Visualization of Forward Light Scatter in Opacified Intraocular Lenses and Straylight Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Intraocular Lenses
2.2. Morphological Analysis
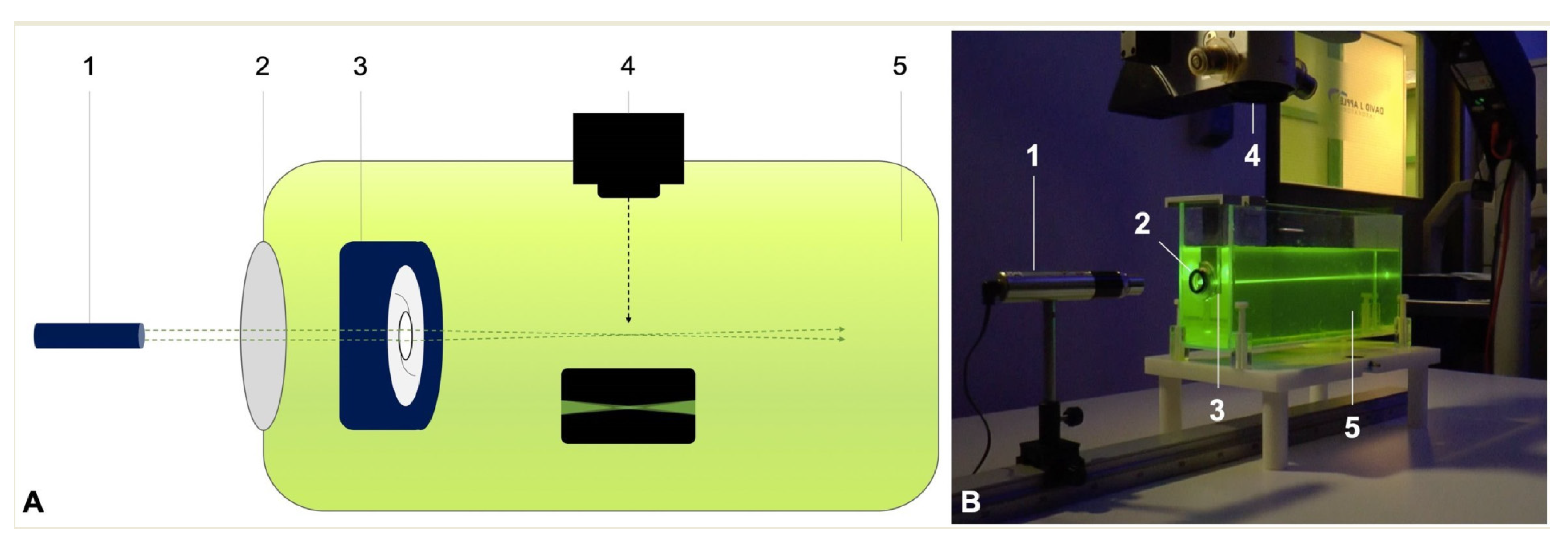
2.3. Ray Propagation Imaging
2.4. Straylight Measurements
3. Results
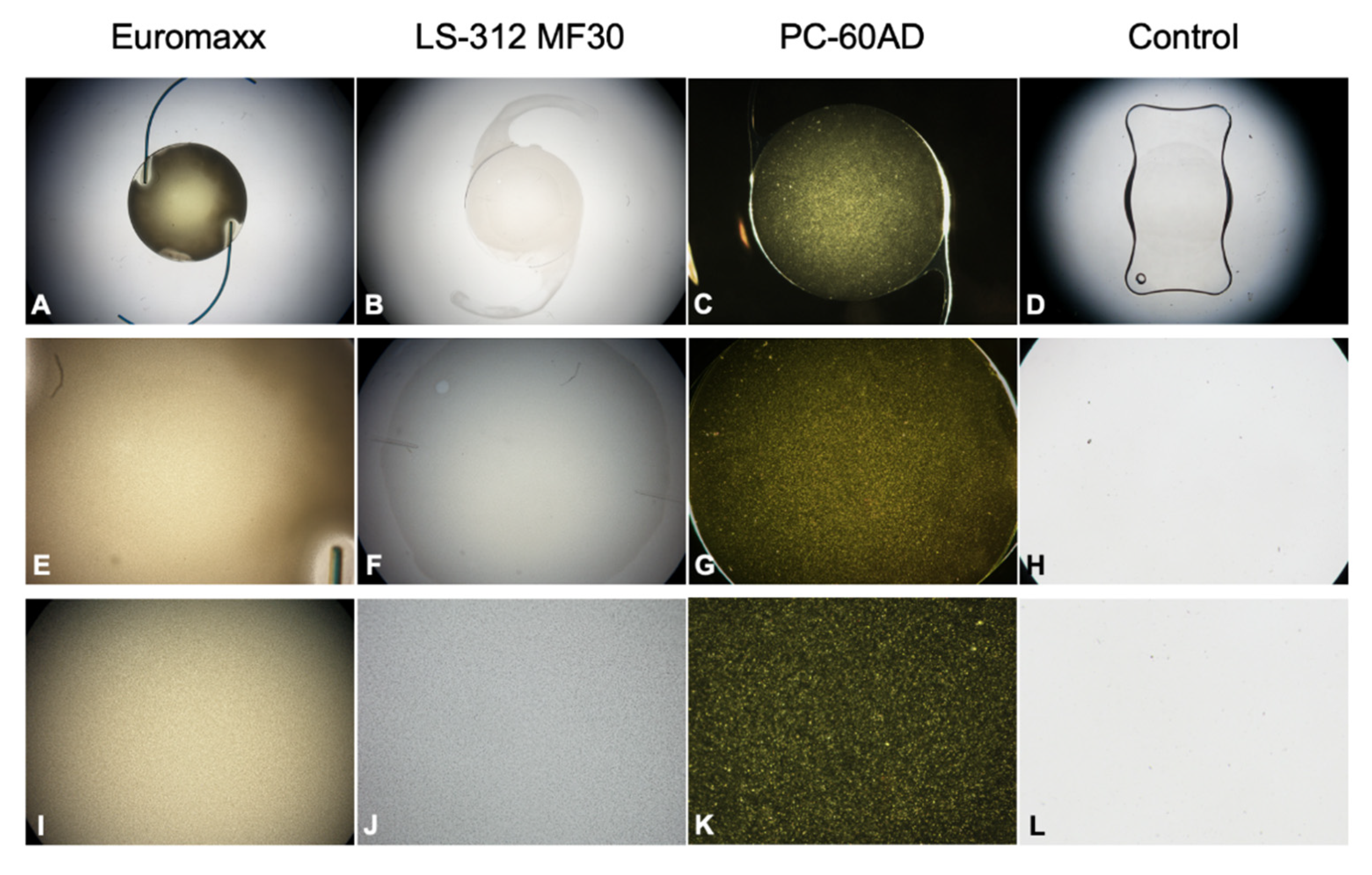
3.1. Morphological Analysis
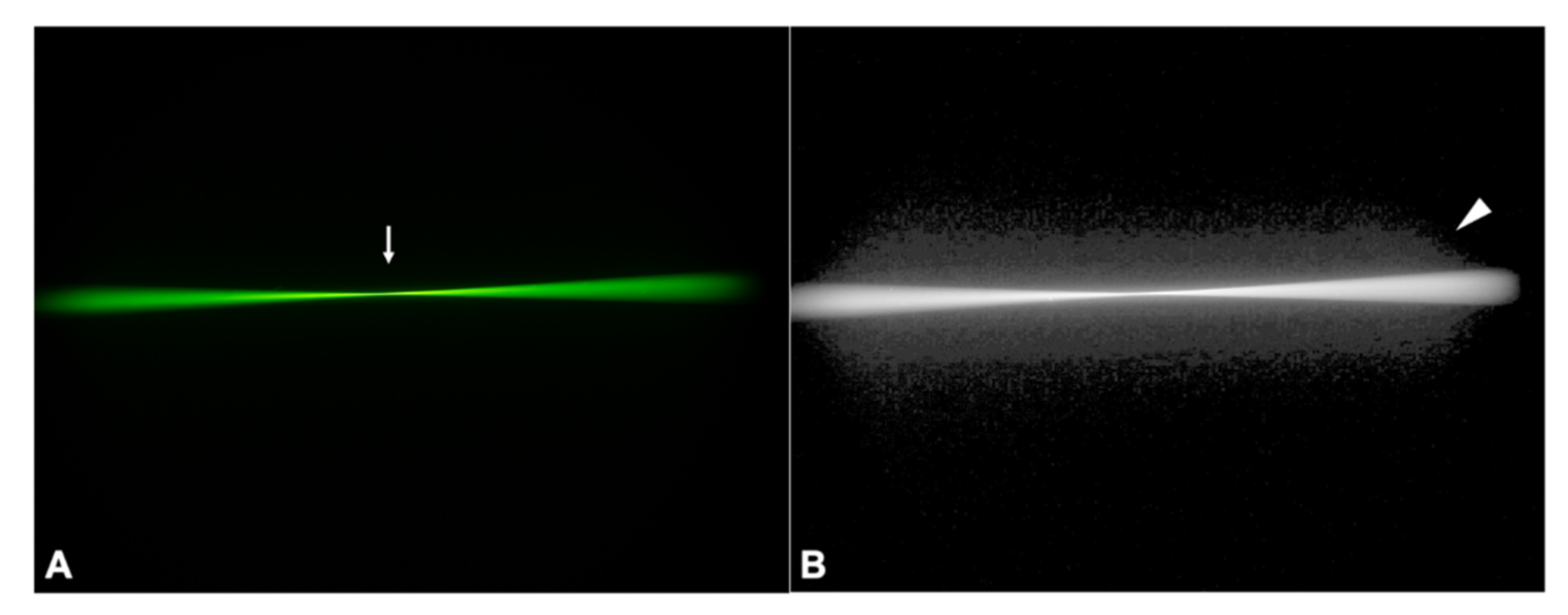
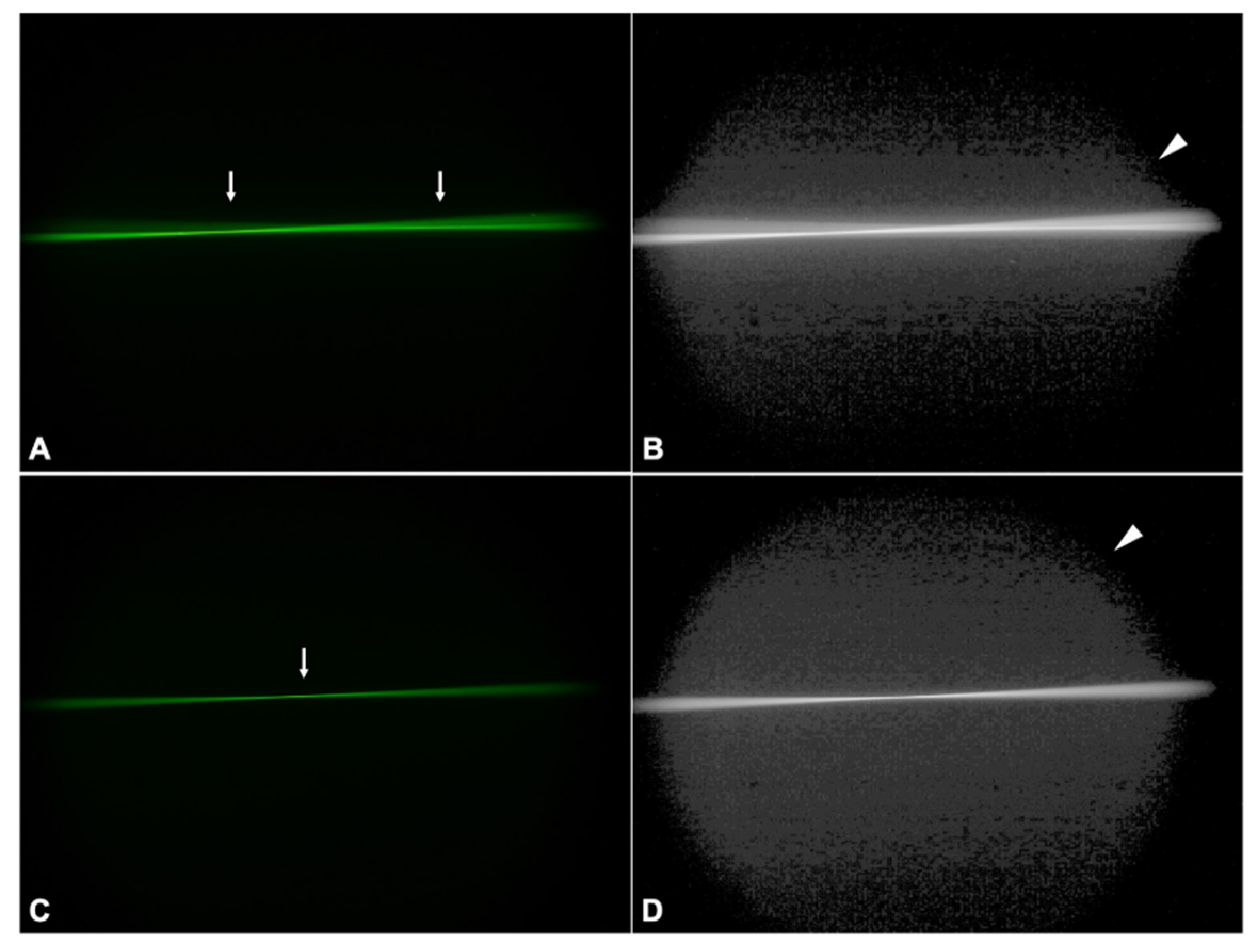
3.2. Ray Propagation Imaging
3.2.1. Control IOL
3.2.2. PC-60AD
3.2.3. LS-312 MF30
3.2.4. Euromaxx ALI313Y
3.3. Straylight Measurements
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yildirim, T.M.; Auffarth, G.U.; Łabuz, G.; Bopp, S.; Son, H.-S.; Khoramnia, R. Material Analysis and Optical Quality Assessment of Opacified Hydrophilic Acrylic Intraocular Lenses After Pars Plana Vitrectomy. Am. J. Ophthalmol. 2018, 193, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Łabuz, G.; Yildirim, T.M.; van den Berg, T.J.T.P.; Khoramnia, R.; Auffarth, G.U. Assessment of Straylight and the Modulation Transfer Function of Intraocular Lenses with Centrally Localized Opacification Associated with the Intraocular Injection of Gas. J. Cataract Refract. Surg. 2018, 44, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, T.M.; Khoramnia, R.; Schickhardt, S.K.; Munro, D.J.; Merz, P.R.; Son, H.-S.; Lieberwirth, I.; Auffarth, G.U. Variation in Intraocular Lens Calcification under Different Environmental Conditions in Eyes with Supplementary Sulcus-Supported Lenses. Am. J. Ophthalmol. Case Rep. 2020, 19, 100797. [Google Scholar] [CrossRef]
