MRI versus Mammography plus Ultrasound in Women at Intermediate Breast Cancer Risk: Study Design and Protocol of the MRIB Multicenter, Randomized, Controlled Trial
Abstract
1. Introduction
2. Study Design and Protocol
2.1. Study Design and Population
2.2. Enrollment
2.3. Participating Centers and Imaging Readers
2.4. Imaging Interpretation
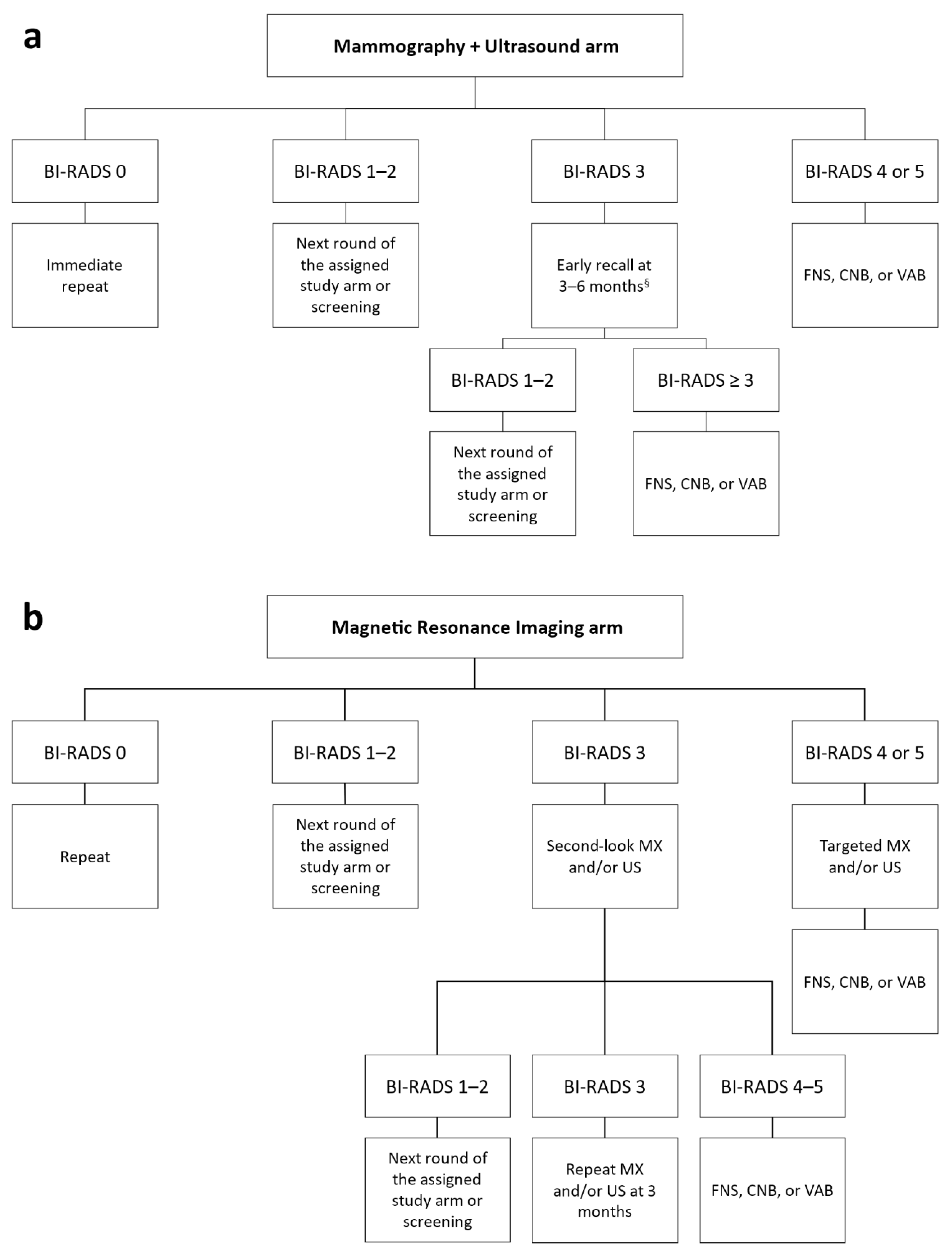
2.5. Diagnostic Workup
2.6. Data Collection
2.7. Study Endpoints
2.8. Sample Size Estimation
3. Characteristics of Enrolled Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weedon-Fekjaer, H.; Romundstad, P.R.; Vatten, L.J. Modern mammography screening and breast cancer mortality: Population study. BMJ 2014, 348, g3701. [Google Scholar] [CrossRef]
- Trimboli, R.M.; Giorgi Rossi, P.; Battisti, N.M.L.; Cozzi, A.; Magni, V.; Zanardo, M.; Sardanelli, F. Do we still need breast cancer screening in the era of targeted therapies and precision medicine? Insights Imaging 2020, 11, 105. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Benbrahim-Tallaa, L.; Bouvard, V.; Bianchini, F.; Straif, K. Breast-Cancer Screening—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2015, 372, 2353–2358. [Google Scholar] [CrossRef]
- Perron, L.; Chang, S.-L.; Daigle, J.-M.; Vandal, N.; Theberge, I.; Diorio, C.; Lemieux, J.; Pelletier, E.; Brisson, J. Breast cancer subtype and screening sensitivity in the Quebec Mammography Screening Program. J. Med. Screen. 2019, 26, 154–161. [Google Scholar] [CrossRef]
- Engmann, N.J.; Golmakani, M.K.; Miglioretti, D.L.; Sprague, B.L.; Kerlikowske, K. Population-Attributable Risk Proportion of Clinical Risk Factors for Breast Cancer. JAMA Oncol. 2017, 3, 1228–1236. [Google Scholar] [CrossRef]
- Masala, G.; Ambrogetti, D.; Assedi, M.; Bendinelli, B.; Caini, S.; Palli, D. Mammographic breast density and breast cancer risk in a Mediterranean population: A nested case–control study in the EPIC Florence cohort. Breast Cancer Res. Treat. 2017, 164, 467–473. [Google Scholar] [CrossRef]
- Boyd, N.F.; Huszti, E.; Melnichouk, O.; Martin, L.J.; Hislop, G.; Chiarelli, A.; Yaffe, M.J.; Minkin, S. Mammographic features associated with interval breast cancers in screening programs. Breast Cancer Res. 2014, 16, 417. [Google Scholar] [CrossRef]
