Complex Regional Pain Syndrome Type I Following Non-Orthopedic Surgery: Case Report and Narrative Review
Abstract
1. Introduction
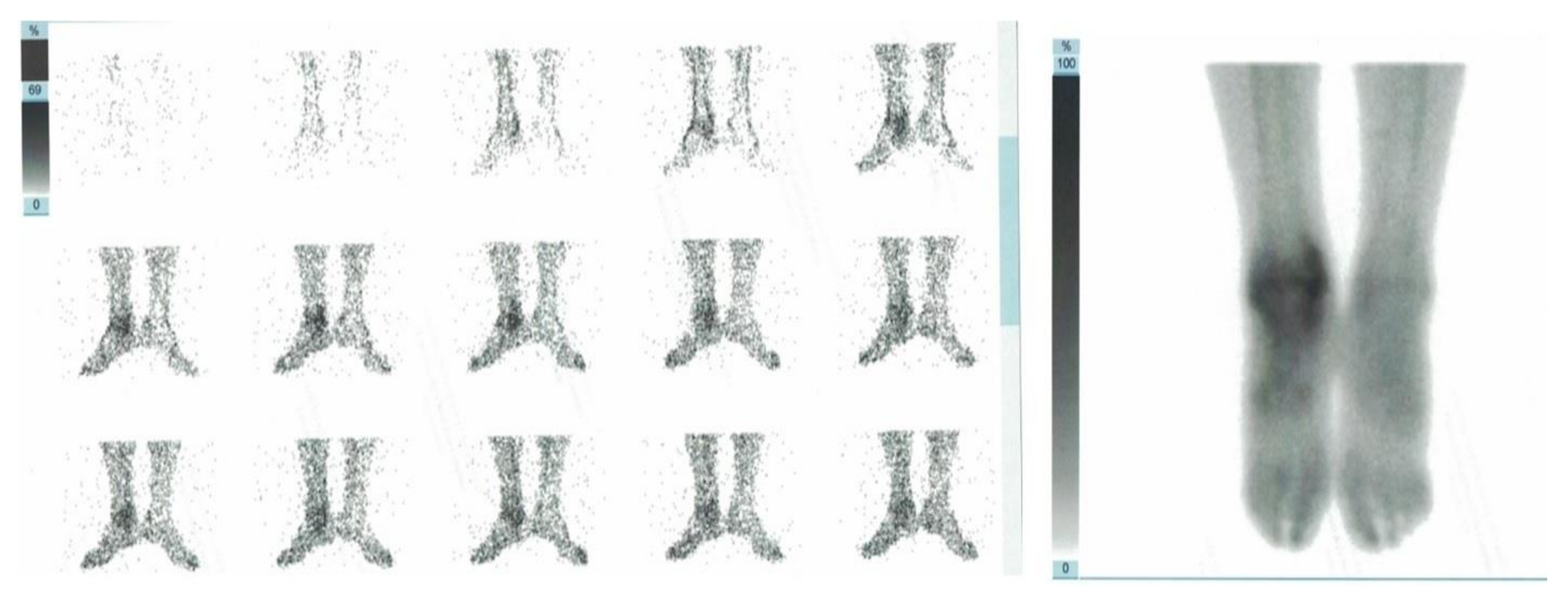
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iolascon, G.; Tarantino, U. Rare diseases in orthopedics: Algodystrophy and aseptic osteonecrosis. Clin. Cases Miner. Bone Metab. 2016, 12, 2–3. [Google Scholar]
- De Sire, A.; Paoletta, M.; Moretti, A.; Brandi, M.L.; Iolascon, G. Complex regional pain syndrome: Facts on causes, diagnosis and therapy. Clin. Cases Miner. Bone Metab. 2018, 15, 166–172. [Google Scholar]
- Birklein, F.; Schmelz, M. Neuropeptides, neurogenic inflammation and complex regional pain syndrome (CRPS). Neurosci. Lett. 2008, 437, 199–202. [Google Scholar] [CrossRef]
- Groeneweg, J.G.; Huygen, F.J.P.M.; Heijmans-Antonissen, C.; Niehof, S.; Zijlstra, F.J. Increased endothelin-1 and diminished nitric oxide levels in blister fluids of patients with intermediate cold type complex regional pain syndrome type 1. BMC Musculoskelet. Disord. 2006, 7, 91. [Google Scholar] [CrossRef]
- Birklein, F.; O’Neill, D.; Schlereth, T. Complex regional pain syndrome: An optimistic perspective. Neurology 2014, 84, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sandroni, P.; Benrud-Larson, L.M.; McClelland, R.L.; Low, P.A. Complex regional pain syndrome type I: Incidence and prevalence in Olmsted county, a population-based study. Pain 2003, 103, 199–207. [Google Scholar] [CrossRef]
- De Mos, M.; de Bruijn, A.G.; Huygen, F.J.; Dieleman, J.P.; Stricker, B.H.; Sturkenboom, M.C. The incidence of complex regional pain syndrome: A population-based study. Pain 2007, 129, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Harden, N.R.; Bruehl, S.; Perez, R.S.; Birklein, F.; Marinus, J.; Maihofner, C.; Lubenow, T.R.; Buvanendran, A.; Mackey, S.; Graciosa, J.R.; et al. Validation of proposed diagnostic criteria (the “Budapest Criteria”) for Complex Regional Pain Syndrome. Pain 2010, 150, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R. The short-form McGill Pain Questionnaire. Pain 1987, 30, 191–197. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care. 