Renal and Inflammation Markers—Renalase, Cystatin C, and NGAL Levels in Asymptomatic and Symptomatic SARS-CoV-2 Infection in a One-Month Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. COVID-19 Patient Characteristics
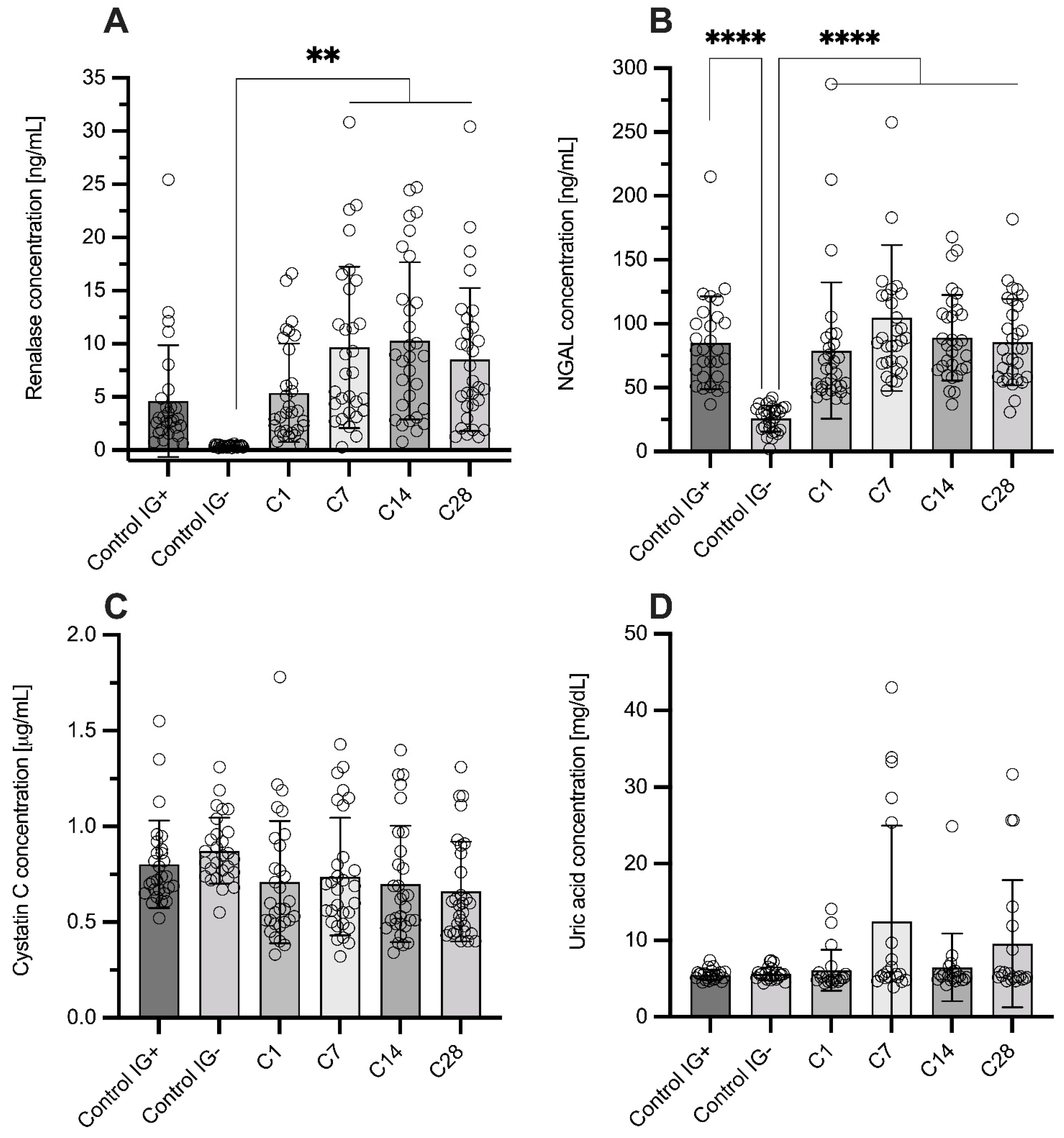
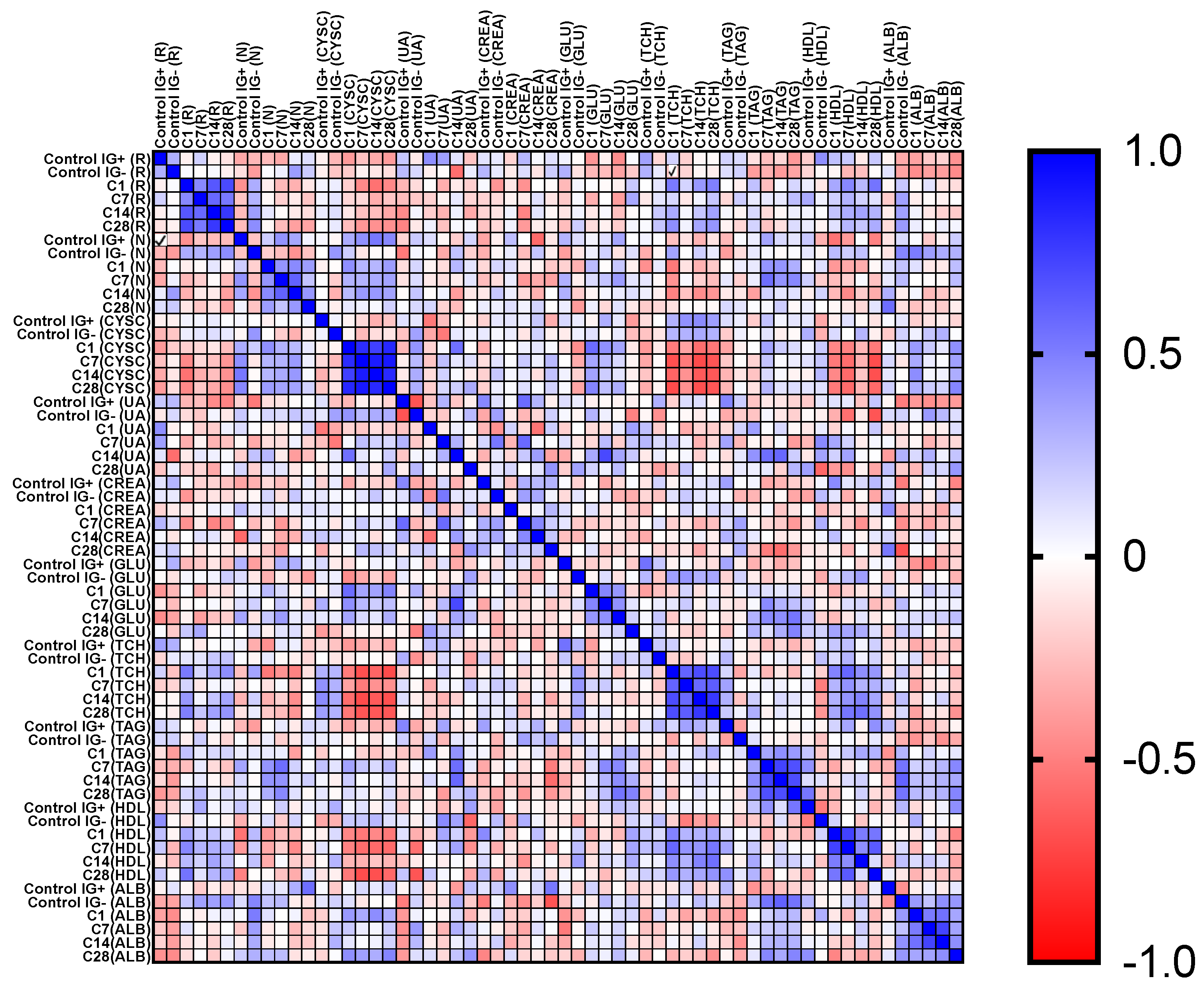
3.2. Biomarker Measurement
4. Discussion
5. Conclusions
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bhattaryya, S. Inflammation during Virus Infection: Swings and Roundabouts | SpringerLink. In Dynamics of Immune Activation in Viral Diseases; Bramhachari, P.V., Ed.; Springer Link: Berlin/Heidelberg, Germany, 2020; pp. 43–59. [Google Scholar]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, H.; Skonieczna-Żydecka, K.; Parczewski, M.; Niścigorska-Olsen, J.; Karpińska, E.; Hornung, M.; Jurczyk, K.; Witak-Jędra, M.; Laurans, Ł.; Maciejewska, K.; et al. Hepatotropic Properties of SARS-CoV-2—Preliminary Results of Cross-Sectional Observational Study from the First Wave COVID-19 Pandemic. J. Clin. Med. 2021, 10, 672. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Laukkanen, J.A. Renal complications in COVID-19: A systematic review and meta-analysis. Ann. Med. 2020, 52, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, E.; Khatibi, S.M.H.; Soofiyani, S.R.; Abediazar, S.; Shoja, M.M.; Ardalan, M.; Vahed, S.Z. COVID-19 and kidney injury: Pathophysiology and molecular mechanisms. Rev. Med. Virol. 2021, 31, e2176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.-H.; Cheng, Y.-C.; Luo, R.; Zhang, C.-X.; Ge, S.-W.; Xu, G. Recovery of new-onset kidney disease in COVID-19 patients discharged from hospital. BMC Infect. Dis. 2021, 21, 397. [Google Scholar] [CrossRef]
- Tissue Expression of RNLS-Summary-The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000184719-RNLS/tissue (accessed on 21 September 2021).