- Khoramnia, R.; Yildirim, T.M.; Łabuz, G.; Mayer, C.S.; Auffarth, G.U. Eintrübung von Intraokularlinsen: Erkenntnisse aus dem Labor und der Klinik. Ophthalmologe 2021, 118, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.Y.; Ting, D.S.J.; Thomas, S.; Auffarth, G.U.; Merz, P. Opacification of Hydrophilic Acrylic Intraocular Lens Following Vitreoretinal Surgery: A Clinicopathological Report. Can. J. Ophthalmol. 2021, 56, e9–e11. [Google Scholar] [CrossRef]
- Neuhann, T.; Yildirim, T.M.; Son, H.-S.; Merz, P.R.; Khoramnia, R.; Auffarth, G.U. Reasons for Explantation, Demographics, and Material Analysis of 200 Intraocular Lens Explants. J. Cataract Refract. Surg. 2020, 46, 20–26. [Google Scholar] [CrossRef]
- Yildirim, T.M.; Labuz, G.; Khoramnia, R.; Son, H.-S.; Schickhardt, S.K.; Lieberwirth, I.; Knorz, M.C.; Auffarth, G.U. Impact of Primary Calcification in Segmented Refractive Bifocal Intraocular Lenses on Optical Performance Including Straylight. J. Refract. Surg. 2020, 36, 20–27. [Google Scholar] [CrossRef]
- Marcovich, A.L.; Tandogan, T.; Bareket, M.; Eting, E.; Kaplan-Ashiri, I.; Bukelman, A.; Auffarth, G.U.; Khoramnia, R. Opacification of Hydrophilic Intraocular Lenses Associated with Vitrectomy and Injection of Intraocular Gas. BMJ Open Ophth. 2018, 3, e000157. [Google Scholar] [CrossRef]
- Roland, S.; Khoramnia, R.; Auffarth, G.U.; Son, H.-S.; Yildirim, T.M.; Schoenherr, U. Eintrübung einer hydrophilen Intraokularlinse nach multiplen Bevacizumab-Injektionen. Ophthalmologe 2019, 116, 882–886. [Google Scholar] [CrossRef]
- Giers, B.C.; Tandogan, T.; Auffarth, G.U.; Choi, C.Y.; Auerbach, F.N.; Sel, S.; Mayer, C.; Khoramnia, R. Hydrophilic Intraocular Lens Opacification after Posterior Lamellar Keratoplasty—A Material Analysis with Special Reference to Optical Quality Assessment. BMC Ophthalmol. 2017, 17, 150. [Google Scholar] [CrossRef] [PubMed]