- Kerlikowske, K.; Zhu, W.; Tosteson, A.N.A.; Sprague, B.L.; Tice, J.A.; Lehman, C.D.; Miglioretti, D.L. Identifying Women with Dense Breasts at High Risk for Interval Cancer. Ann. Intern. Med. 2015, 162, 673–681. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Li, S.; Dite, G.S.; Aung, Y.K.; Evans, C.F.; Trinh, H.N.; Baglietto, L.; Stone, J.; Song, Y.; Sung, J.; et al. Interval breast cancer risk associations with breast density, family history and breast tissue aging. Int. J. Cancer 2020, 147, 375–382. [Google Scholar] [CrossRef]
- Pattacini, P.; Nitrosi, A.; Giorgi Rossi, P.; Iotti, V.; Ginocchi, V.; Ravaioli, S.; Vacondio, R.; Braglia, L.; Cavuto, S.; Campari, C. Digital Mammography versus Digital Mammography Plus Tomosynthesis for Breast Cancer Screening: The Reggio Emilia Tomosynthesis Randomized Trial. Radiology 2018, 288, 375–385. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Strobel, K.; Bieling, H.; Leutner, C.; Schild, H.H.; Schrading, S. Supplemental Breast MR Imaging Screening of Women with Average Risk of Breast Cancer. Radiology 2017, 283, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, A.; Schiaffino, S.; Sardanelli, F. The emerging role of contrast-enhanced mammography. Quant. Imaging Med. Surg. 2019, 9, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Koay, E.J.; Borowsky, A.D.; De Marzo, A.M.; Ghosh, S.; Wagner, P.D.; Kramer, B.S. Cancer overdiagnosis: A biological challenge and clinical dilemma. Nat. Rev. Cancer 2019, 19, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.; Weigel, S.; Schrading, S.; Arand, B.; Bieling, H.; König, R.; Tombach, B.; Leutner, C.; Rieber-Brambs, A.; Nordhoff, D.; et al. Prospective Multicenter Cohort Study to Refine Management Recommendations for Women at Elevated Familial Risk of Breast Cancer: The EVA Trial. J. Clin. Oncol. 2010, 28, 1450–1457. [Google Scholar] [CrossRef]
- Sardanelli, F.; Podo, F.; Santoro, F.; Manoukian, S.; Bergonzi, S.; Trecate, G.; Vergnaghi, D.; Federico, M.; Cortesi, L.; Corcione, S.; et al. Multicenter Surveillance of Women at High Genetic Breast Cancer Risk Using Mammography, Ultrasonography, and Contrast-Enhanced Magnetic Resonance Imaging (the High Breast Cancer Risk Italian 1 Study). Investigative Radiol. 2011, 46, 94–105. [Google Scholar] [CrossRef]
