Prognostic Role of Minimal Disseminated Disease and NOTCH1/FBXW7 Mutational Status in Children with Lymphoblastic Lymphoma: The AIEOP Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients, Samples and Treatment Protocol
2.2. Multiparametric Flow Citometry Analysis of MDD
2.3. NOTCH1/FBXW7 Mutational Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinical Features
3.1.1. MDD Analysis in LBL Patients
3.1.2. NOTCH1/FBXW7 Mutational Analysis in T-LBL Patients
3.2. Stratification of Patients Based on MDD at Diagnosis
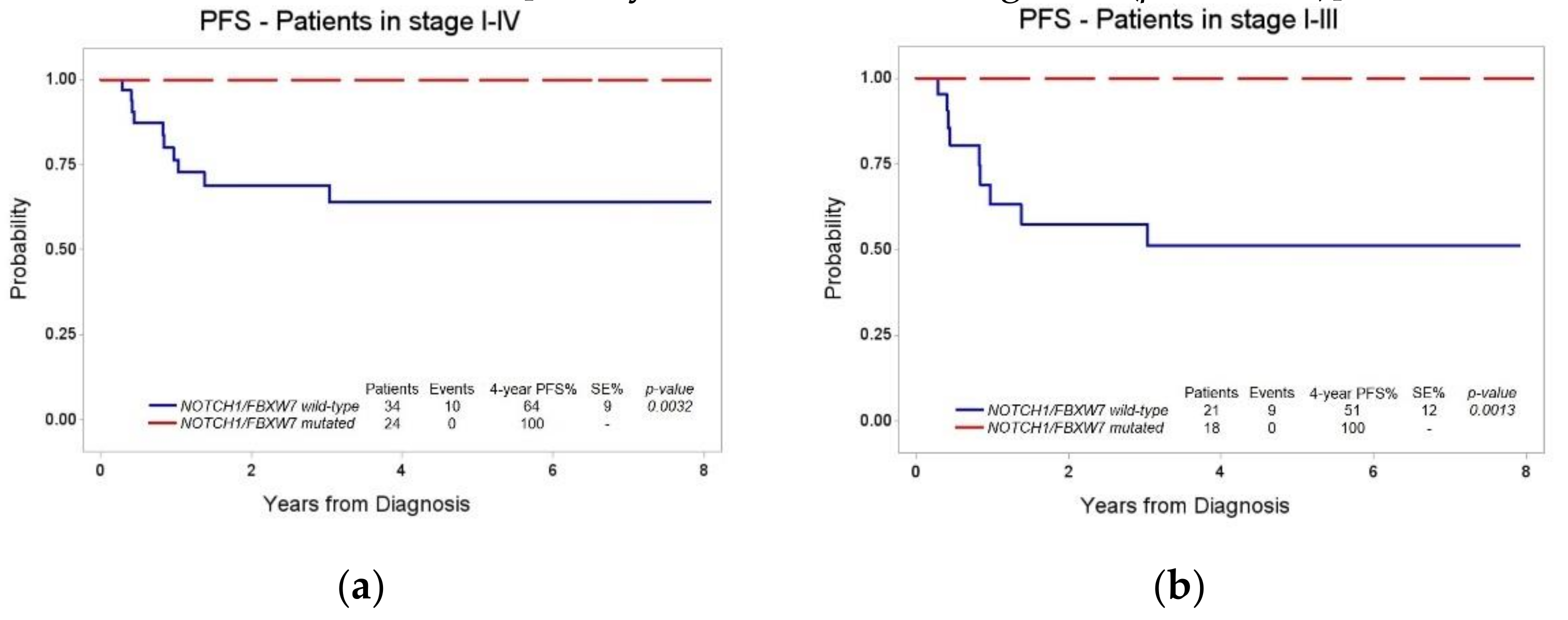
3.3. Stratification of Patients Based on NOTCH1/FBXW7 Mutational Status
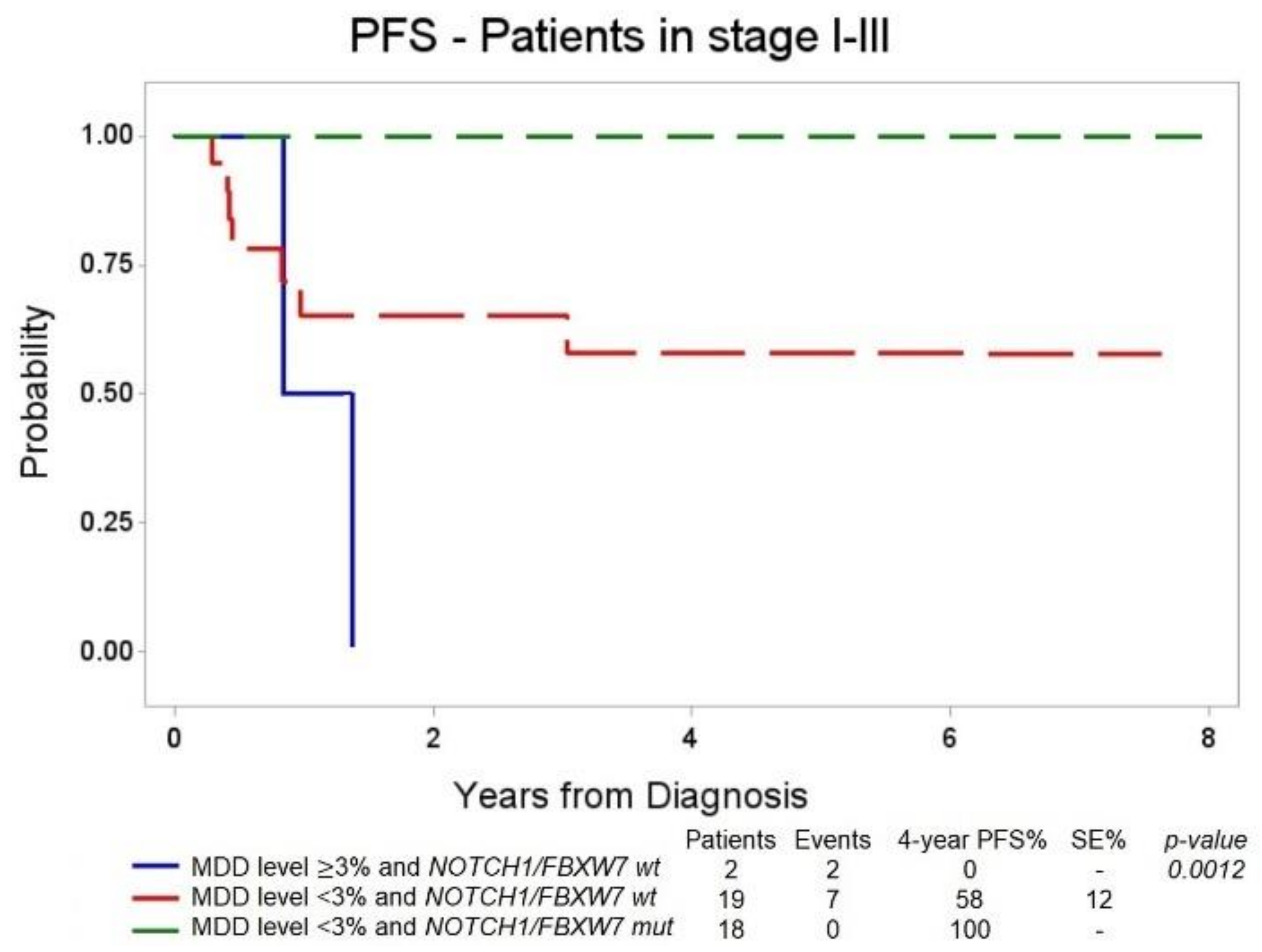
3.4. Combined Stratification of Patients Based on MDD at Diagnosis and NOTCH1/FBXW7 Mutational Status
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Burkhardt, B.; Hermiston, M.L. Lymphoblastic lymphoma in children and adolescents: Review of current challenges and future opportunities. Br. J. Haematol. 2019, 185, 1158–1170. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, B.; Zimmermann, M.; Oschlies, I.; Niggli, F.; Mann, G.; Parwaresch, R.; Riehm, H.; Schrappe, M.; Reiter, A. The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. Br. J. Haematol. 2005, 131, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Oschlies, I.; Burkhardt, B.; Chassagne-Clement, C.; D’Amore, E.S.; Hansson, U.; Hebeda, K.; Mc Carthy, K.; Kodet, R.; Maldyk, J.; Müllauer, L.; et al. Diagnosis and immunophenotype of 188 pediatric lymphoblastic lymphomas treated within a randomized prospective trial: Experiences and preliminary recommendations from the european childhood lymphoma pathology panel. Am. J. Surg. Pathol. 2011, 35, 836–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Termuhlen, A.M.; Smith, L.M.; Perkins, S.L.; Lones, M.; Finlay, J.L.; Weinstein, H.; Gross, T.G.; Abromowitch, M. Disseminated lymphoblastic lymphoma in children and adolescents: Results of the COG A5971 trial: A report from the Children’s Oncology Group. Br. J. Haematol. 