Precise Epigenetic Analysis Using Targeted Bisulfite Genomic Sequencing Distinguishes FSHD1, FSHD2, and Healthy Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Sample Collection and DNA Preparation
2.3. DNA Methylation Analysis
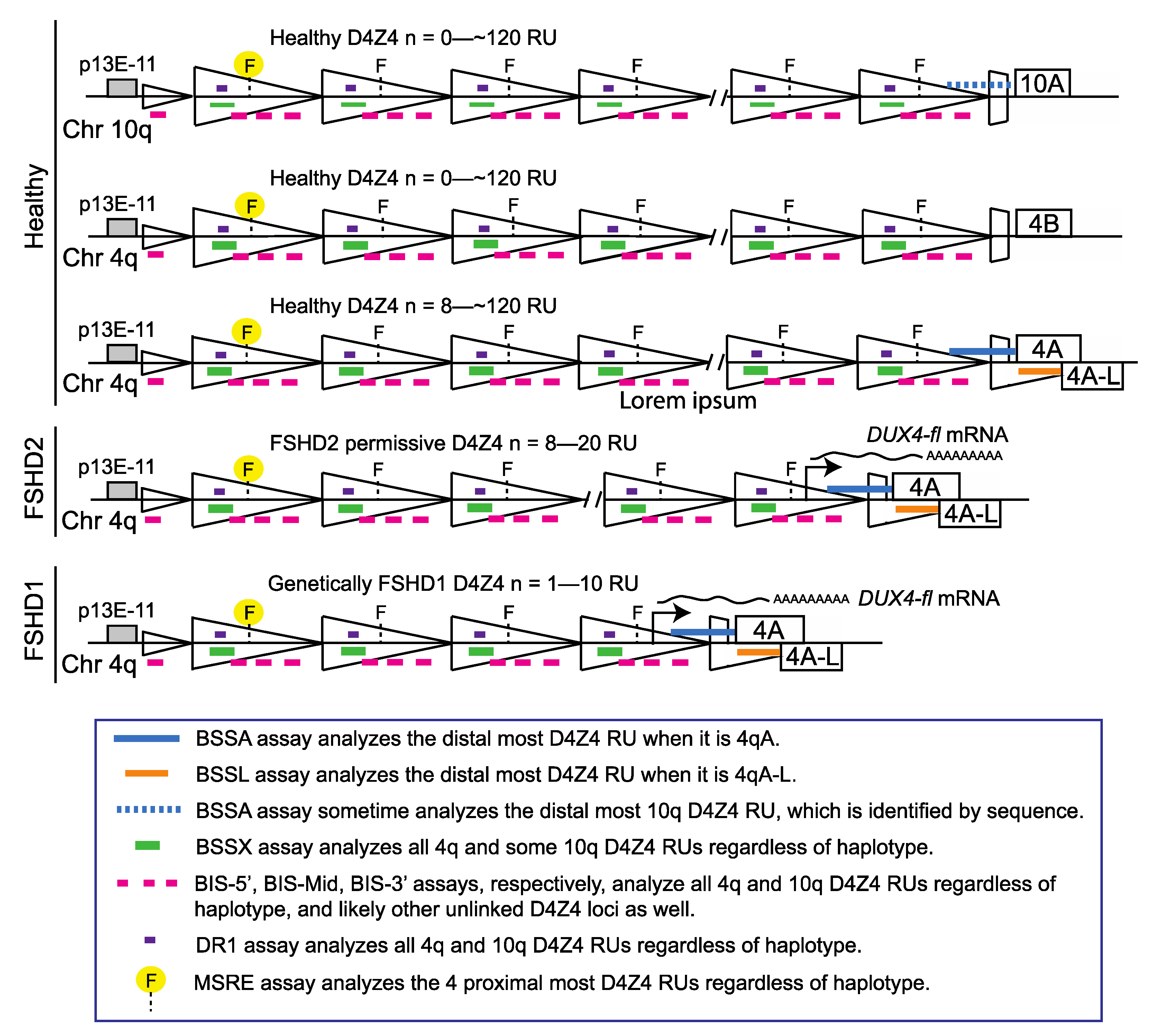
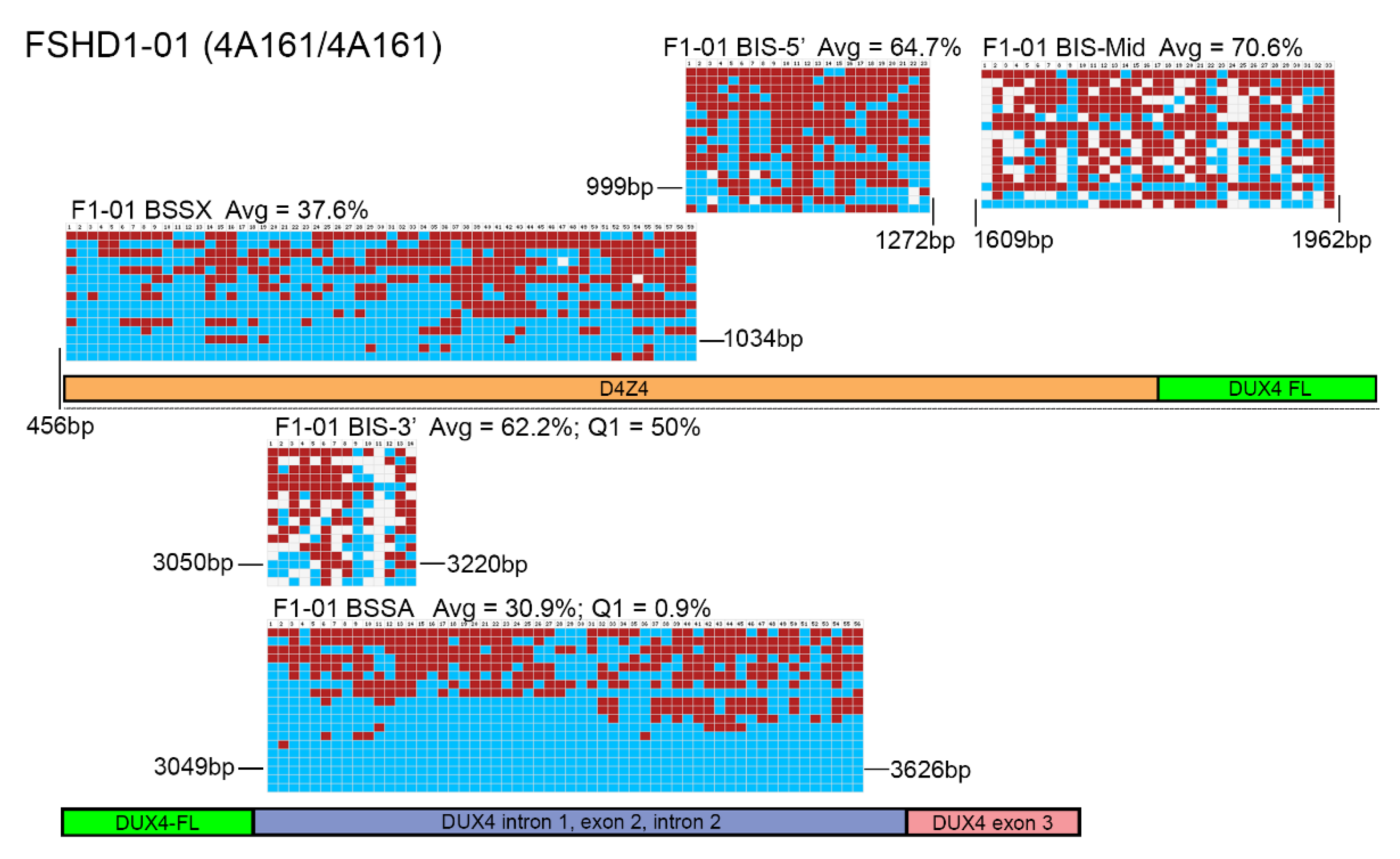
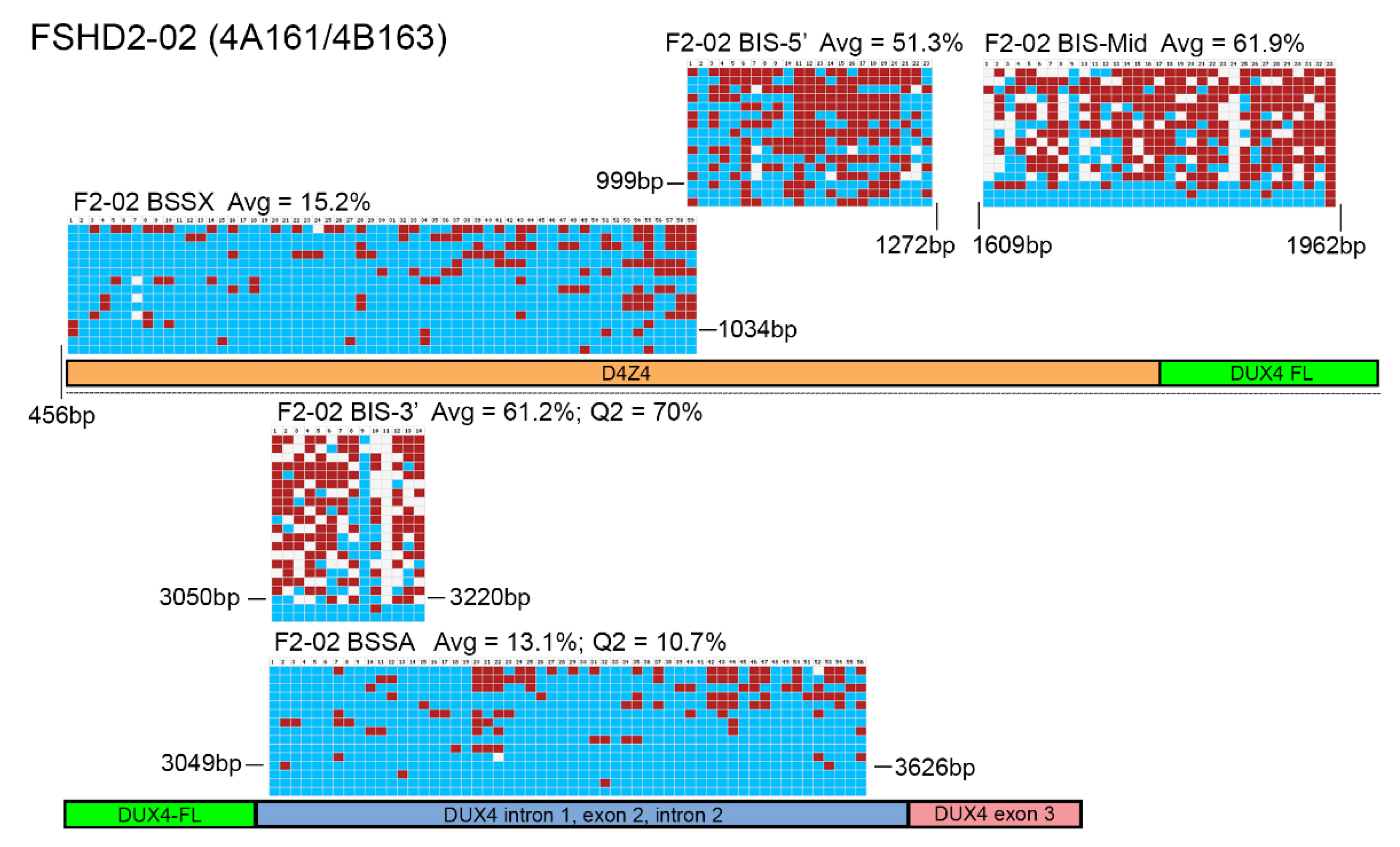
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padberg, G.W. Facioscapulohumeral Disease. Ph.D. Thesis, Leiden University, Leiden, The Netherlands, 1982. [Google Scholar]
- Wang, L.H.; Tawil, R. Facioscapulohumeral Dystrophy. Curr. Neurol. Neurosci. Rep. 2016, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.E.; Statland, J.M. FSHD1 or FSHD2: That is the question: The answer: It’s all just FSHD. Neurology 2019, 92, 881–882. [Google Scholar] [CrossRef]
- Wijmenga, C.; Hewitt, J.E.; Sandkuijl, L.A.; Clark, L.N.; Wright, T.J.; Dauwerse, H.G.; Gruter, A.M.; Hofker, M.H.; Moerer, P.; Williamson, R.; et al. Chromosome 4q DNA rearrangements associated with facioscapulohumeral muscular dystrophy. Nat. Genet. 1992, 2, 26–30. [Google Scholar] [CrossRef]
- van Deutekom, J.C.; Wijmenga, C.; van Tienhoven, E.A.; Gruter, A.M.; Hewitt, J.E.; Padberg, G.W.; van Ommen, G.J.; Hofker, M.H.; Frants, R.R. FSHD associated DNA rearrangements are due to deletions of integral copies of a 3.2 kb tandemly repeated unit. Hum. Mol. Genet. 1993, 2, 2037–2042. [Google Scholar] [CrossRef] [PubMed]
- de Greef, J.C.; Wohlgemuth, M.; Chan, O.A.; Hansson, K.B.; Smeets, D.; Frants, R.R.; Weemaes, C.M.; Padberg, G.W.; van der Maarel, S.M. Hypomethylation is restricted to the D4Z4 repeat array in phenotypic FSHD. Neurology 2007, 69, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Calandra, P.; Cascino, I.; Lemmers, R.J.; Galluzzi, G.; Teveroni, E.; Monforte, M.; Tasca, G.; Ricci, E.; Moretti, F.; van der Maarel, S.M.; et al. Allele-specific DNA hypomethylation characterises FSHD1 and FSHD2. J. Med. Genet. 2016, 53, 348–355. [Google Scholar] [CrossRef]
- Lunt, P.W. 44th ENMC International Workshop: Facioscapulohumeral Muscular Dystrophy: Molecular Studies 19–21 July 1996, Naarden, The Netherlands. Neuromuscul. Disord. 1998, 8, 126–130. [Google Scholar] [CrossRef]
- van Overveld, P.G.; Lemmers, R.J.; Sandkuijl, L.A.; Enthoven, L.; Winokur, S.T.; Bakels, F.; Padberg, G.W.; van Ommen, G.J.; Frants, R.R.; van der Maarel, S.M. Hypomethylation of D4Z4 in 4q-linked and non-4q-linked facioscapulohumeral muscular dystrophy. Nat. Genet. 2003, 35, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Lemmers, R.J.; Tawil, R.; Petek, L.M.; Balog, J.; Block, G.J.; Santen, G.W.; Amell, A.M.; van der Vliet, P.J.; Almomani, R.; Straasheijm, K.R.; et al. Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes facioscapulohumeral muscular dystrophy type 2. Nat. Genet. 2012, 44, 1370–1374. [Google Scholar] [CrossRef] [Green Version]
