MicroRNAs—The Heart of Post-Myocardial Infarction Remodeling
Abstract
1. Introduction
2. miRNAs and Post-MI Remodeling
2.1. LVR Mechanisms
2.2. miRNAs in LVR after MI
2.3. Multifunctional miRNAs
2.4. miRNAs and Remodeling Induced by Pressure Overload
3. miRNAs as Prognostic Biomarkers of Post-MI LVR
4. miRNA as Therapeutic Targets
5. Conclusions
6. Clinical Perspective
Author Contributions
Funding
Conflicts of Interest
References
- OECD/EU. Mortality following acute myocardial infarction (AMI). In Health at a Glance: Europe 2016: State of Health in the EU Cycle; OECD Publishing: Paris, France, 2016. [Google Scholar]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Martin, G.; St John, S.; Sharpe, N. Left Ventricular Remodeling after MI. Circulation 2000, 101, 2981–2988. [Google Scholar]
- Hinderer, S.; Schenke-Layland, K. Cardiac fibrosis—A short review of causes and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Gaggin, H.K.; Barison, A.; Emdin, M.; Januzzi, J.L. Imaging, Biomarker, and Clinical Predictors of Cardiac Remodeling in Heart Failure with Reduced Ejection Fraction. JACC Heart Fail. 2019, 7, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Gravning, J.; Smedsrud, M.K.; Omland, T.; Eek, C.; Skulstad, H.; Aaberge, L.; Bendz, B.; Kjekshus, J.; Mørkrid, L.; Edvardsen, T. Sensitive troponin assays and N-terminal pro–B-type natriuretic peptide in acute coronary syndrome: Prediction of significant coronary lesions and long-term prognosis. Am. Heart J. 2013, 165, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Dobaczewski, M.; Chen, W.; Frangogiannis, N.G. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J. Mol. Cell. Cardiol. 2011, 51, 600–606. [Google Scholar] [CrossRef]
- Ma, Z.-G.; Yuan, Y.-P.; Wu, H.-M.; Zhang, X.; Tang, Q.-Z. Cardiac fibrosis: New insights into the pathogenesis. Int. J. Biol. Sci. 2018, 14, 1645–1657. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Zhang, Y. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-Smad pathways in TGF-β signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Geng, H.H.; Xiao, J.; Qin, X.T.; Wang, F.; Xing, J.H.; Xie, Y.-F.; Mao, Y.; Liang, J.-W.; Ji, X.P. miR-7a/b attenuates post-myocardial infarction remodeling and protects H9c2 cardiomyoblast against hypoxia-induced apoptosis involving Sp1 and PARP-1. Sci. Rep. 2016, 6, 29082. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, Y.; Chen, Y.; Li, J.; Zhang, Z.; Sun, Y.; Shen, H.; Zhao, Z.; Huang, Z.; Zhang, W.; et al. Inhibition of MicroRNA-9-5p Protects Against Cardiac Remodeling Following Myocardial Infarction in Mice. Hum. Gene Ther. 2019, 30, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Hullinger, T.G.; Montgomery, R.L.; Seto, A.G.; Dickinson, B.A.; Semus, H.M.; Lynch, J.M.; Dalby, C.M.; Robinson, K.; Stack, C.; Latimer, P.A.; et al. Inhibition of miR-15 Protects Against Cardiac Ischemic Injury. Circ. Res. 2012, 110, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.L.; Zheng, H.; Chen, Q.R.; Yuan, X.H.; Ren, J.H.; Luo, X.F.; Chen, P.; Lin, Z.Y.; Chen, S.Z.; Wu, X.Q.; et al. Bone marrow-derived mesenchymal stem cells overexpressing MiR-21 efficiently repair myocardial dam-age in rats. Oncotarget 2017, 8, 29161–29173. [Google Scholar] [CrossRef]
- Gao, F.; Kataoka, M.; Liu, N.; Liang, T.; Huang, Z.-P.; Gu, F.; Ding, J.; Liu, J.; Zhang, F.; Ma, Q.; et al. Therapeutic role of miR-19a/19b in cardiac regeneration and protection from myocardial infarction. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Deng, W.; Wang, Y.; Long, X.; Zhao, R.; Wang, Z.; Liu, Z.; Cao, S.; Shi, B. miR-21 Reduces Hydrogen Peroxide-Induced Apoptosis in c-kit+ Cardiac Stem Cells In Vitro through PTEN/PI3K/Akt Signaling. Oxidative Med. Cell. Longev. 2016, 2016, 1–14. [Google Scholar] [CrossRef]
- Sabatel, C.; Malvaux, L.; Bovy, N.; Deroanne, C.; Lambert, V.; Gonzalez, M.-L.A.; Colige, A.; Rakic, J.-M.; Noel, A.; Martial, J.A.; et al. MicroRNA-21 Exhibits Antiangiogenic Function by Targeting RhoB Expression in Endothelial Cells. PLoS ONE 2011, 6, e16979. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, S.; Kong, F.; Cai, X.; Ye, B.; Shan, P.; Huang, W. MicroRNA-21 protects against cardiac hypoxia/reoxygenation injury by inhibiting excessive autophagy in H9c2 cells via the Akt/mTOR pathway. J. Cell. Mol. Med. 2017, 21, 467–474. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, K.; Li, A.Y. Trimetazidine protects against hypoxia-reperfusion-induced cardiomyocyte apoptosis by in-creasing microRNA-21 expression. Int. J. Clin. Exp. Pathol. 2015, 8, 3735–3741. [Google Scholar]