2013, 162, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Landmann, E.; Burkhardt, B.; Zimmermann, M.; Meyer, U.; Woessmann, W.; Klapper, W.; Wrobel, G.; Rosolen, A.; Pillon, M.; Escherich, G.; et al. Results and conclusions of the European intergroup EURO-LB02 trial in children and adolescents with lymphoblastic lymphoma. Haematologica 2017, 120, 2086–2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, E.; Burkhardt, B. Lymphoblastic lymphoma in childhood and adolescence. Pediatr. Hematol. Oncol. 2013, 30, 484–508. [Google Scholar] [CrossRef] [PubMed]
- Coustan-Smith, E.; Sandlund, J.T.; Perkins, S.L.; Chen, H.; Chang, M.; Abromowitch, M.; Campana, D. Minimal disseminated disease in childhood T-cell lymphoblastic lymphoma: A report from the Children’s Oncology Group. J. Clin. Oncol. 2009, 27, 3533–3539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mussolin, L.; Buldini, B.; Lovisa, F.; Carraro, E.; Disarò, S.; Nigro, L.L.; d’Amore, E.S.G.; Pillon, M.; Basso, G. Detection and role of minimal disseminated disease in children with lymphoblastic lymphoma: The AIEOP experience. Pediatr. Blood Cancer 2015, 62, 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- Callens, C.; Baleydier, F.; Lengline, E.; Ben Abdelali, R.; Petit, A.; Villarese, P.; Cieslak, A.; Minard-Colin, V.; Rullier, A.; Moreau, A.; et al. Clinical impact of NOTCH1 and/or FBXW7 mutations, FLASH deletion, and TCR status in pediatric T-cell lymphoblastic lymphoma. J. Clin. Oncol. 2012, 30, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Bonn, B.R.; Rohde, M.; Zimmermann, M.; Krieger, D.; Oschlies, I.; Niggli, F.; Wrobel, G.; Attarbaschi, A.; Escherich, G.; Klapper, W.; et al. Incidence and prognostic relevance of genetic variations in T-cell lymphoblastic lymphoma in childhood and adolescence. Blood 2013, 121, 3153–3160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillon, M.; Aricò, M.; Mussolin, L.; Carraro, E.; Conter, V.; Sala, A.; Buffardi, S.; Garaventa, A.; D’Angelo, P.; Lo Nigro, L.; et al. Long-term results of the AIEOP LNH-97 protocol for childhood lymphoblastic lymphoma. Pediatr. Blood Cancer 2015, 62, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Lee Harris, N.