- van den Boogaard, M.L.; Lemmers, R.J.; Balog, J.; Wohlgemuth, M.; Auranen, M.; Mitsuhashi, S.; van der Vliet, P.J.; Straasheijm, K.R.; van den Akker, R.F.; Kriek, M.; et al. Mutations in DNMT3B Modify Epigenetic Repression of the D4Z4 Repeat and the Penetrance of Facioscapulohumeral Dystrophy. Am. J. Hum. Genet. 2016, 98, 1020–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamanaka, K.; Sikrova, D.; Mitsuhashi, S.; Masuda, H.; Sekiguchi, Y.; Sugiyama, A.; Shibuya, K.; Lemmers, R.; Goossens, R.; Ogawa, M.; et al. Homozygous nonsense variant in LRIF1 associated with facioscapulohumeral muscular dystrophy. Neurology 2020, 94, e2441–e2447. [Google Scholar] [CrossRef] [PubMed]
- de Greef, J.C.; Lemmers, R.J.; van Engelen, B.G.; Sacconi, S.; Venance, S.L.; Frants, R.R.; Tawil, R.; van der Maarel, S.M. Common epigenetic changes of D4Z4 in contraction-dependent and contraction-independent FSHD. Hum. Mutat. 2009, 30, 1449–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriels, J.; Beckers, M.C.; Ding, H.; De Vriese, A.; Plaisance, S.; van der Maarel, S.M.; Padberg, G.W.; Frants, R.R.; Hewitt, J.E.; Collen, D.; et al. Nucleotide sequence of the partially deleted D4Z4 locus in a patient with FSHD identifies a putative gene within each 3.3 kb element. Gene 1999, 236, 25–32. [Google Scholar] [CrossRef]
- Dixit, M.; Ansseau, E.; Tassin, A.; Winokur, S.; Shi, R.; Qian, H.; Sauvage, S.; Matteotti, C.; van Acker, A.M.; Leo, O.; et al. DUX4, a candidate gene of facioscapulohumeral muscular dystrophy, encodes a transcriptional activator of PITX1. Proc. Natl. Acad. Sci. USA 2007, 104, 18157–18162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowaljow, V.; Marcowycz, A.; Ansseau, E.; Conde, C.B.; Sauvage, S.; Matteotti, C.; Arias, C.; Corona, E.D.; Nunez, N.G.; Leo, O.; et al. The DUX4 gene at the FSHD1A locus encodes a pro-apoptotic protein. Neuromuscul. Disord. 2007, 17, 611–623. [Google Scholar] [CrossRef] [Green Version]
- Lemmers, R.J.; van der Vliet, P.J.; Klooster, R.; Sacconi, S.; Camano, P.; Dauwerse, J.G.; Snider, L.; Straasheijm, K.R.; van Ommen, G.J.; Padberg, G.W.; et al. A unifying genetic model for facioscapulohumeral muscular dystrophy. Science 2010, 329, 1650–1653. [Google Scholar] [CrossRef] [Green Version]
