Uterine Fibroids and Infertility
Abstract
:1. Introduction
2. Uterine Fibroids and Infertility
3. Pathophysiology
Uterine Myometrial Peristalsis
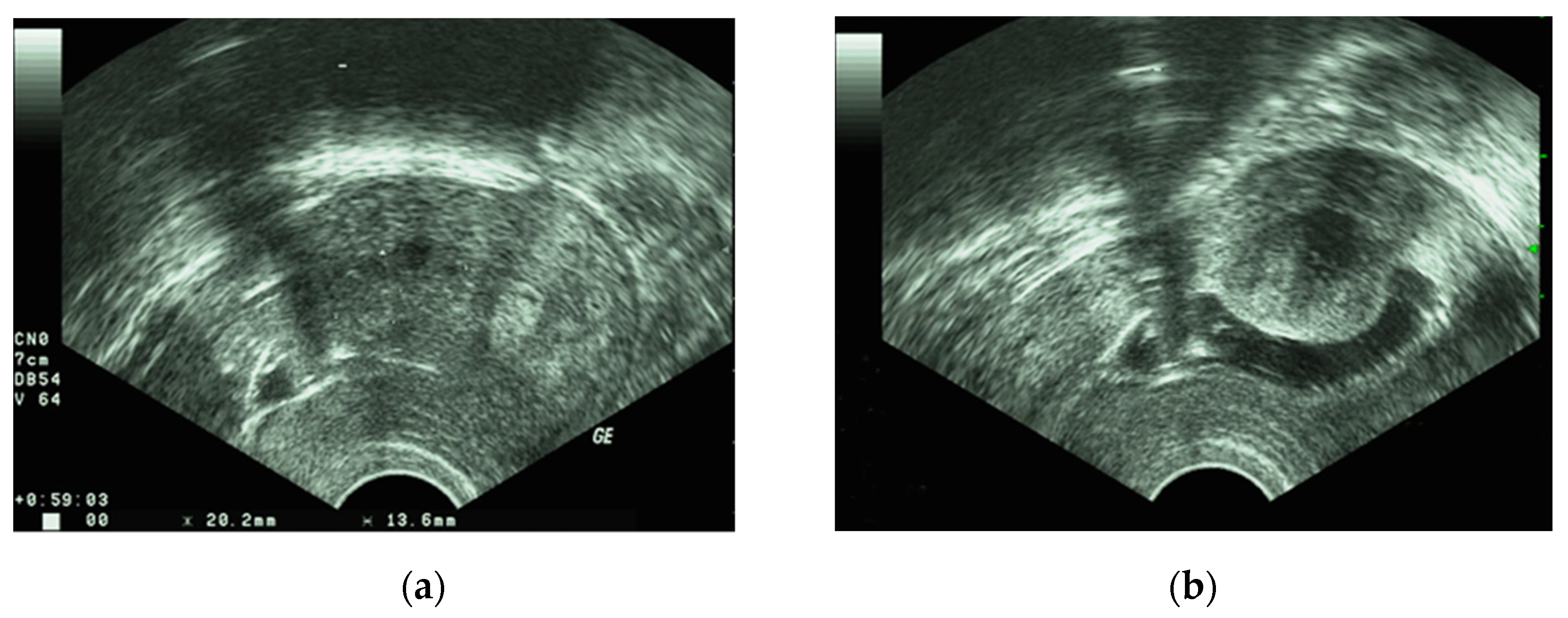
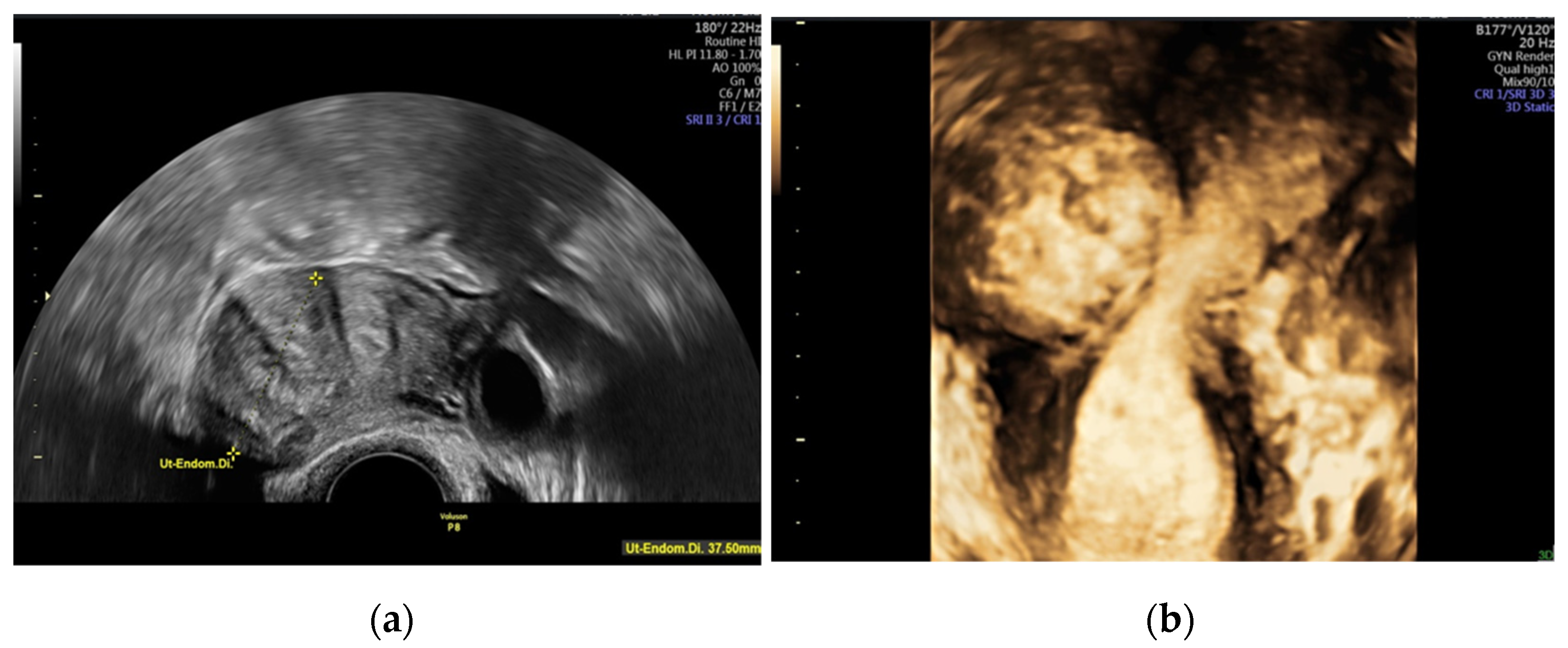
4. Diagnosis
5. Management
6. Recommendation
7. Conclusions
- a.
- Submucosal fibroids should be removed before ART or in cases of habitual abortions.
- b.
- Subserosal fibroids: as they do not seem to affect pregnancy rates, myomectomy does not appear to be necessary.
- c.
- Intramural fibroids: controversial data, lack of homogenous opinion. Intramural fibroids ≥5 cm: perform surgery before ART or in cases of habitual abortion. Intramural fibroids <5 cm: the reported outcome varies between no difference and significantly reduced cumulative pregnancy rates.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowak, I.; Wilczyńska, K.; Wilczyński, J.R.; Malinowski, A.; Radwan, P.; Radwan, M.; Kuśnierczyk, P. KIR, LILRB and their Ligands’ Genes as Potential Biomarkers in Recurrent Implantation Failure. Arch. Immunol. Ther. Exp. 2017, 65, 391–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zegers-Hochschild, F.; Adamson, G.D.; de Mouzon, J.; Ishihara, O.; Mansour, R.; Nygren, K.; Sullivan, E.; van der Poel, S.; on behalf of ICMART and WHO. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Hum. Reprod. 2009, 92, 1520–1524. [Google Scholar]
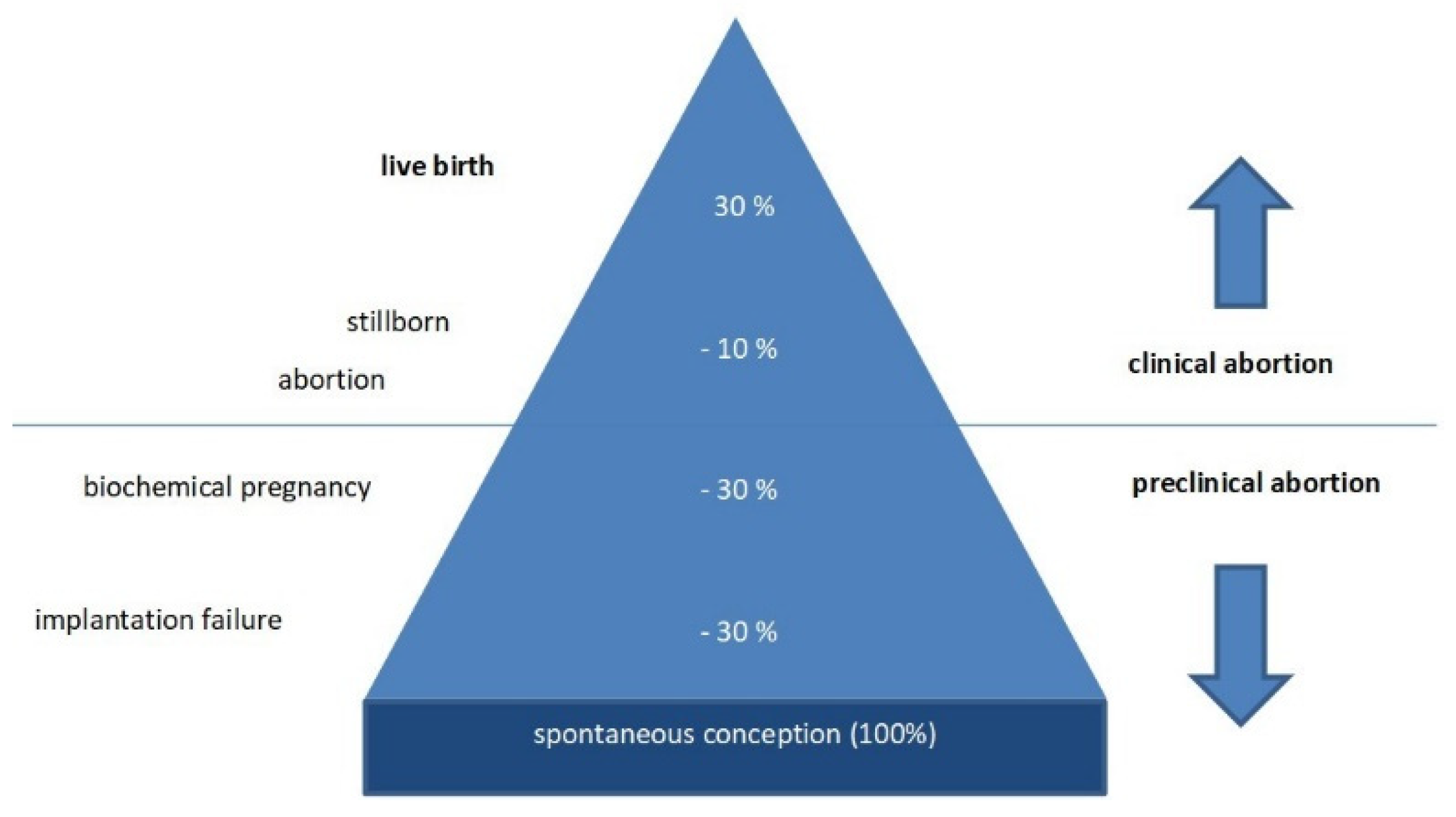
- Macklon, N.S.; Geraedts, J.P.; Fauser, B.C. Conception to ongoing pregnancy: The ‘black box’ of early pregnancy loss. Hum. Reprod. Update 2002, 8, 333–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coughlan, C.; Ledger, W.; Wang, Q.; Liu, F.; Demirol, A.; Gurgan, T.; Cutting, R.; Ong, K.; Sallam, H.; Li, T.C. Recurrent implantation failure: Definition and management. Reprod. Biomed. Online 2014, 28, 14–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koot, Y.E.; Teklenburg, G.; Salker, M.S.; Brosens, J.J.; Macklon, N.S. Molecular aspects of implantation failure. Biochim. Biophys. Acta 2012, 1822, 1943–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penzias, A.S. Recurrent IVF failure: Other factors. Fertil. Steril. 2012, 97, 1033–1038. [Google Scholar] [CrossRef]
- Stewart, E.A.; Laughlin-Tommaso, S.K.; Catherino, W.H.; Lalitkumar, S.; Gupta, D.; Vollenhoven, B. Uterine fibroids. Nat. Rev. Dis. Primers 2016, 2, 16043. [Google Scholar] [CrossRef]