- Yang, F.; Liu, W.; Yan, X.; Zhou, H.; Zhang, H.; Liu, J.; Yu, M.; Zhu, X.; Ma, K. Effects of mir-21 on Cardiac Microvascular Endothelial Cells After Acute Myocardial Infarction in Rats: Role of Phosphatase and Tensin Homolog (PTEN)/Vascular Endothelial Growth Factor (VEGF) Signal Pathway. Med. Sci. Monit. 2016, 22, 3562–3575. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, H.-Y.; Zhong, B.-L.; Zhang, B.; Chen, H. Antiapoptotic Effect of Recombinant HMGB1 A-box Protein via Regulation of microRNA-21 in Myocardial Ischemia-Reperfusion Injury Model in Rats. DNA Cell Biol. 2016, 35, 192–202. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, H.; Ge, D.; Xu, Y.; Xu, H.; Yang, Y.; Gu, M.; Zhou, Y.; Zhu, J.; Ge, T.; et al. Mir-21 Promotes Cardiac Fibrosis After Myocardial Infarction Via Targeting Smad7. Cell. Physiol. Biochem. 2017, 42, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Khanna, S.; Hussain, S.R.A.; Biswas, S.; Azad, A.; Rink, C.; Gnyawali, S.; Shilo, S.; Nuovo, G.J.; Sen, C.K. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprote-ase-2 via phosphatase and tensin homologue. Cardiovasc. Res. 2009, 82, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Tian, S.-S.; Hang, P.-Z.; Sun, C.; Guo, J.; Du, Z.-M. Combination of microRNA-21 and microRNA-146a Attenuates Cardiac Dysfunction and Apoptosis during Acute Myocardial Infarction in Mice. Mol. Ther.-Nucleic Acids 2016, 5, e296. [Google Scholar] [CrossRef]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef]
- Hong, Y.; Cao, H.; Wang, Q.; Ye, J.; Sui, L.; Feng, J.; Cai, X.; Song, H.; Zhang, X.; Chen, X. MiR-22 may Suppress Fibrogenesis by Targeting TGFβR I in Cardiac Fibroblasts. Cell. Physiol. Biochem. 2016, 40, 1345–1353. [Google Scholar] [CrossRef]
- Wang, J.; Huang, W.; Xu, R.; Nie, Y.; Cao, X.; Meng, J.; Xu, X.; Hu, S.; Zheng, Z. MicroRNA-24 regulates cardiac fibrosis after myocardial infarction. J. Cell. Mol. Med. 2012, 16, 2150–2160. [Google Scholar] [CrossRef] [PubMed]
- Icli, B.; Wara, A.; Moslehi, J.; Sun, X.; Plovie, E.; Cahill, M.; Marchini, J.; Schissler, A.; Padera, R.F.; Shi, J.; et al. MicroRNA-26a Regulates Pathological and Physiological Angiogenesis by Targeting BMP/SMAD1 Signaling. Circ. Res. 2013, 113, 1231–1241. [Google Scholar] [CrossRef]
- Xiao, L.; He, H.; Ma, L.; Da, M.; Cheng, S.; Duan, Y.; Wang, Q.; Wu, H.; Song, X.; Duan, W.; et al. Effects of miR-29a and miR-101a Expression on Myocardial Interstitial Collagen Generation After Aerobic Exercise in Myocardial-infarcted Rats. Arch. Med Res. 2017, 48, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Liu, Y.; Li, S.; Chen, Y.; Li, L.; Cao, Y.; Mingyao, M.; Shi, P.; Song, C.; Li, B.; et al. Activation of AMPK Attenuated Cardiac Fibrosis by Inhibiting CDK2 via p21/p27 and miR-29 Family Pathways in Rats. Mol. Ther.-Nucleic Acids 2017, 8, 277–290. [Google Scholar] [CrossRef]
- Van Rooij, E.; Sutherland, L.B.; Thatcher, J.E.; DiMaio, J.M.; Naseem, R.H.; Marshall, W.S.; Hill, J.A.; Olson, E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13027–13032. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ji, Q.; Zhu, H.; Ren, Y.; Fan, Z.; Tian, N. miR-30a attenuates cardiac fibrosis in rats with myocardial infarction by inhibiting CTGF. Exp. Ther. Med. 2018, 15, 4318–4324. [Google Scholar] [CrossRef]
- Duisters, R.F.; Tijsen, A.J.; Schroen, B.; Leenders, J.J.; Lentink, V.; van der Made, I.; Herias, V.; van Leeuwen, R.E.; Schellings, M.W.; Barenbrug, P.; et al. miR-133 and miR-30 Regulate Connective Tissue Growth Factor Implications for a Role of MicroRNAs in Myocardial Matrix Remodeling. Circ. Res. 2009, 104, 170–178. [Google Scholar] [CrossRef]
- Chen, L.W. Relationship between myocardial microRNA-30a expression and myocardial fibrosis in rats post myocar-dial infarction. Chin. J. Cardiovasc. Dis. 2016, 44, 443–449. [Google Scholar]
- Kim, J.O.; Park, J.H.; Kim, T.; Hong, S.E.; Lee, J.Y.; Nho, K.J.; Cho, C.; Kim, Y.S.; Kang, W.S.; Ahn, Y.; et al. A novel system-level approach using RNA-sequencing data identifies miR-30-5p and miR-142a-5p as key regulators of apoptosis in myocardial infarction. Sci. Rep. 2018, 8, 14638. [Google Scholar] [CrossRef]
- Zhang, W.; Chang, H.; Zhang, H.; Zhang, L. MiR-30e Attenuates Isoproterenol-induced Cardiac Fibrosis through Suppressing Snai1/TGF-b Signaling. J. Cardiovasc. Pharmacol. 2017, 70, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Gambacciani, C.; Kusmic, C.; Meghini, F.; Rizzo, M.; Mariani, L.; Pitto, L. miR-29a and miR-30c negatively regulate DNMT 3a in cardiac ischemic tissues: Implications for car-diac remodelling. microRNA Diagn. Ther. 2013, 1, 35–45. [Google Scholar] [CrossRef][Green Version]
- Huang, Y.; Qi, Y.; Du, J.-Q.; Zhang, D.-F. MicroRNA-34a regulates cardiac fibrosis after myocardial infarction by targeting Smad4. Expert Opin. Ther. Targets 2014, 18, 1–11. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, W.; Wang, C.; Zhou, X.; Xu, X.; Geng, P.; Zhu, J. Expression and function of miR-92a in ventricular remodeling after PCI treatment of acute myocardial in-farction. Int. J. Clin. Exp. Pathol. 2017, 10, 1158–1165. [Google Scholar]