- Berg, W.A.; Zhang, Z.; Lehrer, D.; Jong, R.A.; Pisano, E.D.; Barr, R.G.; Böhm-Vélez, M.; Mahoney, M.C.; Evans, W.P.; Larsen, L.H.; et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012, 307, 1394–1404. [Google Scholar] [CrossRef]
- Riedl, C.C.; Luft, N.; Bernhart, C.; Weber, M.; Bernathova, M.; Tea, M.-K.M.; Rudas, M.; Singer, C.F.; Helbich, T.H. Triple-Modality Screening Trial for Familial Breast Cancer Underlines the Importance of Magnetic Resonance Imaging and Questions the Role of Mammography and Ultrasound Regardless of Patient Mutation Status, Age, and Breast Density. J. Clin. Oncol. 2015, 33, 1128–1135. [Google Scholar] [CrossRef]
- Vreemann, S.; van Zelst, J.C.M.; Schlooz-Vries, M.; Bult, P.; Hoogerbrugge, N.; Karssemeijer, N.; Gubern-Mérida, A.; Mann, R.M. The added value of mammography in different age-groups of women with and without BRCA mutation screened with breast MRI. Breast Cancer Res. 2018, 20, 84. [Google Scholar] [CrossRef]
- Sung, J.S.; Stamler, S.; Brooks, J.; Kaplan, J.; Huang, T.; Dershaw, D.D.; Lee, C.H.; Morris, E.A.; Comstock, C.E. Breast Cancers Detected at Screening MR Imaging and Mammography in Patients at High Risk: Method of Detection Reflects Tumor Histopathologic Results. Radiology 2016, 280, 716–722. [Google Scholar] [CrossRef]
- Lee, J.M.; Arao, R.F.; Sprague, B.L.; Kerlikowske, K.; Lehman, C.D.; Smith, R.A.; Henderson, L.M.; Rauscher, G.H.; Miglioretti, D.L. Performance of Screening Ultrasonography as an Adjunct to Screening Mammography in Women across the Spectrum of Breast Cancer Risk. JAMA Intern. Med. 2019, 179, 658–667. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Guidelines Version 1.2021 Breast Cancer Screening and Diagnosis; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2021. [Google Scholar]
- National Institute for Health and Care Excellence (NICE). Familial Breast Cancer: Classification, Care and Managing Breast Cancer and Related Risks in People with a Family History of Breast Cancer. Available online: https://www.nice.org.uk/guidance/cg164/chapter/Recommendations (accessed on 14 June 2021).