- Benson, C.B.; Chow, J.S.; Chang-Lee, W.; Hill, J.A., 3rd; Doubilet, P.M. Outcome of pregnancies in women with uterine leiomyomas identified by sonography in the first trimester. J. Clin. Ultrasound 2001, 29, 261–264. [Google Scholar] [CrossRef]
- Coronado, G.D.; Marshall, L.M.; Schwartz, S.M. Complications in pregnancy, labor, and delivery with uterine leiomyomas: A population-based study. Obs. Gynecol. 2000, 95, 764–769. [Google Scholar] [CrossRef]
- Whynott, R.M.; Vaught, K.C.C.; Segars, J.H. The Effect of Uterine Fibroids on Infertility: A Systematic Review. Semin. Reprod. Med. 2017, 35, 523–532. [Google Scholar] [CrossRef]
- Islam, M.S.; Akhtar, M.M.; Segars, J.H. Vitamin D deficiency and uterine fibroids: An opportunity for treatment or prevention? Fertil. Steril. 2021, 115, 1175–1176. [Google Scholar] [CrossRef]
- Vergara, D.; Catherino, W.H.; Trojano, G.; Tinelli, A. Vitamin D: Mechanism of Action and Biological Effects in Uterine Fibroids. Nutrients 2021, 13, 597. [Google Scholar] [CrossRef] [PubMed]
- Sabry, M.; Halder, S.K.; Allah, A.S.; Roshdy, E.; Rajaratnam, V.; Al-Hendy, A. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: A cross-sectional observational study. Int. J. Womens Health 2013, 5, 93–100. [Google Scholar] [PubMed] [Green Version]
- Harris, S.S. Vitamin D and African Americans. J. Nutr. 2006, 136, 1126–1129. [Google Scholar] [CrossRef] [Green Version]
- Lima, M.S.O.; da Silva, B.B.; de Medeiros, M.L.; Dos Santos, A.R.; do Nascimento Brazil, E.D.; Filho, W.M.N.E.; Ibiapina, J.O.; Brito, A.G.A.; Costa, P.V.L. Evaluation of vitamin D receptor expression in uterine leiomyoma and nonneoplastic myometrial tissue: A cross-sectional controlled study. Reprod. Biol. Endocrinol. 2021, 19, 67. [Google Scholar] [CrossRef]
- Buttram, V.C., Jr.; Reiter, R.C. Uterine leiomyomata: Etiology, symptomatology, and management. Fertil. Steril. 1981, 36, 433–445. [Google Scholar]
- Donnez, J.; Jadoul, P. What are the implications of myomas on fertility? A need for a debate? Hum. Reprod. 2002, 17, 1424–1430. [Google Scholar] [CrossRef]