- Bellera, N.; Barba, I.; Rodriguez-Sinovas, A.; Ferret, E.; Asín, M.A.; Gonzalez-Alujas, T.; Pérez-Rodon, J.; Esteves, M.; Fonseca, C.; Toran, N.; et al. Single Intracoronary Injection of Encapsulated Antagomir-92a Promotes Angiogenesis and Prevents Ad-verse Infarct Remodeling. J. Am. Heart Assoc. 2014, 3, e000946. [Google Scholar] [CrossRef]
- Li, Q.; Xie, J.; Li, J.; Gu, R.; Ding, l.; Wang, L.; Xu, B. Overexpression of microRNA-99a attenuates heart remodelling and improves cardiac performance after myo-cardial infarction. J. Cell. Mol. Med. 2014, 8, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Z.; Liao, X.; Zeng, Q.; Li, Y.; Hu, F.; Liu, Y.; Meng, K.; Qian, C.; Zhang, Q.; et al. MicroRNA-101a Inhibits Cardiac Fibrosis Induced by Hypoxia via Trageting TGFβRI on Cardiac Fibroblasts. Cell. Physiol. Biochem. 2015, 35, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Sun, X.; Shan, H.; Wang, N.; Wang, J.; Ren, J.; Feng, S.; Xie, L.; Lu, C.; Yuan, Y.; et al. MicroRNA-101 Inhibited Postinfarct Cardiac Fibrosis and Improved Left Ventricular Compliance via the FBJ Osteosarcoma Oncogene/Transforming Growth Factor-β1 Pathway. Circulation 2012, 126, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, V.; Rai, R.; Place, A.T.; Murphy, S.B.; Verma, S.K.; Ghosh, A.K.; Vaughan, D.E. MiR-125b Is Critical for Fibroblast-to-Myofibroblast Transition and Cardiac Fibrosis. Circulation 2016, 133, 291–301. [Google Scholar] [CrossRef]
- Bie, Z.D.; Sun, L.Y.; Geng, C.L.; Meng, Q.G.; Lin, X.J.; Wang, Y.F.; Wang, X.B.; Yang, J. MiR-125b Regulates SFRP5 Expression to Promote Growth and Activation of Cardiac Fibroblasts: MiR-125b regulates SFRP5 in cardiac fibroblasts. Cell Biol. Int. 2016, 40, 1224–1234. [Google Scholar] [CrossRef]
- Li, N.; Zhou, H.; Tang, Q. miR-133: A Suppressor of Cardiac Remodeling? Front. Pharmacol. 2018, 9, 903. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, J.; Tuazon, J.P.; Borlongan, C.V.; Yu, G. MicroRNA-133a and Myocardial Infarction. Cell Transplant. 2019, 28, 831–838. [Google Scholar] [CrossRef]
- He, Q.; Wang, F.; Honda, T.; James, J.; Li, J.; Redington, A. Loss of miR-144 signaling interrupts extracellular matrix remodeling after myocardial infarction leading to worsened cardiac function. Sci. Rep. 2018, 8, 16886. [Google Scholar] [CrossRef]
- Li, J.; Cai, S.X.; He, Q.; Zhang, H.; Friedberg, D.; Wang, F.; Redington, A.N. Intravenous miR-144 reduces left ventricular remodeling after myocardial infarction. Basic Res. Cardiol. 2018, 113, 36. [Google Scholar] [CrossRef]
- Ibrahim, A.G.-E.; Cheng, K.; Marbán, E. Exosomes as Critical Agents of Cardiac Regeneration Triggered by Cell Therapy. Stem Cell Rep. 2014, 2, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, Y.; Park, K.M.; Hu, Q.; Teoh, J.P.; Broskova, Z.; Ranganathan, P.; Jayakumar, C.; Li, J.; Su, H.; et al. MicroRNA-150 protects the mouse heart from ischaemic injury by regulating cell death. Cardiovasc. Res. 2015, 106, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ye, P.; Wang, S.; Wu, J.; Sun, Y.; Zhang, A.; Ren, L.; Cheng, C.; Huang, X.; Wang, K.; et al. MicroRNA-150 Protects the Heart from Injury by Inhibiting Monocyte Accumulation in a Mouse Model of Acute Myocardial Infarction. Circ. Cardiovasc. Genet. 2015, 8, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, W.; Huang, Y.; Lu, Y.; Zhu, L.; Lin, L.; Li, J.; Xie, Q. GW28-e0427 MiR-155 in macrophage regulates cardiac function and fibrosis after myocardial infarction possibly through SOCS1-dependent inflammatory response. J. Am. Coll. Cardiol. 2017, 70, C12. [Google Scholar] [CrossRef]
- Duygu, B.; Poels, E.M.; Juni, R.; Bitsch, N.; Ottaviani, L.; Olieslagers, S.; de Windt, L.J.; Martins, P.A.D.C. miR-199b-5p is a regulator of left ventricular remodeling following myocardial infarction. Non-coding RNA Res. 2017, 2, 18–26. [Google Scholar] [CrossRef]
- Shyu, K.-G.; Wang, B.-W.; Cheng, W.-P.; Lo, H.-M. MicroRNA-208a Increases Myocardial Endoglin Expression and Myocardial Fibrosis in Acute Myocardial Infarction. Can. J. Cardiol. 2015, 31, 679–690. [Google Scholar] [CrossRef]
- Hu, S.; Huang, M.; Li, Z.; Jia, F.; Ghosh, Z.; Lijkwan, M.A.; Fasanaro, P.; Sun, N.; Wang, X.; Martelli, F.; et al. MicroRNA-210 as a Novel Therapy for Treatment of Ischemic Heart Disease. Circulation 2010, 122, S124–S131. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qin, Y.; Shao, S.; Yu, Y.; Zhang, C.; Dong, H.; Lv, G.; Dong, S. MicroRNA-214 Inhibits Left Ventricular Remodeling in an Acute Myocardial Infarction Rat Model by Suppressing Cellular Apoptosis via the Phosphatase and Tensin Homolog (PTEN). Int. Heart J. 2016, 57, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, Y.; Deng, Y.; Li, H. MicroRNA-223 Regulates Cardiac Fibrosis After Myocardial Infarction by Targeting RA-SA1. Cell Physiol. Biochem. 2018, 46, 1439–1454. [Google Scholar]