- Milojcic, C.; Latz, C.; Tandogan, T.; Auffarth, G.U.; Holz, F.G.; Choi, C.Y.; Khoramnia, R. Eintrübung einer hydrophilen Acryl-Intraokularlinse nach DMEK: Eine Materialanalyse. Ophthalmologe 2017, 114, 832–837. [Google Scholar] [CrossRef]
- Tandogan, T.; Khoramnia, R.; Choi, C.Y.; Scheuerle, A.; Wenzel, M.; Hugger, P.; Auffarth, G.U. Optical and Material Analysis of Opacified Hydrophilic Intraocular Lenses after Explantation: A Laboratory Study. BMC Ophthalmol. 2015, 15, 170. [Google Scholar] [CrossRef]
- Neuhann, I.M.; Kleinmann, G.; Apple, D.J. A New Classification of Calcification of Intraocular Lenses. Ophthalmology 2008, 115, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Kanclerz, P.; Yildirim, T.M.; Khoramnia, R. A Review of Late Intraocular Lens Opacifications. Curr. Opin. Ophthalmol. 2021, 32, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Kanclerz, P.; Yildirim, T.M.; Khoramnia, R. Microscopic Characteristics of Late Intraocular Lens Opacifications. Arch. Pathol. Lab. Med. 2021, 145, 759–767. [Google Scholar] [CrossRef]
- Yildirim, T.M.; Fang, H.; Schickhardt, S.K.; Wang, Q.; Merz, P.R.; Auffarth, G.U. Glistening Formation in a New Hydrophobic Acrylic Intraocular Lens. BMC Ophthalmol. 2020, 20, 186. [Google Scholar] [CrossRef]
- Weindler, J.N.; Łabuz, G.; Yildirim, T.M.; Tandogan, T.; Khoramnia, R.; Auffarth, G.U. The Impact of Glistenings on the Optical Quality of a Hydrophobic Acrylic Intraocular Lens. J. Cataract Refract. Surg. 2019, 45, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Łabuz, G.; Knebel, D.; Auffarth, G.U.; Fang, H.; van den Berg, T.J.; Yildirim, T.M.; Son, H.