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Varenna, M.; Adami, S.; Rossini, M.; Gatti, D.; Idolazzi, L.; Zucchi, F.; Malavolta, N.; Sinigaglia, L. Treatment of complex regional pain syndrome type I with neridronate: A randomized, double-blind, placebo-controlled study. Rheumatology 2012, 52, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, G.; de Sire, A.; Moretti, A.; Gimigliano, F. Complex regional pain syndrome (CRPS) type I: Historical perspective and critical issues. Clin. Cases Miner. Bone Metab. 2015, 12 (Suppl. 1), 4–10. [Google Scholar] [CrossRef]
- Resmini, G.; Ratti, C.; Canton, G.; Murena, L.; Moretti, A.; Iolascon, G. Treatment of complex regional pain syndrome. Clin. Cases Miner. Bone Metab. 2015, 12 (Suppl. 1), 26–30. [Google Scholar] [CrossRef]
- Bruehl, S.; Maihöfner, C.; Stanton-Hicks, M.; Perez, R.S.; Vatine, J.-J.; Brunner, F.; Birklein, F.; Schlereth, T.; Mackey, S.; Mailis-Gagnon, A.; et al. Complex regional pain syndrome: Evidence for warm and cold subtypes in a large prospective clinical sample. Pain 2016, 157, 1674–1681. [Google Scholar] [CrossRef]
- Atkins, R.; Duckworth, T.; Kanis, J. Features of algodystrophy after Colles’ fracture. J. Bone Jt. Surgery. Br. Vol. 1990, 72, 105–110. [Google Scholar] [CrossRef]
- Veldman, P.; Reynen, H.; Arntz, I.; Goris, R. Signs and symptoms of reflex sympathetic dystrophy: Prospective study of 829 patients. Lancet 1993, 342, 1012–1016. [Google Scholar] [CrossRef]
- Lai, C.-J.; Chou, C.-L.; Liu, T.-J.; Chan, R.-C. Complex Regional Pain Syndrome after Transradial Cardiac Catheterization. J. Chin. Med. Assoc. 2006, 69, 179–183. [Google Scholar] [CrossRef][Green Version]
- Parikh, R. Complex Regional Pain Syndrome after transfemoral coronary balloon angioplasty. Turk Kardiyol. Dernegi Arsivi-Arch. Turk. Soc. Cardiol. 2016, 44, 694–696. [Google Scholar] [CrossRef]
- Saad, A.; Knolla, R.; Gupta, K. Complex regional pain syndrome following transfemoral catheterization. J. Invasive Cardiol. 2011, 23, E267–E270. [Google Scholar]
- Papadimos, T.J.; Hofmann, J.P. Radial artery thrombosis, palmar arch systolic blood velocities, and chronic regional pain syndrome 1 following transradial cardiac catheterization. Catheter. Cardiovasc. Interv. 2002, 57, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Weise, W.J.; Bernard, D.B. Reflex Sympathetic Dystrophy Syndrome of the Hand after Placement of an Arteriovenous Graft for Hemodialysis. Am. J. Kidney Dis. 1991, 18, 406–408. [Google Scholar] [CrossRef]
