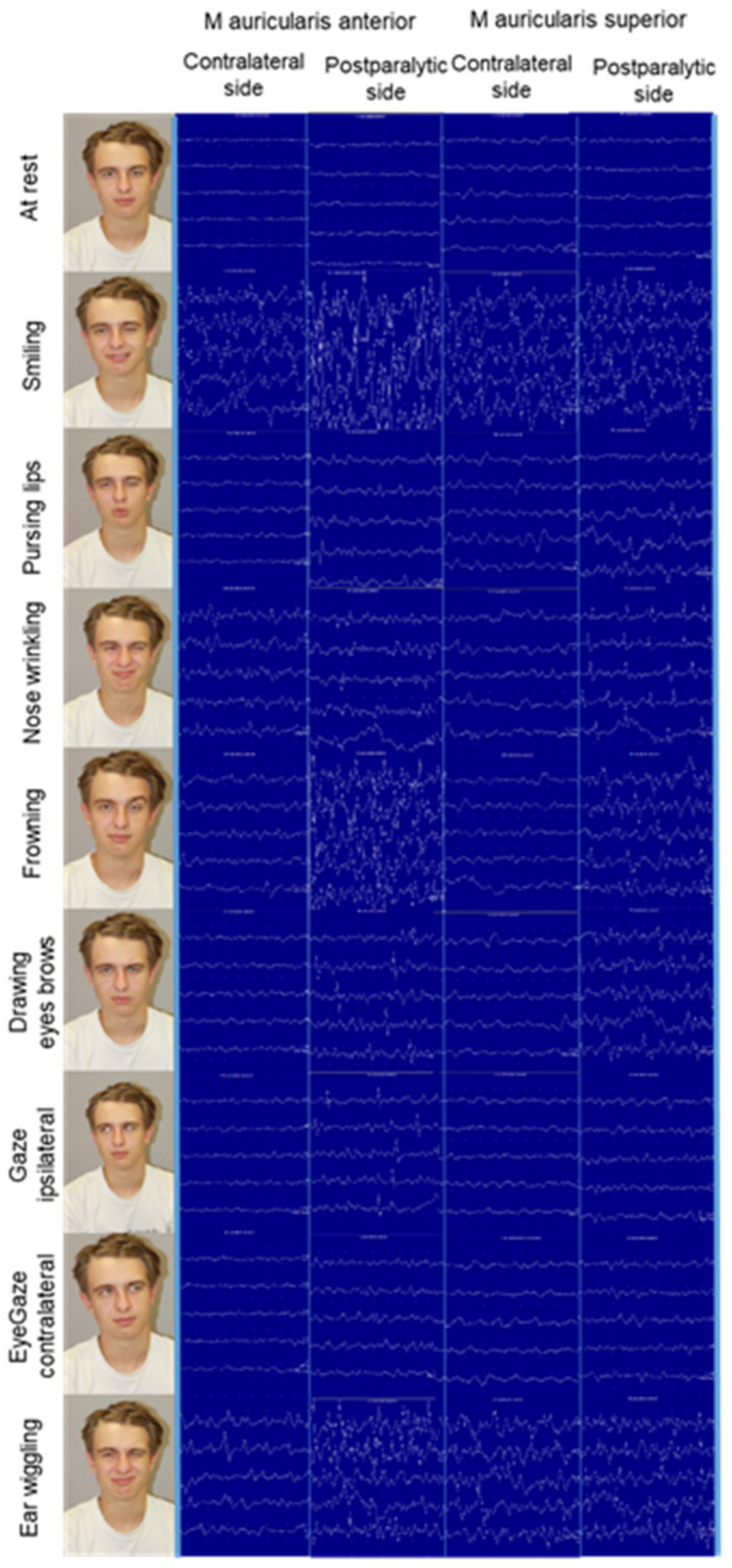
3.4. Multi-Channel Surface Electromyography of the Extrinsic and Intrinsic Ear Muscles in Healthy Probands
3.5. One-Channel Surface Electromyography of the Extrinsic and Intrinsic Ear Muscles in Patients with Postparalytic Facial Synkinesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paulsen, F.; Waschke, J. Atlas of Anatomy. Head, Neck and Neuroanatomy; Elsevier: Amsterdam, The Netherlands, 2017; Volume 3. [Google Scholar]
- Liugan, M.; Zhang, M.; Cakmak, Y.O. Neuroprosthetics for Auricular Muscles: Neural Networks and Clinical Aspects. Front. Neurol. 2017, 8, 752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guntinas-Lichius, O.; Volk, G.F.; Olsen, K.D.; Makitie, A.A.; Silver, C.E.; Zafereo, M.E.; Rinaldo, A.; Randolph, G.W.; Simo, R.; Shaha, A.R.; et al. Facial nerve electrodiagnostics for patients with facial palsy: A clinical practice guideline. Eur. Arch. Otorhinolaryngol. 2020, 277, 1855–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berzin, F.; Fortinguerra, C.R. EMG study of the anterior, superior and posterior auricular muscles in man. Ann. Anat. 1993, 175, 195–197. [Google Scholar] [CrossRef]
- Serra, G.; Tugnoli, V.; Cristofori, M.C.; Eleopra, R.; de Grandis, D. The electromyographic examination of the posterior auricular muscle. Electromyogr. Clin. Neurophysiol. 1986, 26, 661–665. [Google Scholar] [PubMed]
- Hobson, D.E.; Borys, A.E. Oculo-Auricular Synkinesia Post Bell’s Palsy Causing Unilateral Wilson’s Phenomenon. Mov. Disord. Clin. Pract. 2020, 7, 564–566. [Google Scholar] [CrossRef] [PubMed]
- O’Beirne, G.A.; Patuzzi, R.B. Basic properties of the sound-evoked post-auricular muscle response (PAMR). Hear. Res. 1999, 138, 115–132. [Google Scholar] [CrossRef]
- Schmalfuss, L.; Rupp, R.; Tuga, M.R.; Kogut, A.; Hewitt, M.; Meincke, J.; Klinker, F.; Duttenhoefer, W.; Eck, U.; Mikut, R.; et al. Steer by ear: Myoelectric auricular control of powered wheelchairs for individuals with spinal cord injury. Restor. Neurol. Neurosci. 2016, 34, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Kopsch, F. Rauber’s Lehrbuch der Anatomie des Menschen, 8th ed.; Georg Thieme: Leipzig, Germany, 1909. [Google Scholar]