- Wiśniewska, M.; Serwin, N.; Dziedziejko, V.; Marchelek-Myśliwiec, M.; Dołęgowska, B.; Domański, L.; Ciechanowski, K.; Safranow, K.; Pawlik, A. Chronic kidney disease is associated with increased levels of renalase in serum and decreased in erythrocytes. Pol. Arch. Intern. Med. 2019, 129, 790–797. [Google Scholar] [CrossRef]
- Passov, A.; Petäjä, L.; Pihlajoki, M.; Salminen, U.-S.; Suojaranta, R.; Vento, A.; Andersson, S.; Pettilä, V.; Schramko, A.; Pesonen, E. The origin of plasma neutrophil gelatinase-associated lipocalin in cardiac surgery. BMC Nephrol. 2019, 20, 182. [Google Scholar] [CrossRef]
- Cai, L.; Rubin, J.; Han, W.; Venge, P.; Xu, S. The Origin of Multiple Molecular Forms in Urine of HNL/NGAL. Clin. J. Am. Soc. Nephrol. 2010, 5, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Gala-Bladzinska, A.; Kuzniewski, M. Performance neutrophil gelatinase-associated lipocalin in clinical settings. Przegl Lek. 2013, 70, 400–403. [Google Scholar]
- Mishra, J.; Dent, C.; Tarabishi, R.; Mitsnefes, M.M.; Ma, Q.; Kelly, C.; Ruff, S.M.; Zahedi, K.; Shao, M.; Bean, J.; et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005, 365, 1231–1238. [Google Scholar] [CrossRef]
- Bolignano, D.; Donato, V.; Coppolino, G.; Campo, S.; Buemi, A.; Lacquaniti, A.; Buemi, M. Neutrophil Gelatinase–Associated Lipocalin (NGAL) as a Marker of Kidney Damage. Am. J. Kidney Dis. 2008, 52, 595–605. [Google Scholar] [CrossRef]
- Hirsch, R.; Dent, C.; Pfriem, H.; Allen, J.; Beekman, R.H.; Ma, Q.; Dastrala, S.; Bennett, M.; Mitsnefes, M.; Devarajan, P. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr. Nephrol. 2007, 22, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Testani, J.M.; Brisco, M.A. Plasma NGAL: So, it Really Is Just Expensive Creatinine! J. Am. Coll. Cardiol. 2016, 68, 1432–1434. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Villa, P.; Jiménez, M.; Soriano, M.-C.; Manzanares, J.; Casasnovas, P. Serum cystatin C concentration as a marker of acute renal dysfunction in critically ill patients. Crit. Care 2005, 9, R139–R143. [Google Scholar] [CrossRef]
- Zhi, H.; Zhang, M.; Cui, X.; Li, Y. Renal echography and cystatin C for prediction of acute kidney injury: Very different in pa-tients with cardiac failure or sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019, 31, 1258–1263. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Peng, D.; Zhu, H.-M.; Li, B.-Y.; Yang, X.; Sun, X.-L.; Zhang, M. Predictive value of serum cystatin C for risk of mortality in severe and critically ill patients with COVID-19. World J. Clin. Cases 2020, 8, 4726–4734. [Google Scholar] [CrossRef] [PubMed]