- Unek, I.T.; Birlik, M.; Cavdar, C.; Ersoy, R.; Önen, F.; Çelik, A.; Camsari, T. Reflex sympathetic dystrophy syndrome due to arteriovenous fistula. Hemodial. Int. 2005, 9, 344–348. [Google Scholar] [CrossRef]
- Kemler, M.A.; Tordoir, J.H.M. Reflex sympathetic dystrophy following vascular access surgery for hemodialysis: Influence of peripheral ischaemia? Nephrol. Dial. Transplant. 1998, 13, 784–786. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muñoz-Gomez, J.; Collado, A.; Gratacós, J.; Llena, J.; Campistol, J.M.; Andreu, J.; Lomeña, F. Reflex sympathetic dystrophy syndrome of the lower limbs in renal transplant patients treated with cyclosporin A. Arthritis Rheum. 1991, 34, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Ybarra, J.; Crespo, M.; Torregrosa, J.V.; Fuster, D.; Campistol, J.M.; Oppenheimer, F. Reflex sympathetic dystrophy syndrome in renal transplanted patients under immunosuppression with tacrolimus. Transplant. Proc. 2003, 35, 2937–2939. [Google Scholar] [CrossRef]
- Stamatoullas, A.; Ferrant, A.; Manicourt, D. Reflex sympathetic dystrophy after bone marrow transplantation. Ann. Hematol. 1993, 67, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.; McGuigan, C.; Kerr, S.; Taggart, A. Complex regional pain syndrome post mastectomy. Rheumatol. Int. 2001, 21, 165–166. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Chen, L.-C.; Chen, J.-C.; Cheng, Y.-L. Complex Regional Pain Syndrome Following the Nuss Procedure for Severe Pectus Excavatum. Ann. Thorac. Cardiovasc. Surg. 2014, 20, 542–545. [Google Scholar] [CrossRef]
- Torregrosa, J.-V. Reflex sympathetic dystrophy syndrome in renal transplant patients. A mysterious and misdiagnosed entity. Nephrol. Dial. Transplant. 1999, 14, 1364–1365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ott, S.; Maihöfner, C. Signs and Symptoms in 1,043 Patients with Complex Regional Pain Syndrome. J. Pain 2018, 19, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Georgius, P.; Santarelli, D.M. A new hypothesis for the pathophysiology of complex regional pain syndrome. Med Hypotheses 2018, 119, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Birklein, F.; Ajit, S.K.; Goebel, A.; Perez, R.S.G.M.; Sommer, C. Complex regional pain syndrome—Phenotypic characteristics and potential biomarkers. Nat. Rev. Neurol. 2018, 14, 272–284. [Google Scholar] [CrossRef]