- Platzer, W. Pernkopf Anatomie: Kopf und Hals; Urban & Schwarzenberg: Munich, Germany, 1987; Volume 1. [Google Scholar]
- Benninghoff, A.; Drenckhahn, D. Anatomie; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Standring, S. Gray’s Anatomy. The Anatomical Basis of Clinical Practice, 42nd ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Benning, S.D.; Patrick, C.J.; Lang, A.R. Emotional modulation of the post-auricular reflex. Psychophysiology 2004, 41, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Bochenek, W.; Bochenek, Z. Postauricular (12 msec latency) responses to acoustic stimuli in patients with peripheral, facial nerve palsy. Acta Otolaryngol. 1976, 81, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.P.; Marczynski, U.; Hopf, H.C. The oculo-auricular phenomenon. Findings in normals and patients with brainstem lesions. Brain 1993, 116, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.; Patuzzi, R. Rotation of the eyes (not the head) potentiates the postauricular muscle response. Ear Hear. 2014, 35, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, D.F.F.; Bento, R.F.; Goffi Gomez, M.V.S. The Contribution of Surface Electromyographic Assessment for Defining the Stage of Peripheral Facial Paralysis: Flaccid or Sequelae Stage. Int. Arch. Otorhinolaryngol. 2018, 22, 348–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, O.J.; Ross, G.L. Variations in the anatomy of the posterior auricular nerve and its potential as a landmark for identification of the facial nerve trunk: A cadaveric study. Anat. Sci. Int. 2012, 87, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Thoden, U. Co-activation of the M. transversus auris with eye movements (Wilson’s oculo-auricular phenomenon) and with activity in other cranial nerves. Graefes Arch. Klin. Exp. Ophthalmol. 1978, 206, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Leistritz, L.; Hochreiter, J.; Bachl, F.; Volk, G.F. Classification of facial movements in chronic facial palsy based on intramuscular EMG signals recorded from the paretic side. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2020, 2020, 662–665. [Google Scholar] [CrossRef] [PubMed]