- Huzarski, T.; Górecka-Szyld, B.; Huzarska, J.; Psut-Muszyńska, G.; Wilk, G.; Sibilski, R.; Cybulski, C.; Kozak-Klonowska, B.; Siołek, M.; Kilar, E.; et al. Screening with magnetic resonance imaging, mammography and ultrasound in women at average and intermediate risk of breast cancer. Hered. Cancer Clin. Pract. 2017, 15, 4. [Google Scholar] [CrossRef]
- Bakker, M.F.; de Lange, S.V.; Pijnappel, R.M.; Mann, R.M.; Peeters, P.H.M.; Monninkhof, E.M.; Emaus, M.J.; Loo, C.E.; Bisschops, R.H.C.; Lobbes, M.B.I.; et al. Supplemental MRI Screening for Women with Extremely Dense Breast Tissue. N. Engl. J. Med. 2019, 381, 2091–2102. [Google Scholar] [CrossRef]
- Sardanelli, F.; Boetes, C.; Borisch, B.; Decker, T.; Federico, M.; Gilbert, F.J.; Helbich, T.; Heywang-Köbrunner, S.H.; Kaiser, W.A.; Kerin, M.J.; et al. Magnetic resonance imaging of the breast: Recommendations from the EUSOMA working group. Eur. J. Cancer 2010, 46, 1296–1316. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi, C.J.; Sickles, E.A.; Mendelson, E.B.; Morris, E.A. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System, 5th ed.; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Maajani, K.; Jalali, A.; Alipour, S.; Khodadost, M.; Tohidinik, H.R.; Yazdani, K. The Global and Regional Survival Rate of Women with Breast Cancer: A Systematic Review and Meta-analysis. Clin. Breast Cancer 2019, 19, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, A.; Schiaffino, S.; Giorgi Rossi, P.; Sardanelli, F. Breast cancer screening: In the era of personalized medicine, age is just a number. Quant. Imaging Med. Surg. 2020, 10, 2401–2407. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, V.; Houssami, N.; Ghirardi, M.; Ferrari, A.; Speziani, M.; Bellarosa, S.; Remida, G.; Gasparotti, C.; Galligioni, E.; Ciatto, S. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: Interval breast cancers at 1-year follow-up. Eur. J. Cancer 2011, 47, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Rebolj, M.; Assi, V.; Brentnall, A.; Parmar, D.; Duffy, S.W. Addition of ultrasound to mammography in the case of dense breast tissue: Systematic review and meta-analysis. Br. J. Cancer 2018, 118, 1559–1570. [Google Scholar] [CrossRef]
- Melnikow, J.; Fenton, J.J.; Whitlock, E.P.; Miglioretti, D.L.; Weyrich, M.S.; Thompson, J.H.; Shah, K. Supplemental Screening for Breast Cancer in Women with Dense Breasts: A Systematic Review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2016, 164, 268–278. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Prummel, M.V.; Muradali, D.; Majpruz, V.; Horgan, M.; Carroll, J.C.; Eisen, A.; Meschino, W.S.; Shumak, R.S.; Warner, E.; et al. Effectiveness of Screening with Annual Magnetic Resonance Imaging and Mammography: Results of the Initial Screen from the Ontario High Risk Breast Screening Program. J. Clin. Oncol. 2014, 32, 2224–2230. [Google Scholar] [CrossRef]
- Lo, G.; Scaranelo, A.M.; Aboras, H.; Ghai, S.; Kulkarni, S.; Fleming, R.; Bukhanov, K.; Crystal, P. Evaluation of the Utility of Screening Mammography for High-Risk Women Undergoing Screening Breast MR Imaging. Radiology 2017, 285, 36–43. [Google Scholar] [CrossRef]