- Kosek, E.; Cohen, M.; Baron, R.; Gebhart, G.F.; Mico, J.-A.; Rice, A.S.; Rief, W.; Sluka, K. Do we need a third mechanistic descriptor for chronic pain states? Pain 2016, 157, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Cappello, Z.J.; Kasdan, M.L.; Louis, D.S. Meta-analysis of Imaging Techniques for the Diagnosis of Complex Regional Pain Syndrome Type I. J. Hand Surg. 2012, 37, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Park, S.; Kim, Y.; Lee, S.; Nahm, F.; Kim, J.; Kim, H.; Oh, S. Analysis of patterns of three-phase bone scintigraphy for patients with complex regional pain syndrome diagnosed using the proposed research criteria (the ‘Budapest Criteria’). Br. J. Anaesth. 2012, 108, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.A.; Roy, L.; Kaye, A.D.; Pyati, S. Utility of Radionuclide Bone Scintigraphy in Complex Regional Pain Syndrome. Curr. Pain Headache Rep. 2018, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, G.; Moretti, A. Pharmacotherapeutic options for complex regional pain syndrome. Expert Opin. Pharmacother. 2019, 20, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Varenna, M.; Adami, S.; Sinigaglia, L. Bisphosphonates in complex regional pain syndrome type I: How do they work? Clin. Exp. Rheumatol. 2014, 32, 451–454. [Google Scholar] [PubMed]
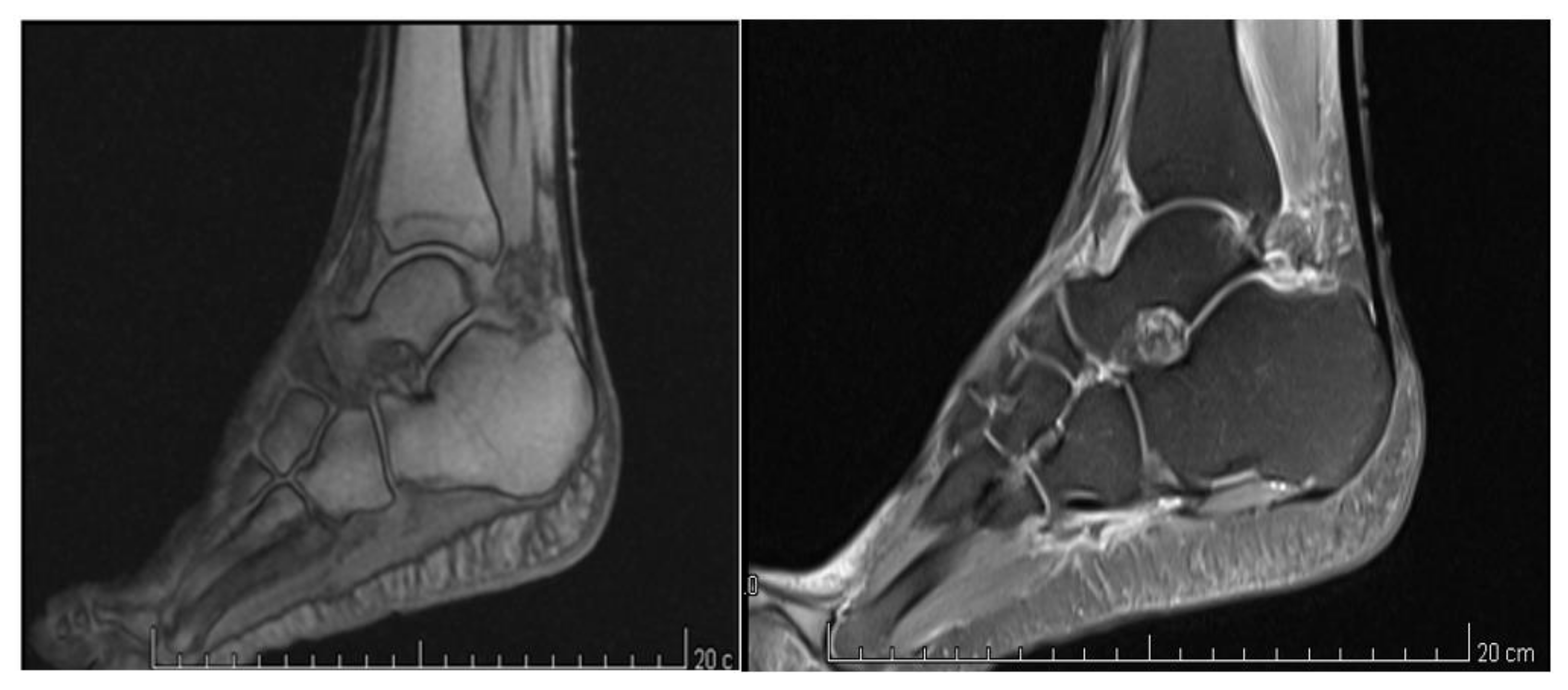
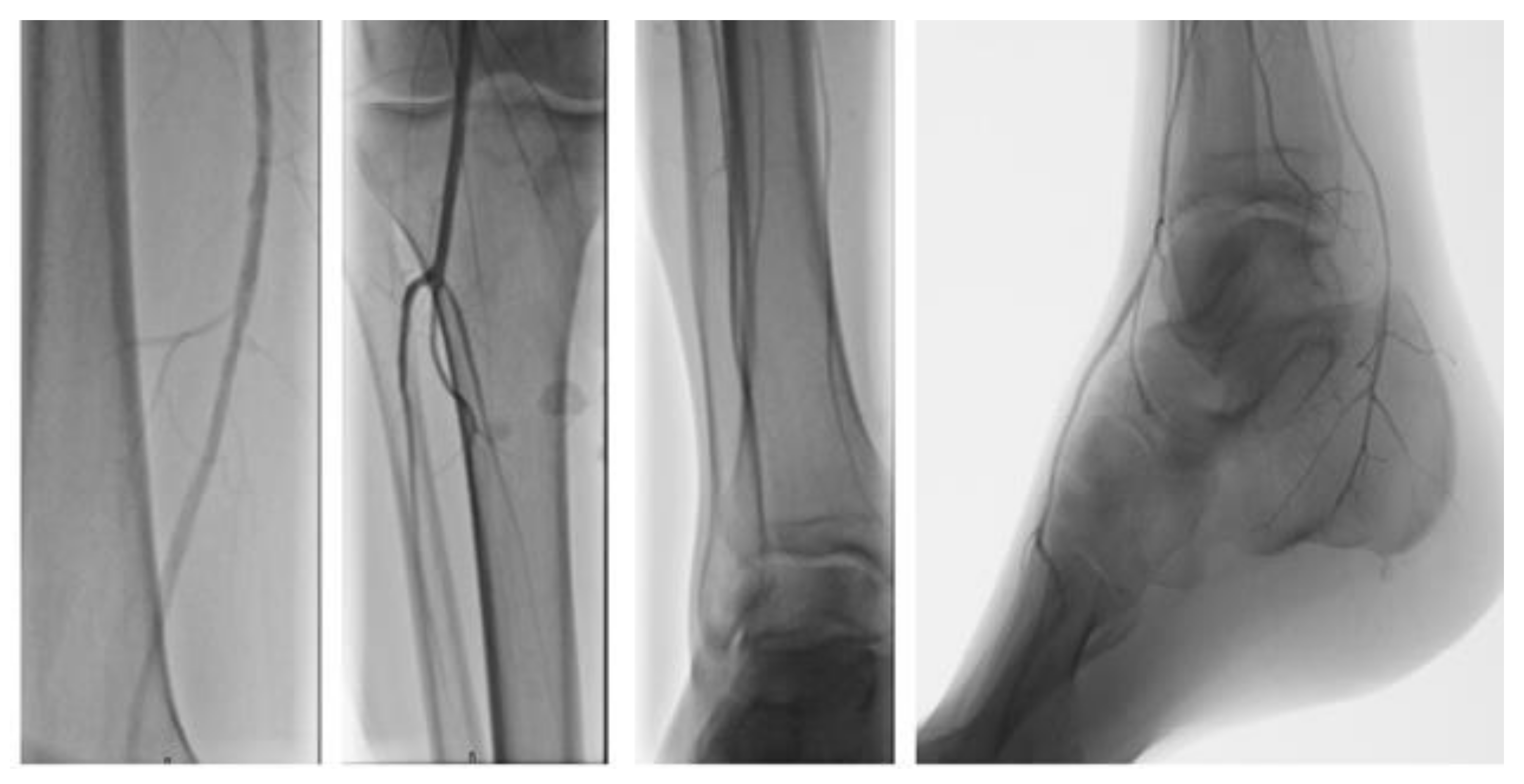
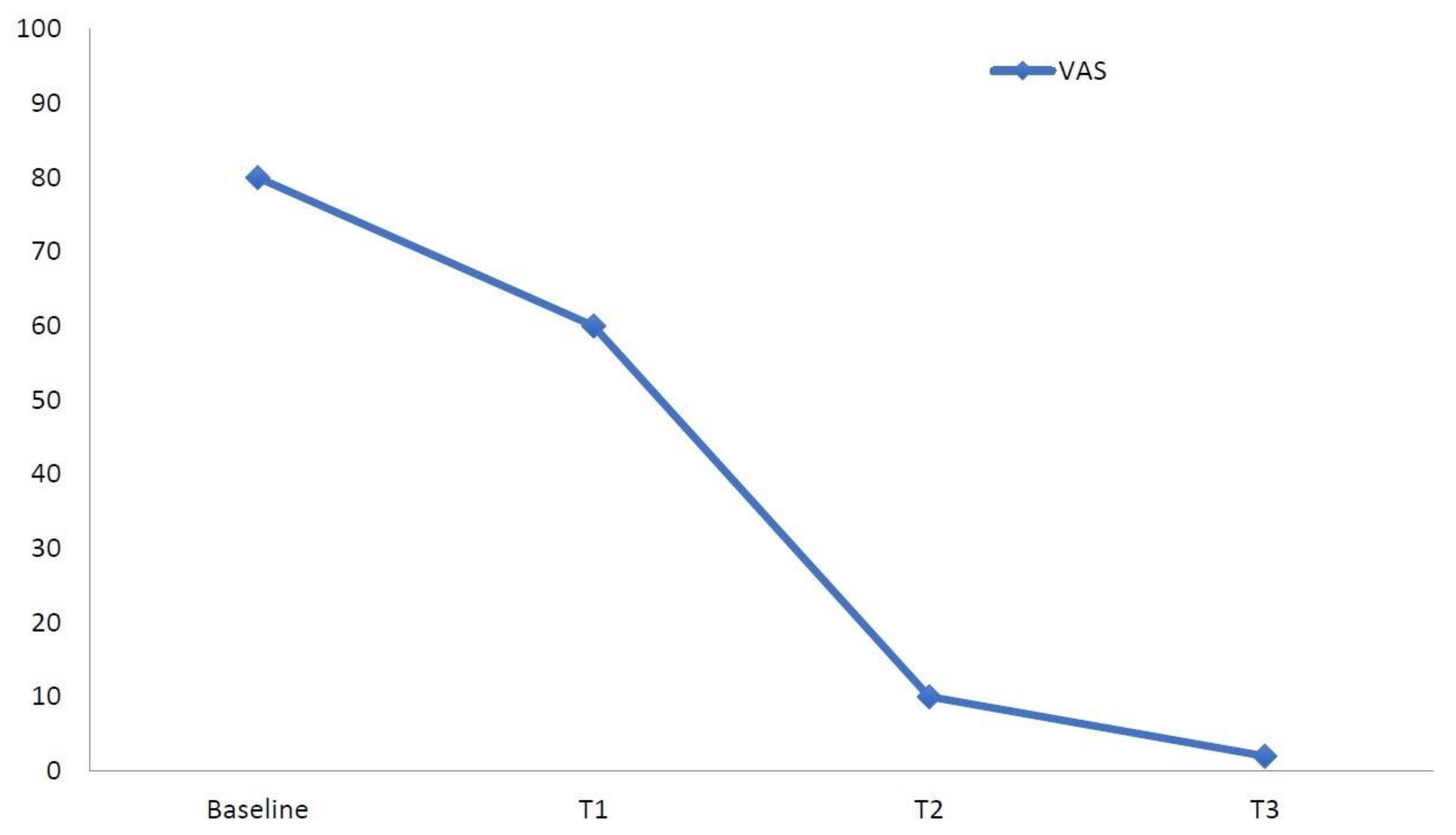
| Outcome Measure | Baseline (T0) | T1 (1 Week) | T2 (1 Month) | T3 (2 Months) |
|---|---|---|---|---|
| VAS Pain | 80 | 60 | 10 | 0 |
| Sensory subscore | 12 | 7 | 2 | 0 |
| Affective subscore | 2 | 2 | 0 | 0 |
| Total score | 14 | 9 | 2 | 0 |
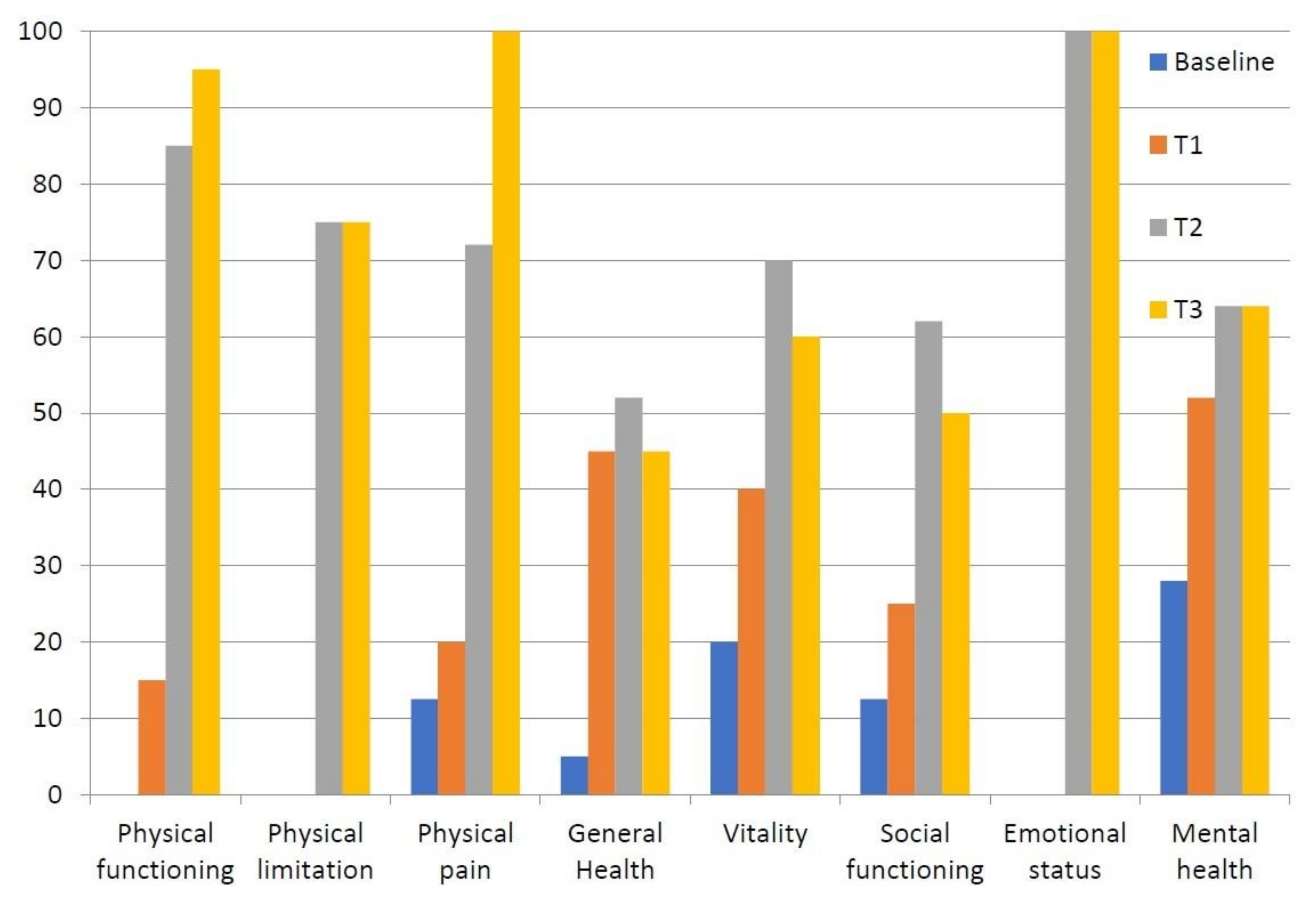
| Outcome Measure (0–100) | Baseline (T0) | T1 (1 Week) | T2 (1 Month) | T3 (2 Months) |
|---|---|---|---|---|
| Physical functioning | 0 | 15 | 95 | 95 |
| Physical limitation | 0 | 0 | 75 | 75 |
| Physical pain | 12.5 | 20 | 72 | 100 |
| General health | 5 | 45 | 52 | 45 |
| Vitality | 20 | 40 | 70 | 60 |