| Muscle/ Exercise | Right Side | Left Side | p | Muscle/ Exercise | Right Side | Left Side | p | Muscle/ Exercise | Right Side | Left Side | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M. auricularis ant. | Mean ± SD | Mean ± SD | M. tragicus | Mean ± SD | Mean ± SD | M. helicis minor | Mean ± SD | Mean ± SD | |||
| Smiling | 2.91 ± 0.28 | 2.83 ± 0.38 | 0.586 | Smiling | 2.90 ± 0.30 | 3.00 ± 0 | 0.341 | Smiling | 2.77 ± 0.44 | 2.77 ± 0.44 | 1.000 |
| Pursing lips | 1.41 ± 0.79 | 1.66 ± 0.88 | 0.339 | Pursing lips | 1.54 ± 0.82 | 1.81 ± 0.60 | 0.277 | Pursing lips | 1.00 ± 1.11 | 1.44 ± 1.01 | 0.272 |
| Nose wrinkling | 0.66 ± 0.65 | 1.41 ± 1.08 | 0.069 | Nose wrinkling | 0.90 ± 0.87 | 0.80 ± 0.78 | 0.780 | Nose wrinkling | 0.11 ± 0.33 | 0.77 ± 1.09 | 0.050 |
| Frowning | 1.91 ± 1.16 | 1.33 ± 1.15 | 0.189 | Frowning | 1 ± 0.89 | 1.45 ± 1.03 | 0.096 | Frowning | 1.55 ± 1.33 | 1.33 ± 1.41 | 0.347 |
| Drawing eyebrows | 0.33 ± 0.88 | 0.58 ± 0.66 | 0.491 | Drawing eyebrows | 0.18 ± 0.60 | 0.90 ± 0.94 | 0.054 | Drawing eyebrows | 0.11 ± 0.33 | 0.44 ± 1.01 | 0.347 |
| Ipsilateral gaze | 0.41 ± 0.90 | 0.58 ± 0.79 | 0.504 | Ipsilateral gaze | 0.27 ± 0.90 | 0.18 ± 0.4 | 0.779 | Ipsilateral gaze | 0 ± 0 | 0.33 ± 0.70 | 0.195 |
| Contralateral gaze | 0 | 0.45 ± 0.52 | 0.016 | Contralateral gaze | 0.18 ± 0.60 | 0.27 ± 0.64 | 0.756 | Contralateral gaze | 0.22 ± 0.44 | 0.22 ± 0.44 | 1.000 |
| Ear wiggling | 2.75 ± 0.7 | 2.75 ± 0.7 | NA | Ear wiggling | 2.42 ± 0.78 | 2.42 ± 0.97 | 1.000 | Ear wiggling | 2.83 ± 0.4 | 2.5 ± 1.22 | 0.363 |
| M. auricularis sup. | M. antitragicus | M. trans. auriculae | |||||||||
| Smiling | 2.83 ± 0.38 | 2.83 ± 0.38 | 1.000 | Smiling | 2.91 ± 0.28 | 2.83 ± 0.57 | 0.339 | Smiling | 3 ± 0 | 2.91 ± 0.28 | 0.339 |
| Pursing lips | 1.16 ± 1.02 | 1.41 ± 0.9 | 0.339 | Pursing lips | 1.41 ± 0.79 | 1.33 ± 0.98 | 0.795 | Pursing lips | 1.16 ± 0.83 | 1.16 ± 0.71 | 1.000 |
| Nose wrinkling | 0.91 ± 1.08 | 0.66 ± 0.77 | 0.389 | Nose wrinkling | 0.58 ± 0.79 | 0.58 ± 0.79 | 1.000 | Nose wrinkling | 0.66 ± 0.88 | 1.08 ± 0.90 | 0.137 |
| Frowning | 2.25 ± 0.96 | 2.25 ± 0.96 | 1.000 | Frowning | 1.45 ± 1.21 | 1.54 ± 1.21 | 0.756 | Frowning | 1.66 ± 1.30 | 1.50 ± 1.24 | 0.504 |