- Gunther, V.; Otte, S.V.; Freytag, D.; Maass, N.; Alkatout, I. Recurrent implantation failure-an overview of current research. Gynecol. Endocrinol. 2021, 37, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Pritts, E.A.; Parker, W.H.; Olive, D.L. Fibroids and infertility: An updated systematic review of the evidence. Fertil. Steril. 2009, 91, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, E.; Vercellini, P.; Daguati, R.; Pasin, R.; De Giorgi, O.; Crosignani, P.G. Fibroids and female reproduction: A critical analysis of the evidence. Hum. Reprod. Update 2007, 13, 465–476. [Google Scholar] [CrossRef] [Green Version]
- Pritts, E.A. Fibroids and infertility: A systematic review of the evidence. Obstet. Gynecol. Surv. 2001, 56, 483–491. [Google Scholar] [CrossRef]
- Surrey, E.S.; Lietz, A.K.; Schoolcraft, W.B. Impact of intramural leiomyomata in patients with a normal endometrial cavity on in vitro fertilization-embryo transfer cycle outcome. Fertil. Steril. 2001, 75, 405–410. [Google Scholar] [CrossRef]
- Garcia, C.R.; Tureck, R.W. Submucosal leiomyomas and infertility. Fertil. Steril. 1984, 42, 16–19. [Google Scholar] [CrossRef]
- Goldenberg, M.; Sivan, E.; Sharabi, Z.; Bider, D.; Rabinovici, J.; Seidman, D.S. Outcome of hysteroscopic resection of submucous myomas for infertility. Fertil. Steril. 1995, 64, 714–716. [Google Scholar] [CrossRef]
- Fernandez, H.; Sefrioui, O.; Virelizier, C.; Gervaise, A.; Gomel, V.; Frydman, R. Hysteroscopic resection of submucosal myomas in patients with infertility. Hum. Reprod. 2001, 16, 1489–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, E.; Gomel, V. The uterus and fertility. Fertil. Steril. 2008, 89, 1–16. [Google Scholar] [CrossRef]
- Block, K.; Kardana, A.; Igarashi, P.; Taylor, H.S. In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing mullerian system. FASEB J. 2000, 14, 1101–1108. [Google Scholar] [CrossRef]
- Rackow, B.W.; Taylor, H.S. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil. Steril. 2010, 93, 2027–2034. [Google Scholar] [CrossRef] [Green Version]
- Taylor, H.S.; Daftary, G.S.; Selam, B. Endometrial HOXA10 expression after controlled ovarian hyperstimulation with recombinant follicle-stimulating hormone. Fertil. Steril. 2003, 80, 839–843. [Google Scholar] [CrossRef]
- Pier, B.D.; Bates, G.W. Potential causes of subfertility in patients with intramural fibroids. Fertil. Res. Pract. 2015, 1, 12. [Google Scholar] [CrossRef] [Green Version]