-S.; Khoramnia, R. Glistening Formation and Light Scattering in Six Hydrophobic-Acrylic Intraocular Lenses. Am. J. Ophthalmol. 2018, 196, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Łabuz, G.; Reus, N.J.; van den Berg, T.J.T.P. Light Scattering Levels from Intraocular Lenses Extracted from Donor Eyes. J. Cataract Refract. Surg. 2017, 43, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Son, H.S.; Labuz, G.; Khoramnia, R.; Merz, P.; Yildirim, T.M.; Auffarth, G.U. Ray Propagation Imaging and Optical Quality Evaluation of Different Intraocular Lens Models. PLoS ONE 2020, 15, e0228342. [Google Scholar] [CrossRef] [PubMed]
- Łabuz, G.; Yildirim, T.M.; Auffarth, G.U.; Son, H.-S.; Khoramnia, R. Laboratory Evaluation of Higher-Order Aberrations and Light Scattering in Explanted Opacified Intraocular Lenses. Eye Vis. 2021, 8, 14. [Google Scholar] [CrossRef]
- Łabuz, G.; Vargas-Martín, F.; van den Berg, T.J.T.P.; López-Gil, N. Method for in Vitro Assessment of Straylight from Intraocular Lenses. Biomed. Opt. Express 2015, 6, 4457. [Google Scholar] [CrossRef] [PubMed]
- De Wit, G.C.; Franssen, L.; Coppens, J.E.; van den Berg, T.J.T.P. Simulating the Straylight Effects of Cataracts. J. Cataract Refract. Surg. 2006, 32, 294–300. [Google Scholar] [CrossRef] [PubMed]
- CIE Central Bureau. CIE Collection 1999: Vision and Colour; Physical Measurement of Light and Radiation; Technical Report/CIE; Internationale Beleuchtungskommission, Ed.; CIE Central Bureau: Vienna, Austria, 1999. [Google Scholar]