| Drawing eyebrows | 0.50 ± 0.90 | 0.75 ± 0.96 | 0.191 | Drawing eyebrows | 0.33 ± 0.77 | 1.00 ± 1.04 | 0.071 | Drawing eyebrows | 0.50 ± 1.00 | 0.41 ± 0.66 | 0.586 |
| Ipsilateral gaze | 0.83 ± 1.19 | 1.00 ± 0.85 | 0.674 | Ipsilateral gaze | 0.50 ± 0.90 | 0.25 ± 0.45 | 0.339 | Ipsilateral gaze | 1.16 ± 1.11 | 1.00 ± 0.85 | 0.615 |
| Contralateral gaze | 0.25 ± 0.62 | 0.33 ± 0.65 | 0.723 | Contralateral gaze | 0.66 ± 0.77 | 0.33 ± 0.49 | 0.220 | Contralateral gaze | 1.25 ± 0.96 | 1.00 ± 0.95 | 0.463 |
| Ear wiggling | 2.87 ± 0.35 | 2.75 ± 0.46 | 0.351 | Ear wiggling | 2.85 ± 0.37 | 2.57 ± 1.13 | 0.356 | Ear wiggling | 2.75 ± 0.46 | 2.50 ± 0.92 | 0.170 |
| M. auricularis post. | M. helicis major | M. obliq. auriculae | |||||||||
| Smiling | 2.41 ± 0.51 | 2.58 ± 0.66 | 0.339 | Smiling | 3.00 ± 0 | 2.81 ± 0.4 | 0.167 | Smiling | 2.90 ± 0.30 | 3 ± 0 | 0.341 |
| Pursing lips | 0.91 ± 0.9 | 1.16 ± 0.93 | 0.389 | Pursing lips | 1.18 ± 0.98 | 0.9 ± 1.13 | 0.465 | Pursing lips | 1.00 ± 0.89 | 1.18 ± 0.98 | 0.506 |
| Nose wrinkling | 0.58 ± 0.79 | 0.75 ± 0.75 | 0.551 | Nose wrinkling | 1.18 ± 1.25 | 0.9 ± 0.83 | 0.341 | Nose wrinkling | 0.72 ± 1.00 | 0.90 ± 1.04 | 0.441 |
| Frowning | 1.41 ± 1.44 | 1.83 ± 1.46 | 0.210 | Frowning | 2.45 ± 0.93 | 1.9 ± 1.04 | 0.052 | Frowning | 1.90 ± 1.22 | 1.90 ± 1.22 | 1.000 |
| Drawing eyebrows | 0.50 ± 0.90 | 0.83 ± 1.19 | 0.266 | Drawing eyebrows | 0.63 ± 0.92 | 0.72 ± 1.00 | 0.810 | Drawing eyebrows | 0.90 ± 1.13 | 0.63 ± 0.80 | 0.082 |
| Ipsilateral gaze | 1.00 ± 0.95 | 0.83 ± 0.57 | 0.586 | Ipsilateral gaze | 0.36 ± 0.8 | 0.36 ± 0.5 | 1.000 | Ipsilateral gaze | 1.27 ± 0.90 | 1.00 ± 0.89 | 0.432 |
| Contralateral gaze | 0.66 ± 1.07 | 0.75 ± 0.75 | 0.809 | Contralateral gaze | 0.18 ± 0.4 | 0.18 ± 0.4 | 1.000 | Contralateral gaze | 1.18 ± 0.98 | 1.27 ± 1.10 | 0.821 |
| Ear wiggling | 2.62 ± 0.74 | 2.75 ± 0.46 | 0.351 | Ear wiggling | 2.86 ± 0.37 | 2.86 ± 0.37 | NA | Ear wiggling | 2.87 ± 0.35 | 2.50 ± 0.92 | 0.197 |
| Exercise | M. risorius | M. auricularis Posterior | M. auricularis Superior | M. tragicus | M. temporalis | M. masseter |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Smiling | 2.64 + 0.50 | 0 + 0 | 2.64 + 0.50 | 1.91 + 1.51 | 2.00 + 0 | 1.64 + 0.50 |