| Social functioning | 12.5 | 25 | 62 | 50 |
| Emotional status | 0 | 0 | 100 | 100 |
| Mental health | 28 | 52 | 64 | 64 |
| Authors | Putative Triggering Event | Case Description |
|---|---|---|
| Lai C.J. et al. (2006) [17] | Primary percutaneous transluminal coronary angioplasty (PTCA) after myocardial infarction | A 73-year-old male with CRPS I of the right hand. Numbness, pain and soft tissue swelling persisted for 3 months after procedure. Characteristics of CRPS were noted with three-phase bone scan (delayed bone phase). The patient was treated with intensive physical therapy and hydrotherapy, manual soft tissue release and occupational therapy for the hand function, with improvement in range of motion, hand function and activity of daily living. |
| Parikh R.P. et al. (2016) [18] | Coronary angioplasty with drug-eluting stent via transfemoral artery approach after post-infarct angina | A 55-year-old man with CRPS I of right foot and ankle. Severe burning pain of the dorsum of the foot and ankle, swelling of the ankle joint and allodynia over the ankle joint few weeks after procedure. Triple-phase bone scan showed delayed uptake of tracer in the distal ends of the tibia, fibula, tarsal and metatarsal bones. Patient was treated with physiotherapy and opioid analgesics. |
| Saad A. et al. (2011) [19] | Transfemoral catheterization-related groin pseudoaneurysm after AV node re-entry tachycardia (AVNRT) ablation | A 36-year-old Caucasian woman with CRPS I of left foot. Numbness, tingling, local edema and mild groin discomfort, swelling and cold sensation after 1 month from procedure. Three-phase bone scan showed cold-type CRPS. Spinal cord stimulator improved symptoms after unsuccessful pharmacological and rehabilitative treatments. |
| Papadimos T.J. and Hofmann J.P. (2002) [20] | Transradial cardiac catheterization associated with prolonged use of a hemostatic device for the left ventriculography and coronary artery injections after chest pain, bradycardia and a right bundle branch block | A 46-year-old man with CRPS I of left hand. Pallor and pain in the left hand presenting 24 h after discharge; several months later, cold intolerance, burning sensations, paresthesia and loss of left radial pulse accompanied pain, intermittent pallor and limited range of motion. Echo color-Doppler showed the occluded left radial artery for 12 cm proximal to the puncture, but perfusion seemed adequate. The patient was treated with physical therapy but limited range of motion with pain persisted. He refused sympathetic blockade and medications. |