- Yuan, H.; Gao, J. The role of miR-370 in fibrosis after myocardial infarction. Mol. Med. Rep. 2017, 15, 3041–3047. [Google Scholar] [CrossRef]
- Seo, H.-H.; Lee, S.; Lee, C.Y.; Lee, J.; Shin, S.; Song, B.-W.; Kim, I.-K.; Choi, J.-W.; Lim, S.; Kim, S.W.; et al. Multipoint targeting of TGF-β/Wnt transactivation circuit with microRNA 384-5p for cardiac fibrosis. Cell Death Differ. 2018, 26, 1107–1123. [Google Scholar] [CrossRef]
- Tao, L.; Bei, Y.; Chen, P.; Lei, Z.; Fu, S.; Zhang, H.; Xu, J.; Che, L.; Chen, X.; Sluijter, J.P.; et al. Crucial Role of miR-433 in Regulating Cardiac Fibrosis. Theranostics 2016, 6, 2068–2083. [Google Scholar] [CrossRef] [PubMed]
- Tolonen, A.M.; Magga, J.; Szabó, Z.; Viitala, P.; Gao, E.; Moilanen, A.M.; Ohukainen, P.; Vainio, L.; Koch, W.J.; Kerkelä, R.; et al. Inhibition of Let-7 microRNA attenuates myocardialremodeling and improves cardiac function postinfarctionin mice. Pharm. Res. Per. 2014, 2, e00056. [Google Scholar]
- Chen, C.-Y.; Choong, O.K.; Liu, L.-W.; Cheng, Y.-C.; Li, S.-C.; Yen, C.Y.; Wu, M.-R.; Chiang, M.-H.; Tsang, T.-J.; Wu, Y.-W.; et al. MicroRNA let-7-TGFBR3 signalling regulates cardiomyocyte apoptosis after infarction. EBioMedicine 2019, 46, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liew, O.W.; Richards, A.M.; Chen, Y.-T. Overview of MicroRNAs in Cardiac Hypertrophy, Fibrosis, and Apoptosis. Int. J. Mol. Sci. 2016, 17, 749. [Google Scholar] [CrossRef] [PubMed]
- Doebele, C.; Bonauer, A.; Fischer, A.; Scholz, A.; Reiss, Y.; Urbich, C.; Hofmann, W.-K.; Zeiher, A.M.; Dimmeler, S. Members of the microRNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic function in endothelial cells. Blood 2010, 115, 4944–4950. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Hao, Q.; Wei, J.; Li, G.-H.; Wu, Y.; Zhao, Y.-F. MicroRNA-19a/b-3p protect the heart from hypertension-induced pathological cardiac hypertrophy through PDE5A. J. Hypertens. 2018, 36, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, K.C.; Wu, W.; Subramaniam, S.; Shyy, J.Y.; Chiu, J.J.; Li, J.Y.; Chien, S. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modu-late flow-induced endothelial inflammation. Proc. Natl. Acad. Sci. USA 2011, 108, 10355–10360. [Google Scholar] [CrossRef]
- Ge, Z.W.; Zhu, X.L.; Wang, B.C.; Hu, J.L.; Sun, J.J.; Wang, S.; Chen, X.J.; Meng, S.P.; Liu, L.; Cheng, Z.Y. MicroRNA-26b relieves inflammatory response and myocardial remodeling of mice with myocardial infarction by suppression of MAPK pathway through binding to PTGS2. Int. J. Cardiol. 2019, 280, 152–159. [Google Scholar] [CrossRef]
- Chiang, M.H.; Liang, C.J.; Lin, L.C.; Yang, Y.F.; Huang, C.C.; Chen, Y.H.; Kao, H.L.; Chen, Y.C.; Ke, S.R.; Lee, C.W.; et al. miR-26a attenuates cardiac apoptosis and fibrosis by targeting ataxia–telangiectasia mutated in myocardial infarction. J. Cell. Physiol. 2020, 235, 6085–6102. [Google Scholar] [CrossRef]
- Jie, Q.; Luo, X.; Ma, Z.; Zhang, B.; Li, S.; Zhang, J. Downregulation of miR-26b-5p, miR-204-5p, and miR-497-3p Expression Facilitates Exercise-Induced Physiological Cardiac Hypertrophy by Augmenting Autophagy in Rats. Front. Genet. 2020, 11, 78. [Google Scholar]
- Liu, Y.; Wang, Z.; Xiao, W. MicroRNA-26a protects against cardiac hypertrophy via inhibiting GATA4 in rat model and cultured cardiomyocytes. Mol. Med. Rep. 2016, 14, 2860–2866. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, M.; Li, C.; Zhou, J.; Li, H.; Zhu, D.; Wang, Z.; Chen, A.; Zhao, Q. MicroRNA-92a Inhibition Attenuates Hypoxia/Reoxygenation-Induced Myocardiocyte Apoptosis by Targeting Smad7. PLoS ONE 2014, 9, e100298. [Google Scholar] [CrossRef]
- Danielson, L.S.; Park, D.S.; Rotllan, N.; Chamorro-Jorganes, A.; Guijarro, M.V.; Fernandez-Hernando, C.; Fishman, G.I.; Phoon, C.K.; Hernando, E. Cardiovascular dysregulation of miR-17-92 causes a lethal hypertrophic cardiomyopathy and ar-rhythmogenesis. FASEB J. 2013, 27, 1460–1467. [Google Scholar] [CrossRef]