- Snider, L.; Geng, L.N.; Lemmers, R.J.; Kyba, M.; Ware, C.B.; Nelson, A.M.; Tawil, R.; Filippova, G.N.; van der Maarel, S.M.; Tapscott, S.J.; et al. Facioscapulohumeral dystrophy: Incomplete suppression of a retrotransposed gene. PLoS Genet. 2010, 6, e1001181. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Snider, L.; Balog, J.; Lemmers, R.J.; Van Der Maarel, S.M.; Tawil, R.; Tapscott, S.J. DUX4-induced gene expression is the major molecular signature in FSHD skeletal muscle. Hum. Mol. Genet. 2014, 23, 5342–5352. [Google Scholar] [CrossRef]
- Tawil, R.; van der Maarel, S.M.; Tapscott, S.J. Facioscapulohumeral dystrophy: The path to consensus on pathophysiology. Skelet Muscle 2014, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Lemmers, R.J.; de Kievit, P.; Sandkuijl, L.; Padberg, G.W.; van Ommen, G.J.; Frants, R.R.; van der Maarel, S.M. Facioscapulohumeral muscular dystrophy is uniquely associated with one of the two variants of the 4q subtelomere. Nat. Genet. 2002, 32, 235–236. [Google Scholar] [CrossRef]
- Lemmers, R.J.; Wohlgemuth, M.; Frants, R.R.; Padberg, G.W.; Morava, E.; van der Maarel, S.M. Contractions of D4Z4 on 4qB subtelomeres do not cause facioscapulohumeral muscular dystrophy. Am. J. Hum. Genet. 2004, 75, 1124–1130. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.; Chen, Y.Y.; Newkirk, D.A.; Wu, B.; Balog, J.; Kong, X.; Ball, A.R., Jr.; Zanotti, S.; Tawil, R.; Hashimoto, N.; et al. Genetic and epigenetic characteristics of FSHD-associated 4q and 10q D4Z4 that are distinct from non-4q/10q D4Z4 homologs. Hum. Mutat. 2014, 35, 998–1010. [Google Scholar] [CrossRef]
- Lyle, R.; Wright, T.J.; Clark, L.N.; Hewitt, J.E. The FSHD-associated repeat, D4Z4, is a member of a dispersed family of homeobox-containing repeats, subsets of which are clustered on the short arms of the acrocentric chromosomes. Genomics 1995, 28, 389–397. [Google Scholar] [CrossRef]
- Lemmers, R.J.; O’Shea, S.; Padberg, G.W.; Lunt, P.W.; van der Maarel, S.M. Best practice guidelines on genetic diagnostics of Facioscapulohumeral muscular dystrophy: Workshop 9th June 2010, LUMC, Leiden, The Netherlands. Neuromuscul. Disord. 2012, 22, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.; Walrafen, P.; Bernard, R.; Attarian, S.; Chaix, C.; Vovan, C.; Renard, E.; Dufrane, N.; Pouget, J.; Vannier, A.; et al. Molecular combing reveals allelic combinations in facioscapulohumeral dystrophy. Ann. Neurol. 2011, 70, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Vasale, J.; Boyar, F.; Jocson, M.; Sulcova, V.; Chan, P.; Liaquat, K.; Hoffman, C.; Meservey, M.; Chang, I.; Tsao, D.; et al. Molecular combing compared to Southern blot for measuring D4Z4 contractions in FSHD. Neuromuscul. Disord. 