| Pursing lips | 2.27 + 1.01 | 0 + 0 | 1.91 + 0.30 | 0.64 + 0.50 | 1.82 + 0.40 | 0.64 + 0.50 |
| Nose wrinkling | 1.09 + 0.30 | 0 + 0 | 1.36 + 0.50 | 0.64 + 0.50 | 1.00 + 0 | 0 + 0 |
| Frowning | 1.64 + 0.50 | 1.36 + 0.50 | 2.91 + 0.30 | 1.36 + 0.50 | 3.00 + 0 | 1.00 + 0 |
| Drawing eyebrows | 2.36 + 0.50 | 0.36 + 0.50 | 2 + 0 | 1.09 + 1.51 | 2.36 + 0.50 | 1.00 + 0 |
| Ipsilateral gaze | 1.82 + 0.98 | 0 + 0 | 1.64 + 0.50 | 0.36 + 0.50 | 1.73 + 1.01 | 1.09 + 1.51 |
| Contralateral gaze | 1.64 + 0.92 | 0 + 0 | 2.36 + 0.50 | 1.00 + 1.41 | 1.73 + 1.01 | 1.09 + 1.51 |
| Ear wiggling | 1.64 + 0.92 | 1.27 + 1.01 | 3.00 + 0 | 2.27 + 0.47 | 3.00 + 0 | 1.64 + 0.92 |
| Showing teeth | 3.00 + 0 | 0.64 + 0.50 | 2.27 + 0.47 | 3.00 + 0 | 2.64 + 0.50 | 1.36 + 0.50 |
| Clenching teeth | 3.00 + 0 | 0 + 0 | 1.36 + 0.50 | 1.09 + 0.30 | 2.64 + 0.50 | 2.27 + 1.01 |
| Chewing ipsilateral | 2.27 + 1.01 | 0 + 0 | 2.18 + 0.98 | 2.09 + 0.94 | 2.18 + 0.98 | 1.73 + 1.42 |
| Chewing contralateral | 2.55 + 0.52 | 0.36 + 0.50 | 3.00 + 0 | 2.27 + 1.01 | 2.91 + 0.3 | 2.27 + 1.01 |
| Muscle/ Exercise | Postparalytic | Contralateral | p | Muscle/ Exercise | Postparalytic | Contralateral | p | Muscle/ Exercise | Postparalytic | Contralateral | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M. auricularis ant. | Mean ± SD | Mean ± SD | M. tragicus | Mean ± SD | Mean ± SD | M. helicis minor | Mean ± SD | Mean ± SD | |||
| Smiling | 2.71 ± 0.48 | 2.85 ± 0.37 | 0.356 | Smiling | 2.28 ± 0.75 | 2.71 ± 0.48 | 0.078 | Smiling | 2.85 ± 0.37 | 2.57 ± 0.53 | 0.172 |
| Pursing lips | 1.71 ± 1.25 | 1.00 ± 0.57 | 0.140 | Pursing lips | 1.57 ± 0.78 | 0.71 ± 0.48 | 0.001 | Pursing lips | 1.85 ± 1.06 | 0.57 ± 0.53 | 0.012 |
| Nose wrinkling | 2 ± 1 | 1.28 ± 1.25 | 0.310 | Nose wrinkling | 1.71 ± 0.75 | 1.00 ± 1.00 | 0.094 | Nose wrinkling | 2 ± 0.57 | 0.71 ± 0.95 | 0.022 |
| Frowning | 2.28 ± 0.75 | 1.85 ± 0.89 | 0.356 | Frowning | 2 ± 0.81 | 1.42 ± 0.97 | 0.280 | Frowning | 2.14 ± 0.69 | 1.28 ± 0.75 | 0.078 |
| Drawing eyebrows | 1.85 ± 1.21 | 0.42 ± 0.53 | 0.016 | Drawing eyebrows | 1.71 ± 0.48 | 0.85 ± 0.89 | 0.045 | Drawing eyebrows | 1.85 ± 0.89 | 0.14 ± 0.37 | 0.007 |