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basso, G.; Buldini, B.; De Zen, L.; Orfao, A. New methodologic approaches for immunophenotyping acute leukemias. Haematologica 2001, 86, 675–692. [Google Scholar] [PubMed]
- Dworzak, M.N.; Gaipa, G.; Ratel, R.; Veltroni, M.; Schumich, A.; Maglia, O.; Karawajew, L.; Benetello, A.; Pötschger, U.; Husak, Z.; et al. Standardization of flow cytometric minimal residual disease evaluation in acute lymphoblastic leukemia: Multicentric assessment is feasible. Cytom. Part B Clin. Cytom. 2008, 74, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 12: Survival analysis. Crit. Care 2004, 8, 389–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mussolin, L.; Pillon, M.; D’Amore, E.S.G.; Conter, V.; Piglione, M.; Lo Nigro, L.; Garaventa, A.; Buffardi, S.; Aricò, M.; Rosolen, A. Minimal disseminated disease in high-risk Burkitt’s lymphoma identifies patients with different prognosis. J. Clin. Oncol. 2011, 29, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Mussolin, L.; Le Deley, M.C.; Carraro, E.; Damm-Welk, C.; Attarbaschi, A.; Williams, D.; Burke, A.; Horibe, K.; Nakazawa, A.; Wrobel, G.; et al. Prognostic factors in childhood anaplastic large cell lymphoma: Long term results of the international alcl99 trial. Cancers 2020, 12, 2747. [Google Scholar] [CrossRef] [PubMed]

| Patients (n = 132) | % | |
|---|---|---|
| Gender | ||
| Male | 92 | 69.7 |
| Female | 40 | 30.3 |
| Median age at diagnosis | ||
| ≤ 8.3 years | 66 | 50 |
| > 8.3 years | 66 | 50 |
| Immunophenotype | ||
| T | 107 | 81.1 |
| pB | 25 | 18.9 |
| Mediastinal involvement | ||
| Yes | 89 | 67.4 |
| No | 43 | 32.6 |
| BM involvement | ||
| Yes | 43 | 32.6 |
| No | 89 | 67.4 |
| Stage | ||
| I | 3 | 2.3 |
| II | 6 | 4.5 |
| III | 74 | 56.1 |
| IV CNS- | 37 | 28 |