- Uchida, H.; Maruyama, T.; Nishikawa-Uchida, S.; Miyazaki, K.; Masuda, H.; Yoshimura, Y. Glycodelin in reproduction. Reprod. Med. Biol. 2013, 12, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Ciavattini, A.; Giuseppe, J.D.; Stortoni, P.; Montik, N.; Giannubilo, S.R.; Litta, P.; Islam, S.; Tranquilli, A.L.; Reis, F.M.; Ciarmela, P. Uterine fibroids: Pathogenesis and interactions with endometrium and endomyometrial junction. Obstet. Gynecol. Int. 2013, 2013, 173184. [Google Scholar] [CrossRef] [PubMed]
- Lash, G.E.; Bulmer, J.N. Do uterine natural killer (uNK) cells contribute to female reproductive disorders? J. Reprod. Immunol. 2011, 88, 156–164. [Google Scholar] [CrossRef]
- Kitaya, K.; Yasuo, T. Leukocyte density and composition in human cycling endometrium with uterine fibroids. Hum. Immunol. 2010, 71, 158–163. [Google Scholar] [CrossRef]
- Tocci, A.; Greco, E.; Ubaldi, F.M. Adenomyosis and ’endometrial-subendometrial myometrium unit disruption disease’ are two different entities. Reprod. Biomed. Online 2008, 17, 281–291. [Google Scholar] [CrossRef]
- Jakimiuk, A.J.; Bogusiewicz, M.; Tarkowski, R.; Dziduch, P.; Adamiak, A.; Wróbel, A.; Haczyński, J.; Magoffin, D.A.; Jakowicki, J.A. Estrogen receptor alpha and beta expression in uterine leiomyomas from premenopausal women. Fertil. Steril. 2004, 82, 1244–1249. [Google Scholar] [CrossRef]
- Ishikawa, H.; Reierstad, S.; Demura, M.; Rademaker, A.W.; Kasai, T.; Inoue, M.; Usui, H.; Shozu, M.; Bulun, S.E. High aromatase expression in uterine leiomyoma tissues of African-American women. J. Clin. Endocrinol. Metab. 2009, 94, 1752–1756. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Togashi, K.; Yamaoka, T.; Nakai, A.; Kido, A.; Nishio, S.; Yamamoto, T.; Kitagaki, H.; Fujii, S. Kinematics of the uterus: Cine mode MR imaging. Radiographics 2004, 24, e19. [Google Scholar] [CrossRef]
- Lyons, E.A.; Taylor, P.J.; Zheng, X.H.; Ballard, G.; Levi, C.S.; Kredentser, J.V. Characterization of subendometrial myometrial contractions throughout the menstrual cycle in normal fertile women. Fertil. Steril. 1991, 55, 771–774. [Google Scholar] [CrossRef]
- Orisaka, M.; Kurokawa, T.; Shukunami, K.; Orisaka, S.; Fukuda, M.T.; Shinagawa, A.; Fukuda, S.; Ihara, N.; Yamada, H.; Itoh, H.; et al. A comparison of uterine peristalsis in women with normal uteri and uterine leiomyoma by cine magnetic resonance imaging. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 135, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, O.; Hayashi, T.; Osuga, Y.; Orisaka, M.; Asada, H.; Okuda, S.; Hori, M.; Furuya, M.; Onuki, H.; Sadoshima, Y.; et al. Decreased pregnancy rate is linked to abnormal uterine peristalsis caused by intramural fibroids. Hum. Reprod. 