| Weise W.J. and Bernard D.B. (1991) [21] | Placement of a left brachiobasilic AV graft of polytetrafluorethylene (WIFE) for hemodialysis | A 58-year-old black woman with CRPS I of left hand and wrist. Pain, swelling and reduction in range of motion, erythema, dysesthesia to light touch of left-hand months after approximately 3 months from beginning hemodialysis and placement of a left brachiobasilic AV graft of polytetrafluorethylene (WIFE). X-rays showed diffuse osteopenia and periarticular demineralization of left hand, allowing to make a diagnosis of RSD. The patient was treated with prednisone (40 mg/die) with improvement of symptoms and physical findings in the next 6 weeks. |
| Unek I.T. et al. (2005) [22] | Placement of an arteriovenous fistula (AVF) between the brachial artery and cephalic vein on left arm for hemodialysis | A 62-year-old patient with CRPS I of the left hand after approximately 1 month of placement AVF. Pain, swelling, cyanosis of the third finger of her left hand and progressive hand pain spreading to other fingers. Physical examination showed impairment of muscle strength (2/5), thenar and hypothenar atrophy, localized edema distal to wrist, flexion contractures on the second and third proximal interphalangeal joints of the left hand. Magnetic resonance angiography showed insufficient flow to distal parts of brachial artery (steal syndrome) at the level of arteriovenous fistula. Bone scan with technetium confirmed diagnosis of RSD at early stage. Patient was treated with calcitonin (400 IU/day) and physical therapy with improvement of swelling and pain. |
| Kemler M.A. and Tordoir J.H.M. (1998) [23] | Vascular access surgery for hemodialysis and thrombosis or insufficient maturation of fistula | A 71-year-old woman, a 59-year-old woman with RDS of left hand after ischemia for fistula thrombosed and a 70-year-old woman with RDS of left hand after ischemia due to insufficient maturation of the fistula. Loss of hand function, pain, atrophy of skin or subcutaneous tissue. Diagnostic work-up and treatments were not reported. |