- Carè, A.; Catalucci, D.; Felicetti, F.; Bonci, D.; Addario, A.; Gallo, P.; Bang, M.-L.; Segnalini, P.; Gu, Y.; Dalton, N.D.; et al. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 2007, 13, 613–618. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Xiao, J.; Ren, A.-J.; Zhang, Y.-F.; Zhang, H.; Chen, M.; Xie, B.; Gao, X.-G.; Wang, Y.-W. Role of miR-1 and miR-133a in myocardial ischemic postconditioning. J. Biomed. Sci. 2011, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Pankratz, F. MicroRNA-155 Exerts Cell-Specific Antiangiogenic but Proarteriogenic Effects during Adaptive Neovasculari-zationm. Circulation 2015, 131, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Huang, H.; Xie, Q.; Wang, Z.; Fan, Y.; Kong, B.; Huang, D.; Xiao, Y. MiR-155 Knockout in Fibroblasts Improves Cardiac Remodeling by Targeting Tumor Protein p53-Inducible Nuclear Protein 1. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Fasanaro, P.; D’Alessandra, Y.; Di Stefano, V.; Melchionna, R.; Romani, S.; Pompilio, G.; Capogrossi, M.C.; Martelli, F. MicroRNA-210 Modulates Endothelial Cell Response to Hypoxia and Inhibits the Receptor Tyrosine Kinase Ligand Ephrin-A3. J. Biol. Chem. 2008, 283, 15878–15883. [Google Scholar] [CrossRef] [PubMed]
- Mutharasan, R.K.; Nagpal, V.; Ichikawa, Y.; Ardehali, H. microRNA-210 is upregulated in hypoxic cardiomyocytes through Akt- and p53-dependent pathways and exerts cytoprotective effects. Am. J. Physiol. Circ. Physiol. 2011, 301, H1519–H1530. [Google Scholar] [CrossRef]
- Tijsen, A.J.; van der Made, I.; van den Hoogenhof, M.M.; Wijnen, W.J.; van Deel, E.D.; de Groot, N.E.; Alekseev, S.; Fluiter, K.; Schroen, B.; Goumans, M.J. The microRNA-15 family inhibits the TGFb-pathway in the heart. Cardiovasc. Res. 2014, 104, 61–71. [Google Scholar] [CrossRef]
- Wei, C.; Kim, I.K.; Kumar, S.; Jayasinghe, S.; Hong, N.; Castoldi, G.; Catalucci, D.; Jones, W.K.; Gupta, S. NF-κB mediated miR-26a regulation in cardiac fibrosis. J. Cell. Physiol. 2013, 228, 1433–1442. [Google Scholar] [CrossRef]
- Bernardo, B.C.; Gao, X.-M.; Winbanks, C.E.; Boey, E.J.H.; Tham, Y.K.; Kiriazis, H.; Gregorevic, P.; Obad, S.; Kauppinen, S.; Du, X.-J.; et al. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc. Natl. Acad. Sci. USA 2012, 109, 17615–17620. [Google Scholar] [CrossRef]
- Martins, P.A.D.C.; Salic, K.; Gladka, M.M.; Armand, A.-S.; Leptidis, S.; El Azzouzit, H.; Hansen, A.; Roo, C.J.C.-D.; Bierhuizen, M.F.A.; Van Der Nagel, R.; et al. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nature 2010, 12, 1220–1227. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.-X.; Li, Y.-L.; Zhang, C.-C.; Zhou, C.-Y.; Wang, L.; Xia, Y.-L.; Du, J.; Li, H.-H. MicroRNA Let-7i Negatively Regulates Cardiac Inflammation and Fibrosis. Hypertension 2015, 66, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ma, Y.; Wang, X.; Li, S.; Yu, T.; Duan, W.; Wu, J.; Wen, Z.; Jiao, Y.; Sun, Z.; et al. Circulating miR-1 as a potential predictor of left ventricular remodeling following acute ST-segment myocar-dial infarction using cardiac magnetic resonance. Quant Imaging Med. Surg. 2020, 10, 1490–1503. [Google Scholar] [CrossRef]
- Grabmaier, U.; Clauss, S.; Gross, L.; Klier, I.; Franz, W.; Steinbeck, G.; Wakili, R.; Theiss, H.; Brenner, C. Diagnostic and prognostic value of miR-1 and miR-29b on adverse ventricular remodeling after acute myocardial infarction—The SITAGRAMI-miR analysis. Int. J. Cardiol. 2017, 244, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Mehurg, S.M.; Arroyo, J.E.; Stroud, R.E.; DeSantis, S.M.; Spinale, F.G. Relationship Between the Temporal Profile of Plasma microRNA and Left Ventricular Remodeling in Pa-tients Following Myocardial Infarction. Circ. Cardiovasc. Genet. 2011, 4, 614–619. [Google Scholar] [CrossRef]
- Devaux, Y.; Vausort, M.; McCann, G.; Kelly, D.; Collignon, O.; Ng, L.; Wagner, D.R.; Squire, I.B. A Panel of 4 microRNAs Facilitates the Prediction of Left Ventricular Contractility after Acute Myocardial Infarction. PLoS ONE 2013, 8, e70644. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Cuvelliez, M.; Fiedler, J.; Charrier, H.; Mulder, P.; Hebbar, E.; Pfanne, A.; Beseme, O.; Chwastyniak, M.; Amouyel, P.; et al. MicroRNAs regulating superoxide dismutase 2 are new circulating biomarkers of heart failure. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Gao, C.; Zhao, D.; Wang, J.; Liu, P.; Xu, B. Clinical significance and correlation of microRNA-21 expression and the neutrophil-lymphocyte ratio in patients with acute myocardial infarction. Clinics 2019, 74, e1237. [Google Scholar] [CrossRef]
- Liu, X.; Dong, Y.; Chen, S.; Zhang, G.; Zhang, M.; Gong, Y.; Li, X. Circulating MicroRNA-146a and MicroRNA-21 Predict Left Ventricular Remodeling after ST-Elevation Myocardial Infarction. Cardiology 2015, 132, 233–241. [Google Scholar] [CrossRef]
- Maciejak, A.; Kostarska-Srokosz, E.; Gierlak, W.; Dluzniewski, M.; Kuch, M.; Marchel, M.; Opolski, G.; Kiliszek, M.; Matlak, K.; Dobrzycki, S.; et al. Circulating miR-30a-5p as a prognostic biomarker of left ventricular dysfunction after acute myocardial infarction. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Zhou, M.; He, J.; Meng, W.; Ma, X.; Dong, S.; Meng, X.; Zhao, X.; Wang, X.; He, F. Circulating miR-208b and miR-34a are associated with left ventricular remodeling after acute myocardial infarction. Int. J. Mol. Sci. 2014, 15, 5774–5788. [Google Scholar] [CrossRef]