- Klisic, A.; Kavaric, N.; Ninic, A. Serum cystatin C levels are associated with triglycerides/high-density lipoprotein cholesterol ratio in adolescent girls ages between 16-19 years old. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10680–10686. [Google Scholar] [PubMed]
- Qing, X.; Furong, W.; Yunxia, L.; Jian, Z.; Xuping, W.; Ling, G. Cystatin C and asymptomatic coronary artery disease in patients with metabolic syndrome and normal glomerular filtration rate. Cardiovasc. Diabetol. 2012, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Brisco, M.A.; Testani, J.M. Novel renal biomarkers to assess cardiorenal syndrome. Curr. Heart Fail. Rep. 2014, 11, 485–499. [Google Scholar] [CrossRef][Green Version]
- Cernaro, V.; Bolignano, D.; Donato, V.; Lacquaniti, A.; Buemi, A.; Crascì, E.; Lucisano, S.; Buemi, M. NGAL is a Precocious Marker of Therapeutic Response. Curr. Pharm. Des. 2011, 17, 844–849. [Google Scholar] [CrossRef]
- Bolignano, D.; Donato, V.; Lacquaniti, A.; Fazio, M.R.; Bono, C.; Coppolino, G.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL) in human neoplasias: A new protein enters the scene. Cancer Lett. 2010, 288, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Kaur, S.; Guha, S.; Batra, S.K. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim. Biophys. Acta BBA Rev. Cancer 2012, 1826, 129–169. [Google Scholar] [CrossRef] [PubMed]
- Komaru, Y.; Doi, K.; Nangaku, M. Urinary Neutrophil Gelatinase-Associated Lipocalin in Critically Ill Patients with Coronavirus Disease 2019. Crit. Care Explor. 2020, 2, e0181. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, Q.; Li, Z.; Shen, L.; Zhang, J.; Wang, P.; Wu, S.; Zhou, T.; Xu, Q.; Chen, X.; et al. Incorporation of Urinary Neutrophil Gelatinase-Associated Lipocalin and Computed Tomography Quantification to Predict Acute Kidney Injury and In-Hospital Death in COVID-19 Patients. Kidney Dis. 2020, 7, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Harmon, S.A.; Sanford, T.H.; Xu, S.; Turkbey, E.B.; Roth, H.; Xu, Z.; Yang, D.; Myronenko, A.; Anderson, V.; Amalou, A.; et al. Artificial intelligence for the detection of COVID-19 pneumonia on chest CT using multinational datasets. Nat. Commun. 2020, 11, 4080. [Google Scholar] [CrossRef] [PubMed]