2015, 25, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, S.; Nakagawa, S.; Takahashi Ueda, M.; Imanishi, T.; Frith, M.C.; Mitsuhashi, H. Nanopore-based single molecule sequencing of the D4Z4 array responsible for facioscapulohumeral muscular dystrophy. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Li, P.; Wang, Z.; Liang, F.; Yang, F.; Fang, L.; Huang, Y.; Huang, S.; Zhou, J.; Wang, D.; et al. Single-molecule optical mapping enables quantitative measurement of D4Z4 repeats in facioscapulohumeral muscular dystrophy (FSHD). J. Med. Genet. 2020, 57, 109–120. [Google Scholar] [CrossRef]
- Lemmers, R.J. Analyzing Copy Number Variation Using Pulsed-Field Gel Electrophoresis: Providing a Genetic Diagnosis for FSHD1. Methods Mol. Biol. 2017, 1492, 107–125. [Google Scholar] [CrossRef]
- van Overveld, P.G.; Enthoven, L.; Ricci, E.; Rossi, M.; Felicetti, L.; Jeanpierre, M.; Winokur, S.T.; Frants, R.R.; Padberg, G.W.; van der Maarel, S.M. Variable hypomethylation of D4Z4 in facioscapulohumeral muscular dystrophy. Ann. Neurol. 2005, 58, 569–576. [Google Scholar] [CrossRef]
- Jones, T.I.; Yan, C.; Sapp, P.C.; McKenna-Yasek, D.; Kang, P.B.; Quinn, C.; Salameh, J.S.; King, O.D.; Jones, P.L. Identifying diagnostic DNA methylation profiles for facioscapulohumeral muscular dystrophy in blood and saliva using bisulfite sequencing. Clin. Epigenetics 2014, 6, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartweck, L.M.; Anderson, L.J.; Lemmers, R.J.; Dandapat, A.; Toso, E.A.; Dalton, J.C.; Tawil, R.; Day, J.W.; van der Maarel, S.M.; Kyba, M. A focal domain of extreme demethylation within D4Z4 in FSHD2. Neurology 2013, 80, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, M.C.; Roche, S.; Dion, C.; Tasmadjian, A.; Bouget, G.; Salort-Campana, E.; Vovan, C.; Chaix, C.; Broucqsault, N.; Morere, J.; et al. Differential DNA methylation of the D4Z4 repeat in patients with FSHD and asymptomatic carriers. Neurology 2014, 83, 733–742. [Google Scholar] [CrossRef]
- Lemmers, R.J.; van den Boogaard, M.L.; van der Vliet, P.J.; Donlin-Smith, C.M.; Nations, S.P.; Ruivenkamp, C.A.; Heard, P.; Bakker, B.; Tapscott, S.; Cody, J.D.; et al. Hemizygosity for SMCHD1 in Facioscapulohumeral Muscular Dystrophy Type 2: Consequences for 18p Deletion Syndrome. Hum. Mutat. 2015, 36, 679–683. [Google Scholar] [CrossRef] [Green Version]
- Jones, T.I.; King, O.D.; Himeda, C.L.; Homma, S.; Chen, J.C.; Beermann, M.L.; Yan, C.; Emerson, C.P., Jr.; Miller, J.B.; Wagner, K.R.; et al. Individual epigenetic status of the pathogenic D4Z4 macrosatellite correlates with disease in facioscapulohumeral muscular dystrophy. Clin. Epigenetics 2015, 7, 1–22. [Google Scholar] [CrossRef] [Green Version]