- Bauters, C.; Kumarswamy, R.; Holzmann, A.; Bretthauer, J.; Anker, S.D.; Pinet, F.; Thum, T. Circulating miR-133a and miR-423-5p fail as biomarkers for left ventricular remodeling after myocardial infarction. Int. J. Cardiol. 2013, 168, 1837–1840. [Google Scholar] [CrossRef]
- Devaux, Y.; Vausort, M.; McCann, G.P.; Zangrando, J.; Kelly, D.; Razvi, N.; Zhang, L.; Ng, L.L.; Wagner, D.R.; Squire, I.B. MicroRNA-150 A Novel Marker of Left Ventricular Remodeling After Acute Myocardial Infarction. Circ. Cardiovasc. Genet. 2013, 6, 290–298. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, L.; Chen, F.; Zhang, L.; Chen, X.; Yang, C.; Han, Z. Circulating miR-208b: A Potentially Sensitive and Reliable Biomarker for the Diagnosis and Prognosis of Acute Myocardial Infarction. Clin. Lab. 2017, 63, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Alavi-Moghaddam, M.; Chehrazi, M.; Alipoor, S.D.; Mohammadi, M.; Baratloo, A.; Mahjoub, M.P.; Movasaghi, M.; Garssen, J.; Adcock, I.M.; Mortaz, E. A Preliminary Study of microRNA-208b after Acute Myocardial Infarction: Impact on 6-Month Survival. Dis. Markers 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo-Calvo, D.; Cediel, G.; Bär, C.; Núñez, J.; Revuelta-Lopez, E.; Gavara, J.; Ríos-Navarro, C.; Llorente-Cortes, V.; Bodí, V.; Thum, T.; et al. Circulating miR-1254 predicts ventricular remodeling in patients with ST-Segment-Elevation Myocardial Infarction: A cardiovascular magnetic resonance study. Sci. Rep. 2018, 8, 15115. [Google Scholar] [CrossRef]
- Vasilescu, C.; Rossi, S.; Shimizu, M.; Tudor, S.; Veronese, A.; Ferracin, M.; Nicoloso, M.; Barbarotto, E.; Popa, M.; Stanciulea, O.; et al. MicroRNA Fingerprints Identify miR-150 as a Plasma Prognostic Marker in Patients with Sepsis. PLoS ONE 2009, 4, e7405. [Google Scholar] [CrossRef] [PubMed]
- Valkov, N.; King, M.E.; Moeller, J.; Liu, H.; Li, X.; Zhang, P. MicroRNA-1-Mediated Inhibition of Cardiac Fibroblast Proliferation Through Targeting Cyclin D2 and CDK6. Front. Cardiovasc. Med. 2019, 6, 65. [Google Scholar] [CrossRef]
- Chung, H. Myocardial Longitudinal Strain in Prediction of Heart Failure after Acute Myocardial Infarction. Korean Circ. J. 2019, 49, 973–974. [Google Scholar] [CrossRef] [PubMed]
- Kleinsinger, F. Working with the non-compliant patient. Perm. J. 2010, 14, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kleinsingerm, F. Understanding Noncompliant Behavior: Definitions and Causes. Perm. J. 2003, 7, 18–21. [Google Scholar]
- Chen, Y.; Zhao, Y.; Chen, W.; Xie, L.; Zhao, Z.A.; Yang, J.; Chen, Y.; Lei, W.; Shen, Z. MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myo-cardial infarction. Stem. Cell Res. Ther. 2017, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Hinkel, R.; Ramanujam, D.P.; Kaczmarek, V.; Howe, A.; Klett, K.; Beck, C.; Dueck, A.; Thum, T.; Laugwitz, K.-L.; Maegdefessel, L.; et al. AntimiR-21 Prevents Myocardial Dysfunction in a Pig Model of Ischemia/Reperfusion Injury. J. Am. Coll. Cardiol. 2020, 75, 1788–1800. [Google Scholar] [CrossRef]
- McDonald, R.A.; Halliday, C.A.; Miller, A.M.; Diver, L.A.; Dakin, R.S.; Montgomery, J.; McBride, M.W.; Kennedy, S.; McClure, J.D.; Robertson, K.E.; et al. Reducing In-Stent Restenosis: Therapeutic Manipulation of miRNA in Vascular Remodeling and Inflammation. J. Am. Coll. Cardiol. 2015, 65, 2314–2327. [Google Scholar] [CrossRef]
- Wang, D.; Deuse, T.; Stubbendorff, M.; Chernogubova, E.; Erben, R.G.; Eken, S.M.; Jin, H.; Li, Y.; Busch, A.; Heeger, C.H.; et al. Local microRNA modulation using a novel anti-miR-21-eluting stent effectively prevents experimental in-stent restenosis. Arter. Thromb. Vasc. Biol. 2015, 35, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Zhang, J.; Jiao, Z.; Dong, N.; Wang, G.; Wang, Z.; Wang, L. Localized injection of miRNA-21-enriched extracellular vesicles effectively restores cardiac function after myocardial infarction. Theranostics 2019, 9, 2346–2360. [Google Scholar] [CrossRef]