- Sardanelli, F.; Cozzi, A.; Trimboli, R.M.; Schiaffino, S. Gadolinium Retention and Breast MRI Screening: More Harm Than Good? Am. J. Roentgenol. 2020, 214, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.M.; Kuhl, C.K.; Moy, L. Contrast-enhanced MRI for breast cancer screening. J. Magn. Reson. Imaging 2019, 50, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.A.; Blume, J.D.; Adams, A.M.; Jong, R.A.; Barr, R.G.; Lehrer, D.E.; Pisano, E.D.; Evans, W.P.; Mahoney, M.C.; Hovanessian Larsen, L.; et al. Reasons Women at Elevated Risk of Breast Cancer Refuse Breast MR Imaging Screening: ACRIN 6666. Radiology 2010, 254, 79–87. [Google Scholar] [CrossRef]
- Mango, V.L.; Goel, A.; Mema, E.; Kwak, E.; Ha, R. Breast MRI screening for average-risk women: A monte carlo simulation cost–benefit analysis. J. Magn. Reson. Imaging 2019, 49, e216–e221. [Google Scholar] [CrossRef] [PubMed]
- Geuzinge, H.A.; Obdeijn, I.-M.; Rutgers, E.J.T.; Saadatmand, S.; Mann, R.M.; Oosterwijk, J.C.; Tollenaar, R.A.E.M.; de Roy van Zuidewijn, D.B.W.; Lobbes, M.B.I.; van’t Riet, M.; et al. Cost-effectiveness of Breast Cancer Screening with Magnetic Resonance Imaging for Women at Familial Risk. JAMA Oncol. 2020, 6, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.K.; Schrading, S.; Strobel, K.; Schild, H.H.; Hilgers, R.-D.; Bieling, H.B. Abbreviated Breast Magnetic Resonance Imaging (MRI): First Postcontrast Subtracted Images and Maximum-Intensity Projection—A Novel Approach to Breast Cancer Screening with MRI. J. Clin. Oncol. 2014, 32, 2304–2310. [Google Scholar] [CrossRef] [PubMed]
- Chhor, C.M.; Mercado, C.L. Abbreviated MRI Protocols: Wave of the Future for Breast Cancer Screening. Am. J. Roentgenol. 2017, 208, 284–289. [Google Scholar] [CrossRef]
- Greenwood, H.I. Abbreviated protocol breast MRI: The past, present, and future. Clin. Imaging 2019, 53, 169–173. [Google Scholar] [CrossRef]
- Broeders, M.; Moss, S.; Nyström, L.; Njor, S.; Jonsson, H.; Paap, E.; Massat, N.; Duffy, S.; Lynge, E.; Paci, E. The Impact of Mammographic Screening on Breast Cancer Mortality in Europe: A Review of Observational Studies. J. Med. Screen. 2012, 19, 14–25. [Google Scholar] [CrossRef]
- Briel, M.; Olu, K.K.; von Elm, E.; Kasenda, B.; Alturki, R.; Agarwal, A.; Bhatnagar, N.; Schandelmaier, S. A systematic review of discontinued trials suggested that most reasons for recruitment failure were preventable. J. Clin. Epidemiol. 2016, 80, 8–15. [Google Scholar] [CrossRef]
- Elshof, L.E.; Rutgers, E.J.T.; Deurloo, E.E.; Loo, C.E.; Wesseling, J.; Pengel, K.E.; Gilhuijs, K.G.A. A practical approach to manage additional lesions at preoperative breast MRI in patients eligible for breast conserving therapy: Results. Breast Cancer Res. Treat. 2010, 124, 707–715. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kolta, M.; Clauser, P.; Kapetas, P.; Bernathova, M.; Pinker, K.; Helbich, T.H.; Baltzer, P.A.T. Can second-look ultrasound downgrade MRI-detected lesions? A retrospective study. Eur. J. Radiol. 2020, 127, 108976. [Google Scholar] [CrossRef]
- Bumberger, A.; Clauser, P.; Kolta, M.; Kapetas, P.; Bernathova, M.; Helbich, T.H.; Pinker, K.; Baltzer, P.A. Can we predict lesion detection rates in second-look ultrasound of MRI-detected breast lesions? A systematic analysis. Eur. J. Radiol. 2019, 113, 96–100. [Google Scholar] [CrossRef]