| Muñoz-Gomez J. et al. (1991) [24] | Renal transplantation and immunosuppression with cyclosporin A | Seven patients (five men and two women) with RSD of lower limbs. Severe pain, periarticular soft tissue swelling, articular mobility conserved and walking impairment. Radiography and scintigraphy (99m-technetium methylene diphosphonate) showed osteoporotic pattern and increased uptake 99m-technetium with a periarticular distribution in the clinically affected areas. Symptoms started approximately 3 months after kidney transplantation in all patients and improved reducing cyclosporin A dosage; the mean duration of symptoms was 8 months. |
| Ybarra J. et al. (2003) [25] | Renal transplantation and immunosuppression with tacrolimus monotherapy or shifted from/to cyclosporin A | Four patients (one man and three women) with RSD of lower limbs. Symptoms and signs (pain, swelling, periarticular soft tissue swelling, difficulty in walking) started between one and four months after immunosuppression or shifted therapy for acute rejection. Bone scintigraphy with (99m-Tc) pyrophosphate confirmed diagnosis. Symptoms spontaneously improved in 3–4 months in two patients and pain gradually resolved 6 months spontaneously or after prednisone in the other two patients. |
| Stamatoullas A., Ferrant A., and Manicourt D. (1993) [26] | Bone marrow transplantation (BMT), immunosuppression, chemotherapy, radiotherapy | Three patients (two men and one woman) with RSD of lower limbs. Painful feet and ankles, difficulty in standing-up or to walking during the early post-transplant period. Bone scintigraphy confirmed diagnosis of RSD. Two patients were unsuccessfully treated with calcitonin (50 U/day for 5 days), replaced by pamidronate (30 mg/d) for 5 days that was effective. Calcitonin (50 U/day for 5 days) improved symptoms in one patient. |