- de Greef, J.C.; Lemmers, R.J.; Camano, P.; Day, J.W.; Sacconi, S.; Dunand, M.; van Engelen, B.G.; Kiuru-Enari, S.; Padberg, G.W.; Rosa, A.L.; et al. Clinical features of facioscapulohumeral muscular dystrophy 2. Neurology 2010, 75, 1548–1554. [Google Scholar] [CrossRef] [Green Version]
- Gordon, C.T.; Xue, S.; Yigit, G.; Filali, H.; Chen, K.; Rosin, N.; Yoshiura, K.I.; Oufadem, M.; Beck, T.J.; McGowan, R.; et al. De novo mutations in SMCHD1 cause Bosma arhinia microphthalmia syndrome and abrogate nasal development. Nat. Genet. 2017, 49, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Shaw, N.D.; Brand, H.; Kupchinsky, Z.A.; Bengani, H.; Plummer, L.; Jones, T.I.; Erdin, S.; Williamson, K.A.; Rainger, J.; Stortchevoi, A.; et al. SMCHD1 mutations associated with a rare muscular dystrophy can also cause isolated arhinia and Bosma arhinia microphthalmia syndrome. Nat. Genet. 2017, 49, 238–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roche, S.; Dion, C.; Broucqsault, N.; Laberthonnière, C.A.-O.; Gaillard, M.C.; Robin, J.A.-O.; Lagarde, A.; Puppo, F.; Vovan, C.; Chaix, C.; et al. Methylation hotspots evidenced by deep sequencing in patients with facioscapulohumeral dystrophy and mosaicism. Neurol. Genet. 2019, 5, 3372. [Google Scholar] [CrossRef] [Green Version]
- Dion, C.; Roche, S.; Laberthonniere, C.; Broucqsault, N.; Mariot, V.; Xue, S.; Gurzau, A.D.; Nowak, A.; Gordon, C.T.; Gaillard, M.C.; et al. SMCHD1 is involved in de novo methylation of the DUX4-encoding D4Z4 macrosatellite. Nucleic Acids Res. 2019, 47, 2822–2839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolic, A.; Jones, T.I.; Govi, M.; Mele, F.; Maranda, L.; Sera, F.; Ricci, G.; Ruggiero, L.; Vercelli, L.; Portaro, S.; et al. Interpretation of the Epigenetic Signature of Facioscapulohumeral Muscular Dystrophy in Light of Genotype-Phenotype Studies. Int. J. Mol. Sci. 2020, 21, 2635. [Google Scholar] [CrossRef]
- Salsi, V.; Magdinier, F.; Tupler, R. Does DNA Methylation Matter in FSHD? Genes 2020, 11, 258. [Google Scholar] [CrossRef] [Green Version]
- Lemmers, R.; van der Stoep, N.; Vliet, P.J.V.; Moore, S.A.; San Leon Granado, D.; Johnson, K.; Topf, A.; Straub, V.; Evangelista, T.; Mozaffar, T.; et al. SMCHD1 mutation spectrum for facioscapulohumeral muscular dystrophy type 2 (FSHD2) and Bosma arhinia microphthalmia syndrome (BAMS) reveals disease-specific localisation of variants in the ATPase domain. J. Med. Genet. 2019, 56, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Bisulfite Sequencing DNA Methylation Analysis (BISMA). Available online: http://services.ibc.uni-stuttgart.de/BDPC/BISMA/ (accessed on 30 June 2021).