- Jatoi, I.; Pinsky, P.F. Breast Cancer Screening Trials: Endpoints and Overdiagnosis. J. Natl. Cancer Inst. 2020, 113, 1131–1135. [Google Scholar] [CrossRef]
- Clauser, P.; Dietzel, M.; Weber, M.; Kaiser, C.G.; Baltzer, P.A. Motion artifacts, lesion type, and parenchymal enhancement in breast MRI: What does really influence diagnostic accuracy? Acta Radiol. 2019, 60, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Dilorenzo, G.; Telegrafo, M.; La Forgia, D.; Stabile Ianora, A.A.; Moschetta, M. Breast MRI background parenchymal enhancement as an imaging bridge to molecular cancer sub-type. Eur. J. Radiol. 2019, 113, 148–152. [Google Scholar] [CrossRef]
- Liao, G.J.; Henze Bancroft, L.C.; Strigel, R.M.; Chitalia, R.D.; Kontos, D.; Moy, L.; Partridge, S.C.; Rahbar, H. Background parenchymal enhancement on breast MRI: A comprehensive review. J. Magn. Reson. Imaging 2020, 51, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Fausto, A.; Bernini, M.; La Forgia, D.; Fanizzi, A.; Marcasciano, M.; Volterrani, L.; Casella, D.; Mazzei, M.A. Six-year prospective evaluation of second-look US with volume navigation for MRI-detected additional breast lesions. Eur. Radiol. 2019, 29, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Den Dekker, B.M.; Bakker, M.F.; de Lange, S.V.; Veldhuis, W.B.; van Diest, P.J.; Duvivier, K.M.; Lobbes, M.B.I.; Loo, C.E.; Mann, R.M.; Monninkhof, E.M.; et al. Reducing False-Positive Screening MRI Rate in Women with Extremely Dense Breasts Using Prediction Models Based on Data from the DENSE Trial. Radiology 2021. [Google Scholar] [CrossRef]
| 1 | Availability of an electronic image storage system for MX, US, and MRI |
| 2 | Full-field digital MX systems with high-resolution electronic display systems available to both the technologist at the time of the examination and to the interpreting physician. Mandatory availability on the display settings of the dedicated workstation of relevant information about the digital images and the examined patient. |
| 3 | Breast US scanners equipped with a multi-frequency linear array transducer operating at a center frequency higher than 10 MHz. |
| 4 | MR units with magnets with intensity field ≥ 1.0 T and gradients ≥ 20 mT/m, equipped with bilateral dedicated coils (preferably multichannel) and an automated power injector system with double syringe for both contrast agent and normal saline solution. The MRI protocol must include a high-contrast bilateral morphologic sequence and a bilateral dynamic two-dimensional or three-dimensional study with spatial in-plane resolution ≤ 1.5 mm2 (preferably ≤ 1 mm2) and temporal resolution ≤ 120 s. |
| Center | Mammography Plus Ultrasound n (%) | Magnetic Resonance Imaging n (%) |
|---|---|---|
| IRCCS Ospedale Policlinico San Martino, Genova | 139 (22.3%) | 139 (22.1%) |
| Ospedale Universitario Sant’Anna, Cona—Università degli Studi di Ferrara, Ferrara | 100 (16.0%) | 101 (16.0%) |
| Azienda Ospedaliera Universitaria Integrata, Verona | 100 (16.0%) | 100 (15.9%) |
| IRCCS Policlinico San Donato, San Donato Milanese | 53 (8.5%) | 54 (8.6%) |
| IRCCS Ospedale San Raffaele, Milano | 50 (8.0%) | 50 (7.9%) |