2010, 25, 2475–2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshino, O.; Nishii, O.; Osuga, Y.; Asada, H.; Okuda, S.; Orisaka, M.; Hori, M.; Fujiwara, T.; Hayashi, T. Myomectomy decreases abnormal uterine peristalsis and increases pregnancy rate. J. Minim. Invasive Gynecol. 2012, 19, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Tinelli, A.; Mynbaev, O.A.; Mettler, L.; Hurst, B.S.; Pellegrino, M.; Nicolardi, G.; Kosmas, I.; Malvasi, A. A combined ultrasound and histologic approach for analysis of uterine fibroid pseudocapsule thickness. Reprod. Sci. 2014, 21, 1177–1186. [Google Scholar] [CrossRef]
- Malvasi, A.; Cavallotti, C.; Nicolardi, G.; Pellegrino, M.; Dell’Edera, D.; Vergara, D.; Kumakiri, J.; Greco, M.; Tinelli, A. NT, NPY and PGP 9.5 presence in myomeytrium and in fibroid pseudocapsule and their possible impact on muscular physiology. Gynecol. Endocrinol. 2013, 29, 177–181. [Google Scholar] [CrossRef]
- Shavell, V.I.; Thakur, M.; Sawant, A.; Kruger, M.L.; Jones, T.B.; Singh, M.; Puscheck, E.E.; Diamond, M.P. Adverse obstetric outcomes associated with sonographically identified large uterine fibroids. Fertil. Steril. 2012, 97, 107–110. [Google Scholar] [CrossRef]
- Seshadri, S.; El-Toukhy, T.; Douiri, A.; Jayaprakasan, K.; Khalaf, Y. Diagnostic accuracy of saline infusion sonography in the evaluation of uterine cavity abnormalities prior to assisted reproductive techniques: A systematic review and meta-analyses. Hum. Reprod. Update 2015, 21, 262–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreotti, R.F.; Fleischer, A.C. Practical applications of 3D sonography in gynecologic imaging. Radiol. Clin. N. Am. 2014, 52, 1201–1213. [Google Scholar] [CrossRef]
- Wong, L.; White, N.; Ramkrishna, J.; Araujo Júnior, E.; Meagher, S.; Costa Fda, S. Three-dimensional imaging of the uterus: The value of the coronal plane. World J. Radiol. 2015, 7, 484–493. [Google Scholar] [CrossRef]
- Van den Bosch, T.; Dueholm, M.; Leone, F.P.; Valentin, L.; Rasmussen, C.K.; Votino, A.; Van Schoubroeck, D.; Landolfo, C.; Installé, A.J.; Guerriero, S.; et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: A consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet. Gynecol. 2015, 46, 284–298. [Google Scholar] [CrossRef]