- Matsa, R.; Ashley, E.; Sharma, V.; Walden, A.P.; Keating, L. Plasma and urine neutrophil gelatinase-associated lipocalin in the diagnosis of new onset acute kidney injury in critically ill patients. Crit. Care 2014, 18, R137. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodpoor, A.; Hamishehkar, H.; Fattahi, V.; Sanaie, S.; Arora, P.; Nader, N.D. Urinary versus plasma neutrophil gelatinase-associated lipocalin (NGAL) as a predictor of mortality for acute kidney injury in intensive care unit patients. J. Clin. Anesthesia 2018, 44, 12–17. [Google Scholar] [CrossRef]
- Duda, I.; Krzych, Ł. Plasma Neutrophil Gelatinase-Associated Lipocalin Is Useful for Predicting Mortality in Critically Ill Patients. J. Clin. Med. 2021, 10, 2576. [Google Scholar] [CrossRef]
- Koeze, J.; Van Der Horst, I.C.C.; Keus, F.; Wiersema, R.; Dieperink, W.; Kootstra-Ros, J.E.; Zijlstra, J.G.; Van Meurs, M. Plasma neutrophil gelatinase-associated lipocalin at intensive care unit admission as a predictor of acute kidney injury progression. Clin. Kidney J. 2020, 13, 994–1002. [Google Scholar] [CrossRef]
- de Geus, H.R.H.; Bakker, J.; Lesaffre, E.M.E.H.; le Noble, J.L.M.L. Neutrophil Gelatinase-associated Lipocalin at ICU Admission Predicts for Acute Kidney Injury in Adult Patients. Am. J. Respir. Crit. Care Med. 2011, 183, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Viau, A.; El Karoui, K.; Laouari, D.; Burtin, M.; Nguyen, C.; Mori, K.; Pillebout, E.; Berger, T.; Mak, T.W.; Knebelmann, B.; et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J. Clin. Investig. 2010, 120, 4065–4076. [Google Scholar] [CrossRef]
- Otto, G.P.; Hurtado-Oliveros, J.; Chung, H.-Y.; Knoll, K.; Neumann, T.; Müller, H.J.; Herbsleb, M.; Kohl, M.; Busch, M.; Sossdorf, M.; et al. Plasma Neutrophil Gelatinase-Associated Lipocalin Is Primarily Related to Inflammation during Sepsis: A Translational Approach. PLoS ONE 2015, 10, e0124429. [Google Scholar] [CrossRef]
- Velavan, T.P.; Meyer, C.G. Mild versus severe COVID-19: Laboratory markers. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020, 95, 304–307. [Google Scholar] [CrossRef]