- Ma, T.; Chen, Y.; Chen, Y.; Meng, Q.; Sun, J.; Shao, L.; Yu, Y.; Huang, H.; Hu, Y.; Yang, Z.; et al. MicroRNA-132, Delivered by Mesenchymal Stem Cell-Derived Exosomes, Promote Angiogenesis in Myocardial Infarction. Stem Cells Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Pan, J. Exosome derived from miR-146a modified adipose-derived stem cells attenuate acute myocardial infarction induced myocardial damage via downregulation of early growth response factor 1. J. Cell. Biochem. 2019, 129, 4433–4443. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Hu, J.; Chen, F.; Qiao, S.; Sun, X.; Gao, L.; Xie, J.; Xu, B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc. Res. 2019, 115, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Charles, C.J.; Li, R.R.; Yeung, T.; Mazlan, S.M.I.; Lai, R.C.; De Kleijn, D.P.V.; Lim, S.K.; Richards, A.M. Systemic Mesenchymal Stem Cell-Derived Exosomes Reduce Myocardial Infarct Size: Characterization with MRI in a Porcine Model. Front. Cardiovasc. Med. 2020, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Yeo, R.W.; Lim, S.K. Mesenchymal stem cell exosomes. Semin. Cell. Dev. Biol. 2015, 40, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Lazar, E.; Benedek, T.; Korodi, S.; Rat, N.; Lo, J.; Benedek, I. Stem cell-derived exosomes—An emerging tool for myocardial regeneration. World J. Stem Cells 2018, 10, 106–115. [Google Scholar] [CrossRef]
- Bejerano, T.; Etzion, S.; Elyagon, S.; Etzion, Y.; Cohen, S. Nanoparticle Delivery of miRNA-21 Mimic to Cardiac Macrophages Improves Myocardial Remodeling after Myocardial Infarction. Nano Lett. 2018, 18, 5885–5891. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Wei, Y.; Xue, X.; Li, Y.; Dong, H.; Guo, X.; Shi, X.; He, B. A Novel Anti-Coagulative Nanocomplex in Delivering miRNA-1 Inhibitor Against Microvascular Obstruc-tion of Myocardial Infarction. Adv. Healthc. Mater. 2020, 9, 1901783. [Google Scholar] [CrossRef]
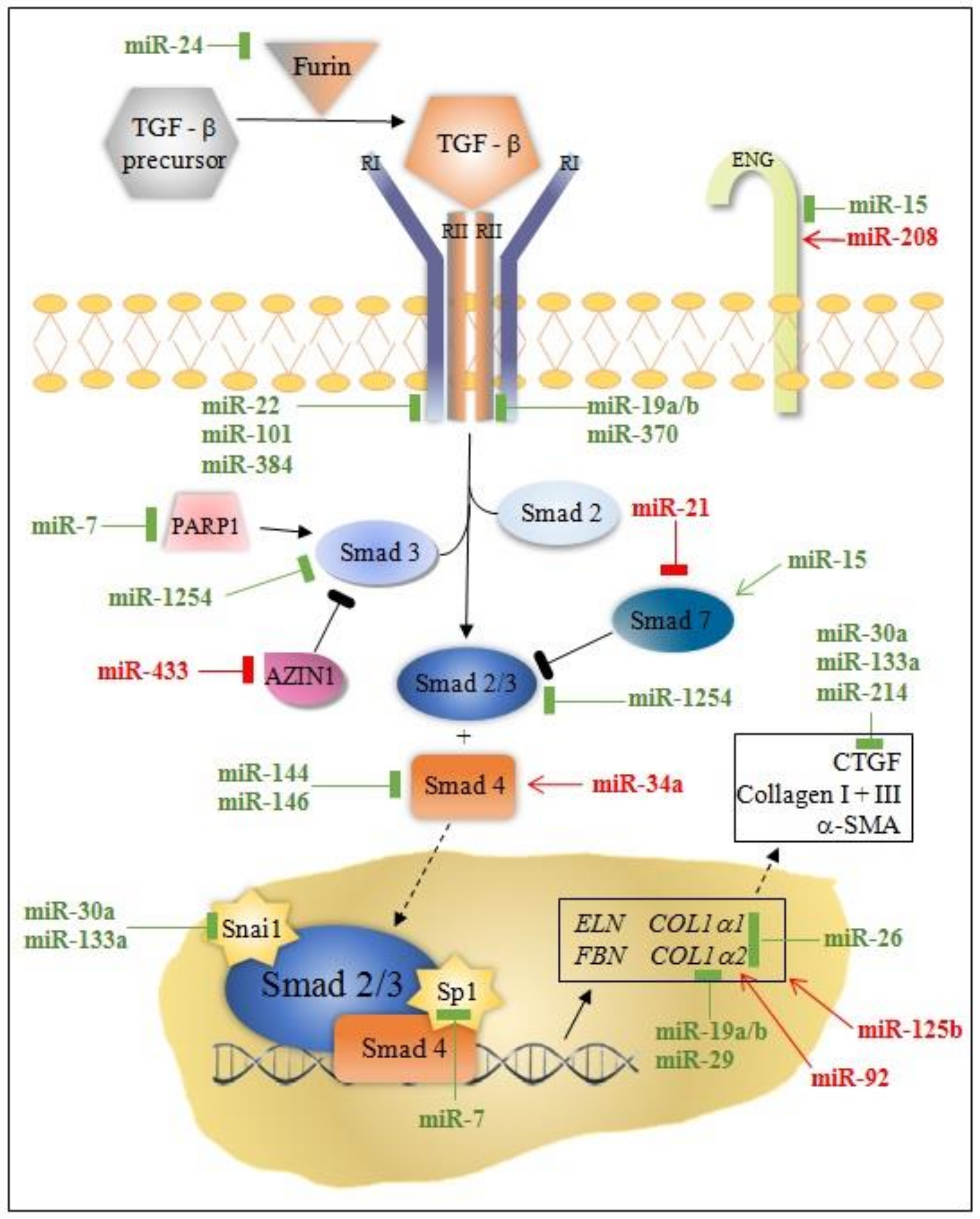
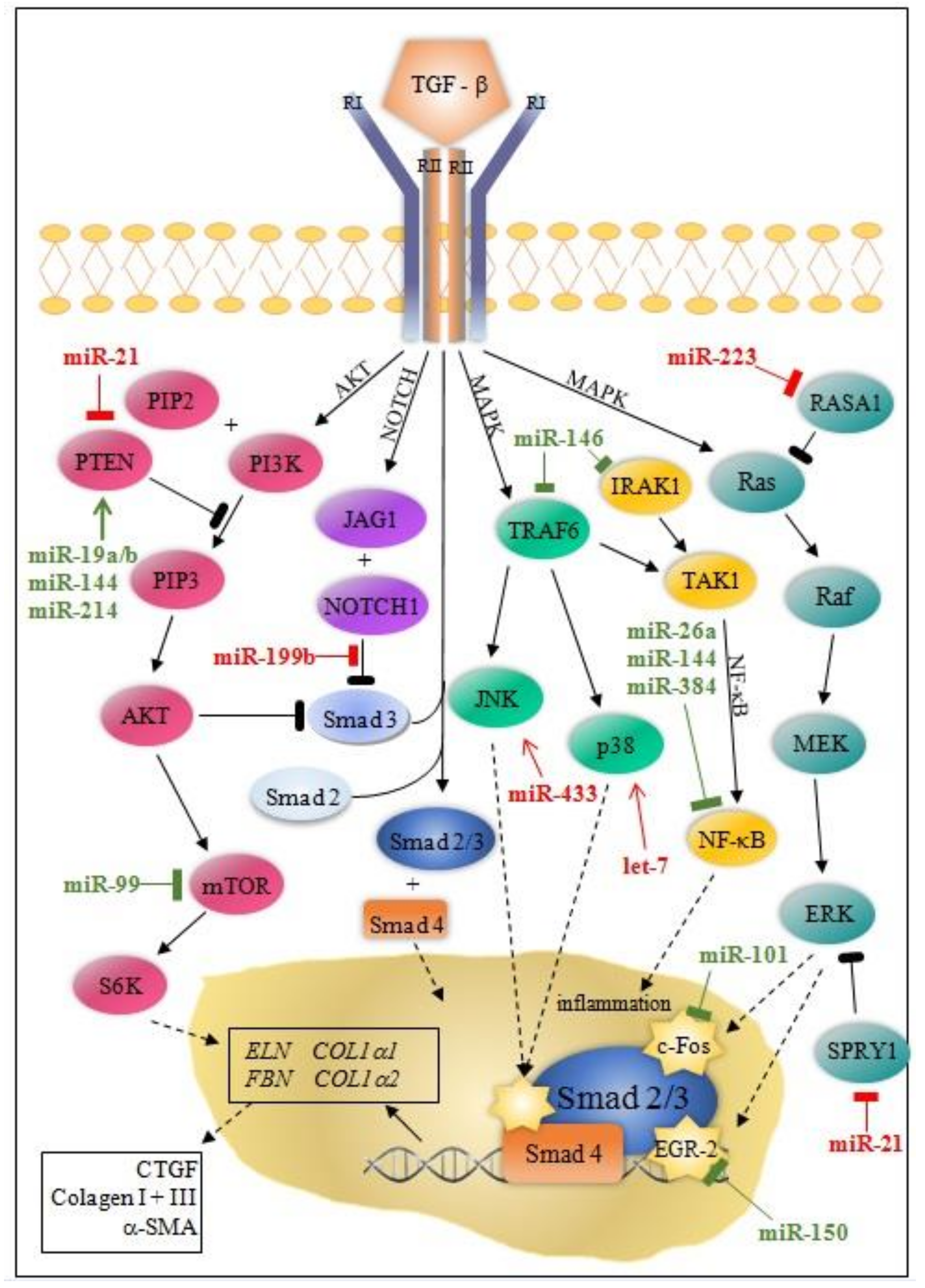
| miRNA | Effect on Fibrosis | Target | Effector Cells | Platforms | Reference |
|---|---|---|---|---|---|
| miR-7a/b | Anti-fibrotic | Sp1 PARP-1 TGFβ | CM | MI mouse H9C2 cell line cardiomyoblast | [13] |
| miR-9 | Pro-fibrotic | Follistatin-like 1 | CM | MI mouse | [14] |
| miR-15 family | Pro-fibrotic | ARL2 BCL2 | CM | MI pig MI mouse | [15] |
| miR-19a/19b | Anti-fibrotic | COL1A1, COL3A1, ELN, FBN1, TGFβRII, PTEN | CM F M | MI mouse Human heart tissue | [16] |
| miR-21 | Pro-fibrotic | Smad7 Spry1 ERK | F CM M | MI mouse and pig NRCFs | [17,18,19,20,21,22,23,24,25,26,27] |
| miR-22 | Anti-fibrotic | TGFβRI | F | MI mouse NRCFs | [28] |
| miR-24 | Anti-fibrotic | Furin | F | MI mouse NRCFs | [29] |
| miR-26a | Pro-fibrotic | Smad1, BMP NF-κB COL1A1 COL1A2 | EC | MI mouse HUVECs Human trials | [30] |
| miR-29 | Anti-fibrotic | FBN1, COL1A1 COL1A2, COL3A1 | F | MI mouse RCFs | [31,32,33] |
| miR-30a-5p | Anti-fibrotic | CTGF TGFβ | F CM EC | MI Rat NRVMs NRCFs | [34,35,36,37,38,39] |
| miR-34a | Pro-fibrotic | Smad4 | F | MI mouse | [40] |
| miR-92 | Pro-fibrotic | Collagen 1 IL-6, TNF-α BCL-2 | F CM M | MI rat Human trials | [41,42] |
| miR-99 | Anti-fibrotic | mTOR/S6K | CM | MI mouse NMVMs | [43] |
| miR-101 | Anti-fibrotic | TGFβR1 c-Fos | F | MI rat NRCFs | [31,44,45] |
| miR-125b | Pro-fibrotic | TGFβ Apelin p53 SFRP5 | F | MI mouse HCFs Human heart tissue | [46,47] |
| miR-133a | Anti-fibrotic | CTGF Snai1 SRF | CM F | MI mouse NRCFs | [48,49] |
| miR-144 | Anti-fibrotic | ZEB1/LOX axis | F | MI mouse NRCFs | [50,51] |
| miR-146 | Anti-fibrotic | Smad4 IRAK1 TRAF6 | F CM | MI mouse NRCMs CDC | [52] |
| miR-150 | Anti-fibrotic | EGR2 P2 × 7R CXCR4 | F CM M | MI mouse Human trials | [53,54] |
| miR-155 | Pro-fibrotic | SOCS1 | M | MI mouse PCMs | [55] |
| miR-199b | Pro-fibrotic | NOTCH1 JAG1 DYRK1A | CM | MI mouse | [56] |
| miR-208a | Pro-fibrotic | Endoglin | F | MI rat RCFs | [57] |
| miR-210 | Anti-fibrotic | EFNA3 PTP1 DAPK1 CTGF | CM F | MI mouse Mouse HL-1 CMs | [58] |
| miR-214 | Anti-fibrotic | PTEN | CM | MI rat | [59] |
| miR-223 | Pro-fibrotic | RASA1 | F | MI mouse Cultured cardiac fibroblasts | [60] |
| miR-370 | Anti-fibrotic | TGFβRII | F CM | MI rat RCFs | [61] |
| miR-384 | Anti-fibrotic | TGFβRI/Wnt(3a)/NF-κb | F | I/R rats NRCFs | [62] |
| miR-433 | Pro-fibrotic | AZIN1 JNK1 | F | MI mouse NRCFs | [63] |
| Let-7c | Pro-fibrotic | OCT4 SOX2 TGFβRIII/p38 | F CM | MI mouse AMCFs MI pig | [64,65] |
| miRNA | Fibrosis | Hypertrophy | Apoptosis | Angiogenesis | Inflammation | Reference |
|---|---|---|---|---|---|---|
| miR-19a/19b | − | − (Ang II model) | − | − | − | [16,67,68] |
| miR-21 | + | + | − | + | − | [19,20,21,22,23,24,25,26,27,69] |
| miR-26 | − | − (TAC, AngII model, exercise-induced) | − | − | − | [30,70,71,72,73] |
| miR-92 | + | + (exercise-induced) | + | + | + | [41,42,74,75] |
| miR-133a | − | − | − | − | − | [48,49,76,77] |
| miR-155 | + | + | + | + arteriogenic | + | [55,78,79] |
| miR-210 | − | − | − | + | − | [58,80,81] |
| Ref. | Signaling Pathway | Fibrosis Induced by MI | miRNA | Fibrosis Induced by Pressure Overload | Signaling Pathway | Ref. |
|---|---|---|---|---|---|---|
| [15] | ARL2 BCL2 | Pro-fibrosis | miR-15b | Anti-fibrosis (TAC) | Smad3 TGFβRI | [82] |
| [16] | COL1α1, COL3α1, ELN, FBN, TGFβRII, PTEN | Anti-fibrosis | miR-19a/b | No modification (TAC) | [16] | |