- Wozniak, A.; Wozniak, S. Ultrasonography of uterine leiomyomas. Prz. Menopauzalny 2017, 16, 113–117. [Google Scholar] [CrossRef]
- Giuliani, E.; As-Sanie, S.; Marsh, E.E. Epidemiology and management of uterine fibroids. Int. J. Gynaecol. Obstet. 2020, 149, 3–9. [Google Scholar] [CrossRef]
- Neis, K.J.; Zubke, W.; Fehr, M.; Römer, T.; Tamussino, K.; Nothacker, M. Hysterectomy for Benign Uterine Disease. Dtsch. Arztebl. Int. 2016, 113, 242–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcone, T.; Parker, W.H. Surgical management of leiomyomas for fertility or uterine preservation. Obstet. Gynecol. 2013, 121, 856–868. [Google Scholar] [CrossRef] [Green Version]
- Zepiridis, L.I.; Grimbizis, G.F.; Tarlatzis, B.C. Infertility and uterine fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 34, 66–73. [Google Scholar] [CrossRef]
- Pakrashi, T. New hysteroscopic techniques for submucosal uterine fibroids. Curr. Opin. Obstet. Gynecol. 2014, 26, 308–313. [Google Scholar] [CrossRef]
- Casadio, P.; Guasina, F.; Morra, C.; Talamo, M.T.; Leggieri, C.; Frisoni, J.; Seracchioli, R. Hysteroscopic myomectomy: Techniques and preoperative assessment. Minerva. Ginecol. 2016, 68, 154–166. [Google Scholar]
- van Kerkvoorde, T.C.; Veersema, S.; Timmermans, A. Long-term complications of office hysteroscopy: Analysis of 1028 cases. J. Minim. Invasive Gynecol. 2012, 19, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Łoziński, T.; Wojtyła, C.; Rawski, W.; Jakiel, G. Complications in modern hysteroscopic myomectomy. Ginekol. Pol. 2018, 89, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Gambadauro, P.; Gudmundsson, J.; Torrejon, R. Intrauterine Adhesions following Conservative Treatment of Uterine Fibroids. Obstet. Gynecol. Int. 2012, 2012, 853269. [Google Scholar] [CrossRef]
- Touboul, C.; Fernandez, H.; Deffieux, X.; Berry, R.; Frydman, R.; Gervaise, A. Uterine synechiae after bipolar hysteroscopic resection of submucosal myomas in patients with infertility. Fertil. Steril. 2009, 92, 1690–1693. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Chen, M.J.; Chen, C.D.; Chen, S.U.; Ho, H.N.; Yang, Y.S. Optimal waiting period for subsequent fertility treatment after various hysteroscopic surgeries. Fertil. Steril. 2013, 99, 2092–2096. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Dolmans, M.M. Uterine fibroid management: From the present to the future. Hum. Reprod. Update 2016, 22, 665–686. [Google Scholar] [CrossRef]