- Terwee, T.; Weeber, H.; van der Mooren, M.; Piers, P. Visualization of the Retinal Image in an Eye Model With Spherical and Aspheric, Diffractive, and Refractive Multifocal Intraocular Lenses. J. Refract. Surg. 2008, 24, 223–232. [Google Scholar] [CrossRef]
- Reiß, S.; Forbrig, J.; Guthoff, R.; Terwee, T.; Stolz, H.; Siewert, S.; El-Tamer, A.; Hinze, U.; Chichkov, B.; Stachs, O. Optimierung der Visualisierungstechnik für Strahlenverläufe durch Intraokularlinsen zur Charakterisierung multifokaler Abbildungseigenschaften von Fresnel-Zonenplatten. Klin Mon. Augenheilkd 2014, 231, 1183–1186. [Google Scholar] [CrossRef]
- Eppig, T.; Rubly, K.; Rawer, A.; Langenbucher, A. Visualization of Light Propagation with Multifocal Intraocular Lenses Using the Ouzo Effect. BioMed Res. Int. 2019, 2019, 6425040. [Google Scholar] [CrossRef]
- van der Meulen, I.J.E.; Porooshani, H.; van den Berg, T.J.T.P. Light-Scattering Characteristics of Explanted Opacified Aquasense Intraocular Lenses. Br. J. Ophthalmol. 2009, 93, 830–832. [Google Scholar] [CrossRef][Green Version]
- van den Berg, T.J.T.P.; Franssen, L.; Kruijt, B.; Coppens, J.E. History of Ocular Straylight Measurement: A Review. Z. Med. Phys. 2013, 23, 6–20. [Google Scholar] [CrossRef]
- Van Den Berg, T.J.T.P. On the Relation between Glare and Straylight. Doc. Ophthalmol. 1991, 78, 177–181. [Google Scholar] [CrossRef]
- Van Den Berg, T.J.T.P. Analysis of Intraocular Straylight, Especially in Relation to Age. Optom. Vis. Sci. 1995, 72, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Łabuz, G.; Reus, N.J.; van den Berg, T.J.T.P. Straylight from Glistenings in Intraocular Lenses: In Vitro Study. J. Cataract Refract. Surg. 2017, 43, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.J.; Oprysa, A. Fluorescence and Light Scattering. J. Chem. Educ. 2004, 81, 705. [Google Scholar] [CrossRef]