- Lemmers, R.J.; Goeman, J.J.; van der Vliet, P.J.; van Nieuwenhuizen, M.P.; Balog, J.; Vos-Versteeg, M.; Camano, P.; Ramos Arroyo, M.A.; Jerico, I.; Rogers, M.T.; et al. Inter-individual differences in CpG methylation at D4Z4 correlate with clinical variability in FSHD1 and FSHD2. Hum. Mol. Genet. 2015, 24, 659–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bock, C.; Reither, S.; Mikeska, T.; Paulsen, M.; Walter, J.; Lengauer, T. BiQ Analyzer: Visualization and quality control for DNA methylation data from bisulfite sequencing. Bioinformatics 2005, 21, 4067–4068. [Google Scholar] [CrossRef]
- Himeda, C.L.; Jones, P.L. The Genetics and Epigenetics of Facioscapulohumeral Muscular Dystrophy. Annu. Rev. Genomics Hum. Genet. 2019, 20, 265–291. [Google Scholar] [CrossRef] [PubMed]
- Preston, M.K.; Tawil, R.; Wang, L. Facioscapulohumeral Muscular Dystrophy. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1443/ (accessed on 21 June 2021).
- Mitsuhashi, S.; Boyden, S.E.; Estrella, E.A.; Jones, T.I.; Rahimov, F.; Yu, T.W.; Darras, B.T.; Amato, A.A.; Folkerth, R.D.; Jones, P.L.; et al. Exome sequencing identifies a novel SMCHD1 mutation in facioscapulohumeral muscular dystrophy 2. Neuromuscul. Disord. 2013, 23, 975–980. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, M.C.; Puppo, F.; Roche, S.; Dion, C.; Campana, E.S.; Mariot, V.; Chaix, C.; Vovan, C.; Mazaleyrat, K.; Tasmadjian, A.; et al. Segregation between SMCHD1 mutation, D4Z4 hypomethylation and Facio-Scapulo-Humeral Dystrophy: A case report. BMC Med. Genet. 2016, 17, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Geel, M.; Dickson, M.C.; Beck, A.F.; Bolland, D.J.; Frants, R.R.; van der Maarel, S.M.; de Jong, P.J.; Hewitt, J.E. Genomic analysis of human chromosome 10q and 4q telomeres suggests a common origin. Genomics 2002, 79, 210–217. [Google Scholar] [CrossRef]
- Lemmers, R.J.; Wohlgemuth, M.; van der Gaag, K.J.; van der Vliet, P.J.; van Teijlingen, C.M.; de Knijff, P.; Padberg, G.W.; Frants, R.R.; van der Maarel, S.M. Specific sequence variations within the 4q35 region are associated with facioscapulohumeral muscular dystrophy. Am. J. Hum. Genet. 2007, 81, 884–894. [Google Scholar] [CrossRef] [Green Version]
- Sharim, H.; Grunwald, A.; Gabrieli, T.; Michaeli, Y.; Margalit, S.; Torchinsky, D.; Arielly, R.; Nifker, G.; Juhasz, M.; Gularek, F.; et al. Long-read single-molecule maps of the functional methylome. Genome Res. 2019, 29, 646–656. [Google Scholar] [CrossRef] [PubMed]


| Subject | Sample ID | Genetic Diagnosis | Haplotype | DRA (E/B) | D4Z4 RUs | SMCHD1 |
|---|---|---|---|---|---|---|
| C-01 | PLJ-10171 | Healthy | 4A161/4A161L | >48 kb | ND | ND |
| C-02 | PLJ-10186 | Healthy | 4A161/4A161L | >48 kb | ND | ND |
| C-03 | PLJ-10167 | Healthy | 4A161/4B163 | >48 kb | ND | ND |
| C-04 | PLJ-10212 | Healthy | 4A161/4B162 | >48 kb | ND | ND |
| C-05 | PLJ-20084 | Healthy | 4A161/4A161 | >48 kb | ND | ND |