- Johansson, C.; Kirsebom, F.C.M. Neutrophils in respiratory viral infections. Mucosal Immunol. 2021, 14, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Serwin, N.M.; Wiśniewska, M.; Cecerska-Heryć, E.; Safranow, K.; Skwirczynska, E.; Dołęgowska, B. Serum-to-urine renalase ratio and renalase fractional excretion in healthy adults and chronic kidney disease patients. BMC Nephrol. 2020, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Guo, X.; Chun, H.J.; Lee, A.I.; Cha, C.; Gorelick, F.; Desir, G.V. Decreased plasma levels of the survival factor renalase are associated with worse outcomes in COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Knop, W.; Serwin, N.M.; Cecerska-Heryć, E.; Grygorcewicz, B.; Dołęgowska, B.; Gomółka, A.; Wiśniewska, M.; Ciechanowski, K. Elevated Levels of Renalase, the β-NAD(P)H Isomerase, Can Be Used as Risk Factors of Major Adverse Cardiovascular Events and All-Cause Death in Patients with Chronic Kidney Disease. Biomolecules 2021, 11, 1514. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, Y.; Yang, L. Acute Kidney Injury in COVID-19: The Chinese Experience. Semin. Nephrol. 2020, 40, 430–442. [Google Scholar] [CrossRef]
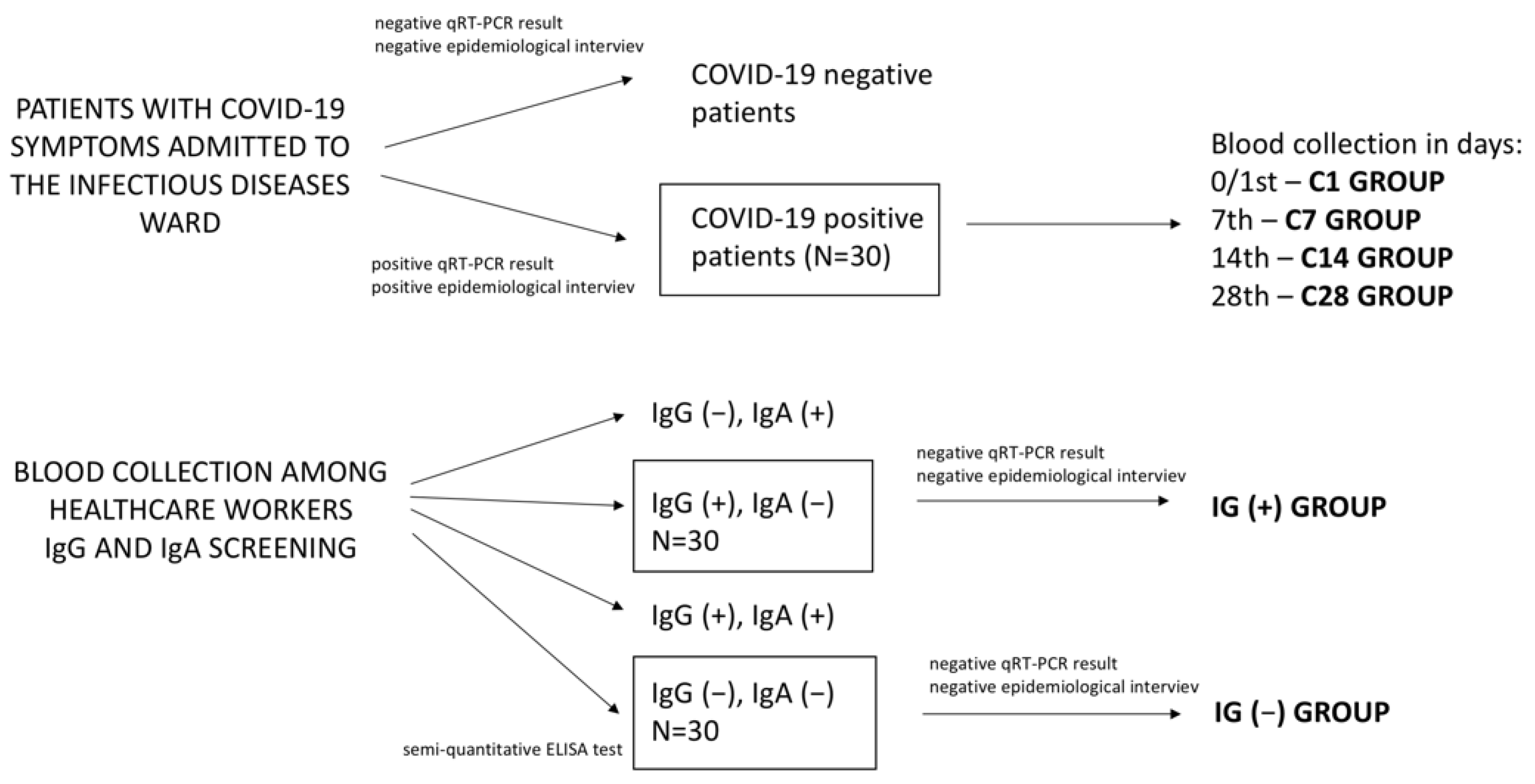

| GROUP/ PARAMETER | IG(−) | IG(+) | C1 | C7 | C14 | C28 |
|---|---|---|---|---|---|---|
| Sex (M/F) | 18/10 | 9/19 | 16/14 | |||
| Age (years) | 48 ± 9 | 42 ± 12 | 56 ± 15 | |||
| 49 (39–57) | 42 (30–50) | 58 (47–68) | ||||
| RNLS (ng/mL) | 0.36 ± 0.09 | 4.62 ± 5.26 | 5.39 ± 4.63 | 9.66 ± 7.60 | 10.29 ± 7.39 | 8.51 ± 6.72 |
| 0.39 (0.27–0.41) | 2.86 (1.76–4.43) | 3.55 (1.79–9.47) | 7.22 (3.73–15.16) | 8.82 (3.88–14.20) | 6.20 (4.11–11.51) | |
| NGAL (ng/mL) | 25.84 ± 10.28 | 85.15 ± 36.28 | 78.94 ± 53.42 | 104.54 ± 57.14 | 89.11 ± 33.55 | 85.65 ± 33.83 |
| 29.01 (17.29–33.75) | 79.71 (58.56–102.81) | 62.83 (50.66–84.26) | 87.98 (69.96–122.58) | 83.39 (64.42–107.84) | 80.20 (58.34–113.88) | |
| CYS C (mg/L) | 0.87 ± 0.17 | 0.80 ± 0.23 | 0.71 ± 0.32 | 0.74 ± 0.31 | 0.70 ± 0.31 | 0.66 ± 0.26 |
| 0.84 (0.75–0.96) | 0.73 (0.66–0.87) | 0.59 (0.50–0.90) | 0.68 (0.50–0.84) | 0.60 (0.49–0.87) | 0.60 (0.45–0.86) | |
| CREA (mg/dL) | 0.76 ± 0.29 | 1.20 ± 0.96 | 1.20 ± 0.73 | 1.51 ± 1.16 | 1.24 ± 0.79 | 1.45 ± 1.02 |
| 0.78 (0.65–0.91) | 0.90 (0.53–1.83) | 1.03 (0.75–1.63) | 0.93 (0.66–2.3) | 1.01 (0.70–1.91) | 1.22 (0.70–1.91) | |
| UA (mg/dL) | 5.6 ± 0.8 | 5.5 ± 0.7 | 5.3 ± 1.1 | 5.7 ± 1.4 | 5.5 ± 0.9 | 5.5 ± 1 |
| 5.5 (5.1–5.8) | 5.5 (5.0–5.8) | 5.1 (4.8–5.3) | 5.3 (4.8–5.9) | 5.3 (4.9–5.9) | 5.2 (4.9–5.7) | |
| GLU (mg/dL) | 101 ± 19 | 82 ± 25 | 96 ± 31 | 110 ± 40 | 100 ± 32 | 90 ± 32 |
| 93 (88–105) | 78 (64–96) | 88 (74–117) | 116 (71–135) | 99 (71–123) | 81 (70–108) | |