| Dipartimento di Scienze Radiologiche, Oncologiche, Patologiche—Università La Sapienza, Roma | 45 (7.2%) | 45 (7.1%) |
| Azienda Ospedaliera Universitaria “Santa Maria della Misericordia”, Udine | 39 (6.2%) | 39 (6.2%) |
| Fondazione IRCCS Istituto Nazionale dei Tumori, Milano | 34 (5.4%) | 38 (6.0%) |
| Fondazione Policlinico Universitario Agostino Gemelli IRCCS—Università Cattolica del Sacro Cuore, Roma | 34 (5.4%) | 34 (5.4%) |
| Istituto Nazionale Tumori IRCCS Fondazione G. Pascale, Napoli | 30 (4.8%) | 30 (4.8%) |
| Total | 624 (100.0%) | 630 (100.0%) |
| Mammography Plus Ultrasound n (%) | Magnetic Resonance Imaging n (%) | |
|---|---|---|
| Total | 624 (100.0%) | 630 (100.0%) |
| Age classes | ||
| 40–44 | 214 (34.3%) | 206 (32.7%) |
| 45–49 | 236 (37.8%) | 249 (39.5%) |
| 50–54 | 130 (20.8%) | 121 (19.2%) |
| 55–59 | 44 (7.1%) | 54 (8.6%) |
| Age at menarche (years) | ||
| ≤11 | 118 (18.9%) | 141 (22.4%) |
| 12–13 | 336 (53.8%) | 311 (49.4%) |
| ≥14 | 165 (26.4%) | 171 (27.1%) |
| Unknown | 5 (0.8%) | 7 (1.1%) |
| Number of full-term pregnancies | ||
| 0 | 170 (27.2%) | 176 (27.9%) |
| 1 | 208 (45.8%) | 176 (38.8%) |
| 2 | 211 (46.5%) | 231 (50.9%) |
| ≥3 | 35 (7.7%) | 47 (10.4%) |
| Age at first birth (years) | ||
| <20 | 4 (0.9%) | 9 (2.0%) |
| 20–24 | 65 (14.3%) | 62 (13.7%) |
| 25–29 | 124 (27.3%) | 140 (30.9%) |
| 30–34 | 141 (31.1%) | 128 (28.3%) |
| ≥35 | 101 (22.2%) | 98 (21.6%) |
| Unknown | 19 (4.2%) | 16 (3.5%) |
| Menopausal status | ||
| Premenopausal | 282 (45.2%) | 277 (44.0%) |
| Perimenopausal | 150 (24.0%) | 147 (23.3%) |
| Postmenopausal | 117 (18.8%) | 130 (20.6%) |
| Unknown | 75 (12.0%) | 76 (12.1%) |
| Contraceptive pill use | ||
| Never | 303 (48.6%) | 298 (47.3%) |
| Current | 59 (9.5%) | 60 (9.5%) |
| Discontinued < 5 years | 39 (6.2%) | 46 (7.3%) |
| Discontinued ≥ 5 years | 223 (35.7%) | 226 (35.9%) |
| HRT use in post-menopause | ||
| Never | 104 (88.9%) | 105 (80.8%) |
| Past | 9 (7.7%) | 21 (16.1%) |
| Current | 4 (3.4%) | 4 (3.1%) |
| Body mass index (kg/m2) | ||
| <25 | 523 (83.8%) | 525 (83.3%) |
| 25–29 | 68 (10.9%) | 63 (10.0%) |
| ≥30 | 12 (1.9%) | 17 (2.7%) |
| Unknown | 21 (3.4%) | 25 (4.0%) |
| Cigarette smoking | ||
| Never | 420 (67.3%) | 425 (67.5%) |
| Past | 107 (17.1%) | 97 (15.4%) |
| Current | 97 (15.5%) | 108 (7.1%) |
| Alcohol consumption | ||
| Never | 446 (71.5%) | 441 (70.0%) |
| Past | 39 (6.2%) | 41 (6.5%) |
| Current | 139 (22.3%) | 148 (23.5%) |
| First-degree family history of breast cancer and ovarian cancer | ||
| None | 383 (61.4%) | 413 (65.6%) |
| Breast cancer | 218 (34.9%) | 197 (31.3%) |
| Ovarian cancer | 17 (2.7%) | 16 (2.5%) |
| Breast cancer and ovarian cancer | 6 (1.0%) | 4 (0.6%) |
| Breast density at pre-trial MX | ||
| a | 6 (1.0%) | 9 (1.4%) |
| b | 47 (7.5%) | 53 (8.4%) |
| c | 56 (9.0%) | 41 (6.5%) |
| d | 486 (77.9%) | 498 (79.0%) |
| Unknown | 29 (4.6%) | 29 (4.6%) |
| LTR of breast cancer | ||
| <15% | 358 (57.4%) | 382 (60.6%) |
| 15–30% | 266 (42.6%) | 248 (39.4%) |
| Risk profile | ||
| LTR ≥ 15%, breast density (a–c) | 109 (17.5%) | 103 (16.3%) |
| LTR < 15%, breast density (d) | 358 (57.4%) | 382 (60.6%) |
| LTR ≥ 15%, breast density (d) | 128 (20.5%) | 116 (18.4%) |
| LTR ≥ 15%, unknown breast density | 29 (4.6%) | 29 (4.6%) |