| C-06 | PLJ-10111 | Healthy | 4A161/4A161 | >48 kb | ND | ND |
| C-07 | PLJ-10255 | Healthy | 4A161/4A161 | >48 kb | ND | ND |
| C-08 | PLJ-20034 | Healthy | 4A161/4A161 | >48 kb | ND | ND |
| F1-01 | PLJ-20043 | FSHD1 | 4A161/4A161 | 12 kb | 3 | ND |
| F1-02 | PLJ-10123 | FSHD1 | 4A161/4A161 | 15 kb | 4 | ND |
| F1-03 | PLJ-10194 | FSHD1 | 4A161/4A166 | 35 kb | 10 | ND |
| F1-04 | PLJ-10218 | FSHD1 | 4A161/4B163 | 25 kb | 7 | ND |
| F1-05 | PLJ-10163 | FSHD1 | 4A161/4B163 | 23 kb | 6 | ND |
| F1-06 | PLJ-10278 | FSHD1 | 4A161/4A161 | 35 kb | 10 | ND |
| F1-07 | PLJ-20052 | FSHD1 | 4A161/4A161 | 12 kb | 3 | ND |
| F1-08 | PLJ-20030 | FSHD1 | 4A161/4A161 | 15 kb | 4 | ND |
| F2-01 | PLJ-10185 | FSHD1 + 2 | 4A161/4B163 | 35 kb | 10 | c.2914−5A>G |
| F2-02 | PLJ-20068 | FSHD2 | 4A161/4B163 | >48 kb | ND | c.2146+1G>A |
| F2-03 | 18MB054 | FSHD2 | 4A161/4A161 | 46 kb | 13 | 1.2Mb deletion |
| F2-04 | 18MB052 | FSHD2 | 4A161/4B163 | 42 kb | 12 | c.3444T>A |
| F2-05 | 19FB042 | FSHD2 | 4A161/4B168 | 43 kb | 12 | c.610A>G |
| F2-06 | 19FB047 | FSHD2 | 4A161/4A166 | 46 kb | 13 | 1.2 Mb deletion |
| Subject | BSSA Avg | BSSA Q1 | BSSA Q2 | BSSA Q3 | BSSX Avg | BSSX Q1 | BSSX Q2 | BSSX Q3 | Epigenetic Diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| C-01 | 58 | 51.8 | 54.5 | 65.2 | 48 | 24.55 | 45.3 | 68.95 | Healthy |
| C-02 | 54.4 | 48.2 | 54.35 | 62.5 | 62 | 46.6 | 71.2 | 76.3 | Healthy |
| C-03 | 61.2 | 56.75 | 61.6 | 67.9 | 60.9 | 52.55 | 66.1 | 72.05 | Healthy |
| C-04 | 61.8 | 52.7 | 61.6 | 70.5 | 53.9 | 38.15 | 55.9 | 67.8 | Healthy |
| C-05 | 52.8 | 42.9 | 56.25 | 64.3 | 56.8 | 34.2 | 64.4 | 78 | Healthy |
| C-06 | 64.6 | 59.8 | 69.1 | 73.2 | 55.3 | 46.65 | 52.5 | 65.25 | Healthy |
| C-07 | 58.2 | 52.25 | 55.4 | 65.15 | 48.1 | 31.6 | 43.25 | 61.85 | Healthy |
| C-08 | 52.1 | 42.9 | 51.8 | 60.7 | 37.5 | 20.03 | 35.05 | 44.95 | Healthy |
| F1-01 | 30.9 | 0.9 | 28.6 | 58.95 | 37.6 | 22 | 33.9 | 58.95 | FSHD1 |
| F1-02 | 48.1 | 7.1 | 39.3 | 67 | 50.6 | 35.6 | 48.3 | 68.65 | FSHD1 |
| F1-03 | 27.3 | 23.2 | 25.9 | 33.9 | 53.6 | 10.2 | 71.2 | 86.4 | FSHD1 |
| F1-04 | 21.4 | 11.7 | 20.5 | 26.15 | 37.21 | 20.3 | 37.3 | 60.7 | FSHD1 |
| F1-05 | 15.4 | 10.7 | 16.1 | 21.4 | 61.6 | 57.8 | 68.25 | 73.3 | FSHD1 |
| F1-06 | 35.2 | 8.9 | 28.55 | 62.5 | 51.3 | 33.1 | 55.2 | 71.2 | FSHD1 |
| F1-07 | 31.9 | 1.8 | 27.95 | 68.75 | 64.2 | 49.15 | 69.5 | 78 | FSHD1 |
| F1-08 | 32.9 | 8 | 14.3 | 60.35 | 63.8 | 61 | 67.8 | 79.7 | FSHD1 |
| F2-01 | 16.9 | 6.25 | 17.85 | 26.8 | 27.6 | 15.3 | 20.5 | 36.2 | FSHD2 |
| F2-02 | 13.1 | 4.55 | 10.7 | 19.6 | 15.2 | 8.55 | 13.6 | 17.75 | FSHD2 |
| F2-03 | 4.8 | 0.9 | 1.8 | 3.6 | 9.1 | 4.25 | 7.65 | 13.6 | FSHD2 |
| F2-04 | 21.1 | 10.7 | 17 | 32.1 | 12.2 | 5.1 | 7.75 | 16.9 | FSHD2 |
| F2-05 | 6.4 | 1.85 | 5.4 | 7.1 | 8.4 | 3.4 | 6.8 | 12.75 | FSHD2 |
| F2-06 | 5.5 | 3.6 | 5.4 | 7.1 | 13 | 3.4 | 8.5 | 20.3 | FSHD2 |