| [16,18,19,20,21,22,23,24,25,26] | Smad7 Spry1 ERK | Pro-fibrosis | miR-21 | Pro-fibrosis (TAC) | Spry1 ERK | [27] |
| [30] | Smad1, BMP NF-κB COL1α1 COL1α2 | Pro-fibrosis | miR-26a | Anti-fibrosis (TAC + AngII) | CTGF NF-κB COL1α1 | [83] |
| [40] | Smad4 | Pro-fibrosis | miR-34 | Pro-fibrosis (TAC) | VEGF POFUT1 NOTCH1 Vinculin Semaphorin 4B | [84] |
| [56] | NOTCH1 JAG1 DYRK1A | Pro-fibrosis | miR-199b | Pro-fibrosis (TAC) | DYRK1A | [85] |
| [64,65] | OCT4 SOX2 TGFβRIII/p38 | Pro-fibrosis | Let-7c Let-7i | Anti-fibrosis (AngII) | IL-6 COL1α2 COL3α1 COL4α1 COL5α2 | [86] |
| miRNA | Effect on LVR | Study Population | Blood Sample Collection | Method for Assessing LVR | Definition of LVR | Reference |
|---|---|---|---|---|---|---|
| miR-1 | + | 80 patients with STEMI | On admission | CMR at 1 and 6 months | ↑ >10% of ∆LVEDV | [87] |
| − | 44 patients with MI (SITAGRAMI trial) | d4, d9, 6 m | CMR at 4 d, 6 m | Absolute change for LVEDV | [88] | |
| No effect | 12 patients with MI | d2, 5, 28, 90 | Echo on d1, 5, 28, 90 | Absolute change for LVEDV | [89] | |
| miR-16/27a/101 /150 | + for miR-16/27a − for miR 150/101 | 150 patients with MI | At discharge | Echo at discharge and at a median of 176 d (range 128–262 d) after MI | Wall Motion Index Score > 1.2 | [90] |
| miR-21 | + | 246 patients with anterior MI (REVE trial) | At discharge, 1 m, 3 m, 1 y | Echo on d3, 7, 3 m, 1 y | ((EDV1year—EDVbaseline)/EDVbaseline) | [91] |
| + | 184 patients with MI | On admission | - | Death within 30 days | [92] | |
| + | 198 patients with MI | d5 | Echo on d5, 1 y | ↑ >20% of LVEDV | [93] | |
| No effect | 44 patients with MI (SITAGRAMI trial) | d4, d9, 6 m | CMR at 4 d, 6 m | Absolute change for LVEDV | [88] | |
| No effect | 12 patients with MI | d2, 5, 28, 90 | Echo on d1, 5, 28, 90 | Absolute change for LVEDV | [89] | |
| miR-29a | + | 12 patients with MI | d2, 5, 28, 90 | Echo on d1, 5, 28, 90 | Absolute change for LVEDV | [89] |
| miR-29b | − | 44 patients with MI (SITAGRAMI trial) | d4, d9, 6 m | CMR at 4 d, 6 m | Absolute change for LVEDV | [88] |
| miR-30a-5p | + | 14 patients with STEMI | On admission, 6 m | Echo on admission, 6 m | LVEF ≤ 50% NT-proBNP ≥ 150pg/ml | [94] |
| miR-34a | + | 359 patients with MI | On admission | Echo after admission, 6 m | ↑ >10% of LVEDV | [95] |
| miR-92a | + | 60 patients with MI | On admission and after PCI | Echo on admission, 3 m | Absolute change for LVEDV, LVESV, LVEF | [41] |
| miR-92 | No effect | 44 patients with MI (SITAGRAMI trial) | d4, d9, 6 m | CMR at 4 d, 6 m | Absolute change for LVEDV | [88] |
| miR-133a | No effect | 246 patients with anterior MI (REVE trial) | At discharge, 1 m, 3 m, 1 y | Echo on d3, 7, 3 m, 1 y | ((EDV1year—EDVbaseline)/EDVbaseline) | [96] |
| No effect | 12 patients with MI | d2, 5, 28, 90 | Echo on d1, 5, 28, 90 | Absolute change for LVEDV | [89] | |
| miR-146a | + | 198 patients with MI | d5 | Echo on d5, 1 y | ↑ >20% of LVEDV | [92] |
| miR-150 | − | 60 patients with MI | At discharge | Echo at discharge, 6 m | ∆EDV > 0 | [97] |
| miR-208b | + | 359 patients with MI | On admission | Echo after admission, 6 m | ↑ >10% of LVEDV | [95] |
| + | 100 patients with MI, 80 patients with UA 80 healthy controls | On admission and after PCI | Echo on admission, 6 m | ↑ >10% of LVEDV | [98] | |
| + | 21 patients with STEMI | On admission | - | Death within 6m | [99] | |
| miR-208 | No effect | 12 patients with MI | d2, 5, 28, 90 | Echo on d1, 5, 28, 90 | Absolute change for LVEDV | [89] |
| miR-423-5p | No effect | 246 patients with anterior MI (REVE trial) | At discharge, 1 m, 3 m, 1 y | Echo on d3, 7, 3 m, 1 y | ((EDV1year—EDVbaseline)/EDVbaseline) | [96] |
| miR-1254 | − | 70 patients with STEMI | On admission | CMR at 1 w, 6 m | Absolute change for LVEDV, LVESV, LVEF | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maries, L.; Marian, C.; Sosdean, R.; Goanta, F.; Sirbu, I.O.; Anghel, A. MicroRNAs—The Heart of Post-Myocardial Infarction Remodeling. Diagnostics 2021, 11, 1675. https://doi.org/10.3390/diagnostics11091675
Maries L, Marian C, Sosdean R, Goanta F, Sirbu IO, Anghel A. MicroRNAs—The Heart of Post-Myocardial Infarction Remodeling. Diagnostics. 2021; 11(9):1675. https://doi.org/10.3390/diagnostics11091675
Chicago/Turabian StyleMaries, Liana, Cătălin Marian, Raluca Sosdean, Flavia Goanta, Ioan Ovidiu Sirbu, and Andrei Anghel. 2021. "MicroRNAs—The Heart of Post-Myocardial Infarction Remodeling" Diagnostics 11, no. 9: 1675. https://doi.org/10.3390/diagnostics11091675
APA StyleMaries, L., Marian, C., Sosdean, R., Goanta, F., Sirbu, I. O., & Anghel, A. (2021). MicroRNAs—The Heart of Post-Myocardial Infarction Remodeling. Diagnostics, 11(9), 1675. https://doi.org/10.3390/diagnostics11091675