| Breast Cancer LTR < 15% | Breast Cancer LTR 15–30% | |||
|---|---|---|---|---|
| Centers | Breast Density (d) n (%) | Breast Density Not Assessed n (%) | Breast Density (a–c) n (%) | Breast Density (d) n (%) |
| IRCCS Ospedale Policlinico San Martino, Genova | 230 (82.7%) | 2 (0.7%) | 1 (0.4%) | 45 (16.2%) |
| Ospedale Universitario Sant’Anna, Cona—Università degli Studi di Ferrara, Ferrara | 115 (57.2%) | 0 (0.0%) | 53 (26.4%) | 33 (16.4%) |
| Azienda Ospedaliera Universitaria Integrata, Verona | 132 (66.0%) | 0 (0.0%) | 3 (1.5%) | 65 (32.5%) |
| IRCCS Policlinico San Donato, San Donato Milanese | 72 (67.3%) | 0 (0.0%) | 26 (24.3%) | 9 (8.4%) |
| IRCCS Ospedale San Raffaele, Milano | 21 (21.0%) | 39 (39.0%) | 20 (20.0%) | 20 (20.0%) |
| Dipartimento di Scienze Radiologiche, Oncologiche, Patologiche—Università La Sapienza, Roma | 58 (64.4%) | 15 (16.7%) | 4 (4.4%) | 13 (14.4%) |
| Azienda Ospedaliera Universitaria “Santa Maria della Misericordia”, Udine | 33 (42.3%) | 2 (2.6%) | 23 (29.5%) | 20 (25.6%) |
| Fondazione IRCCS Istituto Nazionale dei Tumori, Milano | 30 (41.7%) | 0 (0.0%) | 22 (30.6%) | 20 (27.8%) |
| Fondazione Policlinico Universitario Agostino Gemelli IRCCS—Università Cattolica del Sacro Cuore, Roma | 49 (72.1%) | 0 (0.0%) | 0 (0.0%) | 19 (27.9%) |
| Istituto Nazionale Tumori IRCCS Fondazione G. Pascale, Napoli | 0 (0.0%) | 0 (0.0%) | 60 (100.0%) | 0 (0.0%) |
| Total | 740 (59.0%) | 58 (4.6%) | 212 (16.9%) | 244 (19.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonelli, L.A.; Calabrese, M.; Belli, P.; Corcione, S.; Losio, C.; Montemezzi, S.; Pediconi, F.; Petrillo, A.; Zuiani, C.; Camera, L.; et al. MRI versus Mammography plus Ultrasound in Women at Intermediate Breast Cancer Risk: Study Design and Protocol of the MRIB Multicenter, Randomized, Controlled Trial. Diagnostics 2021, 11, 1635. https://doi.org/10.3390/diagnostics11091635
Bonelli LA, Calabrese M, Belli P, Corcione S, Losio C, Montemezzi S, Pediconi F, Petrillo A, Zuiani C, Camera L, et al. MRI versus Mammography plus Ultrasound in Women at Intermediate Breast Cancer Risk: Study Design and Protocol of the MRIB Multicenter, Randomized, Controlled Trial. Diagnostics. 2021; 11(9):1635. https://doi.org/10.3390/diagnostics11091635
Chicago/Turabian StyleBonelli, Luigina Ada, Massimo Calabrese, Paolo Belli, Stefano Corcione, Claudio Losio, Stefania Montemezzi, Federica Pediconi, Antonella Petrillo, Chiara Zuiani, Lucia Camera, and et al. 2021. "MRI versus Mammography plus Ultrasound in Women at Intermediate Breast Cancer Risk: Study Design and Protocol of the MRIB Multicenter, Randomized, Controlled Trial" Diagnostics 11, no. 9: 1635. https://doi.org/10.3390/diagnostics11091635
APA StyleBonelli, L. A., Calabrese, M., Belli, P., Corcione, S., Losio, C., Montemezzi, S., Pediconi, F., Petrillo, A., Zuiani, C., Camera, L., Carbonaro, L. A., Cozzi, A., De Falco Alfano, D., Gristina, L., Panzeri, M., Poirè, I., Schiaffino, S., Tosto, S., Trecate, G., ... Sardanelli, F. (2021). MRI versus Mammography plus Ultrasound in Women at Intermediate Breast Cancer Risk: Study Design and Protocol of the MRIB Multicenter, Randomized, Controlled Trial. Diagnostics, 11(9), 1635. https://doi.org/10.3390/diagnostics11091635