| Ipsilateral gaze | 1.28 ± 0.75 | 0.42 ± 0.53 | 0.078 | Ipsilateral gaze | 0.42 ± 0.53 | 1.00 ± 1.15 | 0.103 | Ipsilateral gaze | 1.14 ± 0.89 | 0.28 ± 0.48 | 0.045 |
| Contralateral gaze | 0.42 ± 0.78 | 0.57 ± 0.78 | 0.604 | Contralateral gaze | 0.42 ± 0.53 | 0.71 ± 1.11 | 0.604 | Contralateral gaze | 0.28 ± 0.48 | 0.42 ± 0.53 | 0.604 |
| Ear wiggling | 1.33 ± 1.15 | 2.66 ± 0.57 | 0.270 | Ear wiggling | 1.33 ± 1.52 | 2.33 ± 0.57 | 0.225 | Ear wiggling | 1.33 ± 1.52 | 2.66 ± 0.57 | 0.184 |
| M auricularis sup. | M. antitragicus | M. trans. auriculae | |||||||||
| Smiling | 2.57 ± 0.78 | 2.85 ± 0.37 | 0.356 | Smiling | 2.42 ± 0.53 | 2.71 ± 0.48 | 0.172 | Smiling | 2.71 ± 0.48 | 2.57 ± 0.53 | 0.356 |
| Pursing lips | 2.28 ± 1.11 | 1.28 ± 0.75 | 0.018 | Pursing lips | 1.85 ± 1.06 | 0.57 ± 0.78 | 0.035 | Pursing lips | 1.85 ± 0.69 | 1.00 ± 0.81 | 0.078 |
| Nose wrinkling | 2.28 ± 0.75 | 1.00 ± 1.15 | 0.093 | Nose wrinkling | 2.28 ± 0.75 | 0.57 ± 1.13 | 0.011 | Nose wrinkling | 2.42 ± 0.53 | 1.14 ± 1.06 | 0.063 |
| Frowning | 2.28 ± 0.75 | 1.85 ± 1.06 | 0.510 | Frowning | 2 ± 0.81 | 1.14 ± 0.37 | 0.017 | Frowning | 1.71 ± 0.95 | 1.42 ± 0.53 | 0.522 |
| Drawing eyebrows | 2.28 ± 1.11 | 0.42 ± 0.53 | 0.004 | Drawing eyebrows | 1.28 ± 1.25 | 0.28 ± 0.75 | 0.038 | Drawing eyebrows | 1.71 ± 0.75 | 0.71 ± 0.95 | 0.062 |
| Ipsilateral gaze | 1.28 ± 1.11 | 1.00 ± 1.00 | 0.457 | Ipsilateral gaze | 1 ± 0.57 | 0.57 ± 0.53 | 0.078 | Ipsilateral gaze | 0.85 ± 0.69 | 1.28 ± 0.95 | 0.289 |
| Contralateral gaze | 0.85 ± 1.21 | 0.71 ± 0.75 | 0.766 | Contralateral gaze | 0.42 ± 0.53 | 0.42 ± 0.78 | 1.000 | Contralateral gaze | 0.57 ± 0.53 | 1.42 ± 1.13 | 0.111 |
| Ear wiggling | 2 ± 1 | 2.33 ± 0.57 | 0.742 | Ear wiggling | 1 ± 1.73 | 2.00 ± 1.00 | 0.225 | Ear wiggling | 1.33 ± 1.52 | 2.00 ± 1.00 | 0.667 |
| M. auricularis post. | M. helicis major | M. obliq. auriculae | |||||||||
| Smiling | 2.71 ± 0.48 | 2.28 ± 0.48 | 0.200 | Smiling | 3 ± 0 | 3.00 ± 0 | NA | Smiling | 2.71 ± 0.48 | 2.57 ± 0.53 | 0.356 |
| Pursing lips | 1.85 ± 0.89 | 0.71 ± 0.75 | 0.005 | Pursing lips | 1.71 ± 1.11 | 0.57 ± 0.53 | 0.047 | Pursing lips | 1.85 ± 1.06 | 0.71 ± 0.95 | 0.103 |