| Subjects | BSS 3′ Avg | BSS 3′ Q1 | BSS 3′ Q2 | BSS 3′ Q3 | BSS Mid Avg | BSS Mid Q1 | BSS Mid Q2 | BSS Mid Q3 | BSS 5′ Avg | BSS 5′ Q1 | BSS 5′ Q2 | BSS 5’ Q3 | Epigenetic Diagnosis | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-01 | 59.1 | 54.5 | 63.05 | 75 | 74.6 | 69.7 | 75 | 81.4 | 69.5 | 54.35 | 76.1 | 87 | Healthy | ||
| C-02 | 60.6 | 37.5 | 70 | 73.85 | 74.2 | 63.8 | 77.3 | 82.4 | 67.8 | 56.5 | 67.4 | 80.45 | Healthy | ||
| C-03 | 59.7 | 32.15 | 76.9 | 81.65 | 77.9 | 72.35 | 77.3 | 84.6 | 63 | 50 | 64.4 | 69.8 | Healthy | ||
| C-04 | 71.3 | 66.7 | 75 | 78.9 | 72.1 | 66.7 | 71.4 | 76 | 73 | 62.05 | 76.1 | 82.6 | Healthy | ||
| C-05 | 62.6 | 50 | 63.05 | 73.85 | 78 | 67.95 | 81.5 | 85.9 | 69.5 | 54.45 | 73.9 | 78.3 | Healthy | ||
| F1-01 | 62.2 | 50 | 68.35 | 79.3 | 70.6 | 63.35 | 75 | 79.75 | 64.7 | 52.3 | 65.2 | 82.65 | Healthy | ||
| F1-02 | 65.9 | 45.85 | 71.4 | 80 | 73.7 | 66.45 | 74.55 | 84.4 | 73.4 | 56.5 | 80.45 | 91.3 | Healthy | ||
| F1-03 | 69.9 | 64.6 | 71.35 | 80 | 75.8 | 66.05 | 77.1 | 85.95 | 73.1 | 63.05 | 73.3 | 89.15 | Healthy | ||
| F1-04 | 61.7 | 47.75 | 66.7 | 73.85 | 69.9 | 64.1 | 70.45 | 76.8 | 75.2 | 65.2 | 80.45 | 91.3 | Healthy | ||
| F1-05 | 56.3 | 47.2 | 52.8 | 65.15 | 77.6 | 66.05 | 76 | 88.95 | 58.7 | 39 | 56.5 | 78.3 | Healthy | ||
| F2-01 | 67.5 | 54.5 | 68.35 | 76.4 | 73 | 64.5 | 71.9 | 81.65 | 53 | 34.75 | 47.8 | 72.25 | Healthy | ||
| F2-02 | 61.2 | 55.6 | 70 | 78.4 | 61.9 | 59.2 | 74 | 82.05 | 51.3 | 42.5 | 52.2 | 58.7 | Healthy | ||
| F2-03 | 60.4 | 52.25 | 61.25 | 70 | 53.5 | 50 | 57.7 | 75.95 | 28.1 | 8.75 | 26.1 | 55.5 | H | H | F |
| F2-04 | 61.4 | 43.75 | 64.6 | 72.5 | 67 | 60.5 | 69.1 | 78.75 | 26.4 | 8.7 | 19.55 | 43.5 | H | H | F |
| F2-05 | 57.7 | 45.5 | 61.8 | 72.5 | 59.2 | 48 | 61.05 | 74.1 | 20.8 | 4.3 | 19.55 | 34.8 | H | H | F |
| F2-06 | 58.3 | 41.45 | 59.3 | 66.7 | 62.5 | 58.3 | 66.7 | 73.9 | 27.8 | 10.85 | 34.8 | 47.7 | H | H | F |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gould, T.; Jones, T.I.; Jones, P.L. Precise Epigenetic Analysis Using Targeted Bisulfite Genomic Sequencing Distinguishes FSHD1, FSHD2, and Healthy Subjects. Diagnostics 2021, 11, 1469. https://doi.org/10.3390/diagnostics11081469
Gould T, Jones TI, Jones PL. Precise Epigenetic Analysis Using Targeted Bisulfite Genomic Sequencing Distinguishes FSHD1, FSHD2, and Healthy Subjects. Diagnostics. 2021; 11(8):1469. https://doi.org/10.3390/diagnostics11081469
Chicago/Turabian StyleGould, Taylor, Takako I. Jones, and Peter L. Jones. 2021. "Precise Epigenetic Analysis Using Targeted Bisulfite Genomic Sequencing Distinguishes FSHD1, FSHD2, and Healthy Subjects" Diagnostics 11, no. 8: 1469. https://doi.org/10.3390/diagnostics11081469
APA StyleGould, T., Jones, T. I., & Jones, P. L. (2021). Precise Epigenetic Analysis Using Targeted Bisulfite Genomic Sequencing Distinguishes FSHD1, FSHD2, and Healthy Subjects. Diagnostics, 11(8), 1469. https://doi.org/10.3390/diagnostics11081469