| Graham L.E. et al. (2002) [27] | Mastectomy in patients with chronic mastalgia unresponsive to all medical treatment | Two women with CRPS I of arm and hand. Pain (in one case, 4-year history of pain in left arm from the operation, and in the other case, after 4 weeks from the bilateral mastectomy operation), stiffness, reduction in articular mobility, hyperalgesia, edema and increased skin temperature. Diagnosis of CRPS was made after plain x-rays, investigations (including inflammatory indices) and in one case, after whole-body isotope bone scan. No improvements in the symptoms after intensive physiotherapy, intravenous infusions of pamidronate, and (in one case) despite two stellate ganglion nerve blocks and localized injections of botulinum toxin. |
| Chen Y.L. et al. (2014) [28] | Nuss procedure for correcting pectus excavatum | A 22-year-old man with CRPS I of right arm. Persistent pain, hyperalgesia, weakness, edema and color and temperature changes on right upper extremity developed 2 weeks after the operation. A three-phase bone scan showed increased uptake in the right elbow joint in the bone phase and slightly increased tracer distribution in the right elbow region during the arterial and blood-pooling phase. Patient was treated with intensive rehabilitation with improvement of the degree of pain, weakness and edema. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretti, A.; Gimigliano, F.; Paoletta, M.; Bertone, M.; Liguori, S.; Toro, G.; Iolascon, G. Complex Regional Pain Syndrome Type I Following Non-Orthopedic Surgery: Case Report and Narrative Review. Diagnostics 2021, 11, 1596. https://doi.org/10.3390/diagnostics11091596
Moretti A, Gimigliano F, Paoletta M, Bertone M, Liguori S, Toro G, Iolascon G. Complex Regional Pain Syndrome Type I Following Non-Orthopedic Surgery: Case Report and Narrative Review. Diagnostics. 2021; 11(9):1596. https://doi.org/10.3390/diagnostics11091596
Chicago/Turabian StyleMoretti, Antimo, Francesca Gimigliano, Marco Paoletta, Matteo Bertone, Sara Liguori, Giuseppe Toro, and Giovanni Iolascon. 2021. "Complex Regional Pain Syndrome Type I Following Non-Orthopedic Surgery: Case Report and Narrative Review" Diagnostics 11, no. 9: 1596. https://doi.org/10.3390/diagnostics11091596
APA StyleMoretti, A., Gimigliano, F., Paoletta, M., Bertone, M., Liguori, S., Toro, G., & Iolascon, G. (2021). Complex Regional Pain Syndrome Type I Following Non-Orthopedic Surgery: Case Report and Narrative Review. Diagnostics, 11(9), 1596. https://doi.org/10.3390/diagnostics11091596