| TC (mg/dL) | 192 ± 27 | 167 ± 17 | 211 ± 68 | 209 ± 65 | 229 ± 62 | 244 ±75 |
| 192 (174–220 | 164 (157–182) | 212 (151–265) | 205 (164–241) | 228 (180–275) | 270 (178–299) | |
| HDL (mg/dL) | 73 ± 22 | 61 ± 10 | 70 ± 5 | 71 ± 6 | 73 ± 5 | 72 ± 7 |
| 70 (56–85) | 64 (58–68) | 71 (68–74) | 71 (69–75) | 73 (71–75) | 73 (70–77) | |
| TG (mg/dL) | 153 ± 29 | 97 ± 41 | 119 ± 46 | 174 ± 90 | 181 ± 99 | 149 ± 102 |
| 148 (137–165) | 87 (68–115) | 111 (92–139) | 144 (108–215 | 143 (113–230) | 111 (81–180) | |
| ALB (g/dL) | 4.7 ± 1.2 | 4.8 ± 0.8 | 3.8 ± 0.5 | 3.8 ± 0.6 | 4.1 ± 0.8 | 4.2 ± 0.8 |
| 4.2 (3.8–5.8) | 5.0 (4.5–5.3) | 3.8 (3.5–4.2) | 3.7 (3.4–4.2) | 4.3 (3.4–4.9) | 4.4 (3.5–4.8) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serwin, N.; Cecerska-Heryć, E.; Pius-Sadowska, E.; Serwin, K.; Niedźwiedź, A.; Wiśniewska, M.; Roszak, M.; Grygorcewicz, B.; Skwirczyńska, E.; Machaliński, B.; et al. Renal and Inflammation Markers—Renalase, Cystatin C, and NGAL Levels in Asymptomatic and Symptomatic SARS-CoV-2 Infection in a One-Month Follow-Up Study. Diagnostics 2022, 12, 108. https://doi.org/10.3390/diagnostics12010108
Serwin N, Cecerska-Heryć E, Pius-Sadowska E, Serwin K, Niedźwiedź A, Wiśniewska M, Roszak M, Grygorcewicz B, Skwirczyńska E, Machaliński B, et al. Renal and Inflammation Markers—Renalase, Cystatin C, and NGAL Levels in Asymptomatic and Symptomatic SARS-CoV-2 Infection in a One-Month Follow-Up Study. Diagnostics. 2022; 12(1):108. https://doi.org/10.3390/diagnostics12010108
Chicago/Turabian StyleSerwin, Natalia, Elżbieta Cecerska-Heryć, Ewa Pius-Sadowska, Karol Serwin, Anna Niedźwiedź, Magda Wiśniewska, Marta Roszak, Bartłomiej Grygorcewicz, Edyta Skwirczyńska, Bogusław Machaliński, and et al. 2022. "Renal and Inflammation Markers—Renalase, Cystatin C, and NGAL Levels in Asymptomatic and Symptomatic SARS-CoV-2 Infection in a One-Month Follow-Up Study" Diagnostics 12, no. 1: 108. https://doi.org/10.3390/diagnostics12010108
APA StyleSerwin, N., Cecerska-Heryć, E., Pius-Sadowska, E., Serwin, K., Niedźwiedź, A., Wiśniewska, M., Roszak, M., Grygorcewicz, B., Skwirczyńska, E., Machaliński, B., & Dołęgowska, B. (2022). Renal and Inflammation Markers—Renalase, Cystatin C, and NGAL Levels in Asymptomatic and Symptomatic SARS-CoV-2 Infection in a One-Month Follow-Up Study. Diagnostics, 12(1), 108. https://doi.org/10.3390/diagnostics12010108