| Nose wrinkling | 2.57 ± 0.53 | 1.14 ± 1.21 | 0.008 | Nose wrinkling | 2.42 ± 0.53 | 1.42 ± 1.13 | 0.086 | Nose wrinkling | 2.28 ± 0.48 | 1.14 ± 1.21 | 0.066 |
| Frowning | 2.14 ± 0.69 | 1.57 ± 1.27 | 0.413 | Frowning | 2.57 ± 0.53 | 1.71 ± 0.95 | 0.017 | Frowning | 2 ± 1 | 1.85 ± 0.89 | 0.604 |
| Drawing eyebrows | 2 ± 1.15 | 0.28 ± 0.48 | 0.007 | Drawing eyebrows | 2.14 ± 0.69 | 0.28 ± 0.75 | 0.0001 | Drawing eyebrows | 1.71 ± 0.75 | 0.43 ± 0.54 | 0.022 |
| Ipsilateral gaze | 1.14 ± 1.06 | 1.00 ± 0.81 | 0.736 | Ipsilateral gaze | 0.85 ± 0.69 | 0.28 ± 0.48 | 0.030 | Ipsilateral gaze | 0.71 ± 0.48 | 1.28 ± 1.11 | 0.231 |
| Contralateral gaze | 0.85 ± 0.69 | 1.00 ± 0.81 | 0.604 | Contralateral gaze | 0.42 ± 0.53 | 0.42 ± 0.53 | 1.000 | Contralateral gaze | 0.71 ± 0.48 | 1.71 ± 1.25 | 0.111 |
| Ear wiggling | 2 ± 1 | 2.66 ± 0.57 | 0.423 | Ear wiggling | 1.33 ± 1.52 | 2.33 ± 1.15 | 0.225 | Ear wiggling | 1.33 ± 1.52 | 2.66 ± 0.57 | 0.184 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rüschenschmidt, H.; Volk, G.F.; Anders, C.; Guntinas-Lichius, O. Electromyography of Extrinsic and Intrinsic Ear Muscles in Healthy Probands and Patients with Unilateral Postparalytic Facial Synkinesis. Diagnostics 2022, 12, 121. https://doi.org/10.3390/diagnostics12010121
Rüschenschmidt H, Volk GF, Anders C, Guntinas-Lichius O. Electromyography of Extrinsic and Intrinsic Ear Muscles in Healthy Probands and Patients with Unilateral Postparalytic Facial Synkinesis. Diagnostics. 2022; 12(1):121. https://doi.org/10.3390/diagnostics12010121
Chicago/Turabian StyleRüschenschmidt, Hanna, Gerd Fabian Volk, Christoph Anders, and Orlando Guntinas-Lichius. 2022. "Electromyography of Extrinsic and Intrinsic Ear Muscles in Healthy Probands and Patients with Unilateral Postparalytic Facial Synkinesis" Diagnostics 12, no. 1: 121. https://doi.org/10.3390/diagnostics12010121
APA StyleRüschenschmidt, H., Volk, G. F., Anders, C., & Guntinas-Lichius, O. (2022). Electromyography of Extrinsic and Intrinsic Ear Muscles in Healthy Probands and Patients with Unilateral Postparalytic Facial Synkinesis. Diagnostics, 12(1), 121. https://doi.org/10.3390/diagnostics12010121