- Spiezio, V.; Walker, B.N.; Calogero, D.; Ilev, I.K. Quantitative Multiparameter Evaluation of Vacuoles in Intraocular Lenses Employing a High-Magnification Digital Microscopy Method. J. Ophthalmol. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Spiezio, V.; Walker, B.N.; Calogero, D.; Ilev, I.K. Experimental and Analytical Quantification of Light Scattering from Vacuoles in Intraocular Lenses. J. Cataract Refract. Surg. 2020, 46, 762–773. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, H.-S.; Łabuz, G.; Khoramnia, R.; Yildirim, T.M.; Choi, C.Y.; Knorz, M.C.; Auffarth, G.U. Visualization of Forward Light Scatter in Opacified Intraocular Lenses and Straylight Assessment. Diagnostics 2021, 11, 1512. https://doi.org/10.3390/diagnostics11081512
Son H-S, Łabuz G, Khoramnia R, Yildirim TM, Choi CY, Knorz MC, Auffarth GU. Visualization of Forward Light Scatter in Opacified Intraocular Lenses and Straylight Assessment. Diagnostics. 2021; 11(8):1512. https://doi.org/10.3390/diagnostics11081512
Chicago/Turabian StyleSon, Hyeck-Soo, Grzegorz Łabuz, Ramin Khoramnia, Timur M. Yildirim, Chul Young Choi, Michael C. Knorz, and Gerd U. Auffarth. 2021. "Visualization of Forward Light Scatter in Opacified Intraocular Lenses and Straylight Assessment" Diagnostics 11, no. 8: 1512. https://doi.org/10.3390/diagnostics11081512
APA StyleSon, H.-S., Łabuz, G., Khoramnia, R., Yildirim, T. M., Choi, C. Y., Knorz, M. C., & Auffarth, G. U. (2021). Visualization of Forward Light Scatter in Opacified Intraocular Lenses and Straylight Assessment. Diagnostics, 11(8), 1512. https://doi.org/10.3390/diagnostics11081512