| IV CNS+ | 12 | 9.1 |
| MFC-MDD | ||
| Positive | 72 | 54.5 |
| Negative | 60 | 45.5 |
| Mutational status * | ||
| N/Fmut | 24 | 41.4 |
| N/Fwt | 34 | 58.6 |
| Events | ||
| Relapse | 16 | 12.1 |
| Refractory disease | 5 | 3.8 |
| Secondary malignancy | 1 | 0.8 |
| Death | ||
| No. Patients | 13 | 9.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lovisa, F.; Gallingani, I.; Varotto, E.; Pasin, C.; Carraro, E.; Michielotto, B.; Garbin, A.; Damanti, C.C.; Pizzi, M.; d’Amore, E.S.G.; et al. Prognostic Role of Minimal Disseminated Disease and NOTCH1/FBXW7 Mutational Status in Children with Lymphoblastic Lymphoma: The AIEOP Experience. Diagnostics 2021, 11, 1594. https://doi.org/10.3390/diagnostics11091594
Lovisa F, Gallingani I, Varotto E, Pasin C, Carraro E, Michielotto B, Garbin A, Damanti CC, Pizzi M, d’Amore ESG, et al. Prognostic Role of Minimal Disseminated Disease and NOTCH1/FBXW7 Mutational Status in Children with Lymphoblastic Lymphoma: The AIEOP Experience. Diagnostics. 2021; 11(9):1594. https://doi.org/10.3390/diagnostics11091594
Chicago/Turabian StyleLovisa, Federica, Ilaria Gallingani, Elena Varotto, Cristiano Pasin, Elisa Carraro, Barbara Michielotto, Anna Garbin, Carlotta Caterina Damanti, Marco Pizzi, Emanuele S. G. d’Amore, and et al. 2021. "Prognostic Role of Minimal Disseminated Disease and NOTCH1/FBXW7 Mutational Status in Children with Lymphoblastic Lymphoma: The AIEOP Experience" Diagnostics 11, no. 9: 1594. https://doi.org/10.3390/diagnostics11091594
APA StyleLovisa, F., Gallingani, I., Varotto, E., Pasin, C., Carraro, E., Michielotto, B., Garbin, A., Damanti, C. C., Pizzi, M., d’Amore, E. S. G., Piglione, M., Muggeo, P., Buffardi, S., Vinti, L., Folsi, V. M., Onofrillo, D., Biffi, A., Buldini, B., Pillon, M., & Mussolin, L. (2021). Prognostic Role of Minimal Disseminated Disease and NOTCH1/FBXW7 Mutational Status in Children with Lymphoblastic Lymphoma: The AIEOP Experience. Diagnostics, 11(9), 1594. https://doi.org/10.3390/diagnostics11091594