- Mettler, L.; Schollmeyer, T.; Tinelli, A.; Malvasi, A.; Alkatout, I. Complications of Uterine Fibroids and Their Management, Surgical Management of Fibroids, Laparoscopy and Hysteroscopy versus Hysterectomy, Haemorrhage, Adhesions, and Complications. Obstet. Gynecol. Int. 2012, 2012, 791248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanos, V.; Berry, K.E.; Frist, M.; Campo, R.; DeWilde, R.L. Prevention and Management of Complications in Laparoscopic Myomectomy. Biomed. Res. Int. 2018, 2018, 8250952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinelli, A.; Favilli, A.; Lasmar, R.B.; Mazzon, I.; Gerli, S.; Xue, X.; Malvasi, A. The importance of pseudocapsule preservation during hysteroscopic myomectomy. Eur. J. Obstet. Gynecol. 2019, 243, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Tinelli, A.; Kosmas, I.; Mynbaev, O.A.; Favilli, A.; Gimbrizis, G.; Sparic, R.; Pellegrino, M.; Malvasi, A. Submucous Fibroids, Fertility, and Possible Correlation to Pseudocapsule Thickness in Reproductive Surgery. Bio. Med. Res. Int. 2018, 2018, 2804830. [Google Scholar] [CrossRef] [Green Version]
- Jeng, C.J.; Ou, K.Y.; Long, C.Y.; Chuang, L.; Ker, C.R. 500 Cases of High.-intensity Focused Ultrasound (HIFU) Ablated Uterine Fibroids and Adenomyosis. Taiwan J. Obs. Gynecol. 2020, 59, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, Q.; Li, W.; Zhu, X.; Jiang, J.; Chen, L.; He, S.; Xue, M.; Ye, M.; Li, X. A comparative analysis of pregnancy outcomes of patients with uterine fibroids after high intensity focused ultrasound ablation and laparoscopic myomectomy: A retrospective study. Int. J. Hyperth. 2021, 38, 79–84. [Google Scholar] [CrossRef]
- Zou, M.; Chen, L.; Wu, C.; Hu, C.; Xiong, Y. Pregnancy outcomes in patients with uterine fibroids treated with ultrasound-guided high-intensity focused ultrasound. BJOG 2017, 124, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.B.; Yu, S.P. Radiofrequency Ablation of Uterine Fibroids: A Review. Curr. Obstet. Gynecol. Rep. 2016, 5, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Bradley, L.D.; Pasic, R.P.; Miller, L.E. Clinical Performance of Radiofrequency Ablation for Treatment of Uterine Fibroids: Systematic Review and Meta-Analysis of Prospective Studies. J. Laparoendosc. Adv. Surg. Tech. A 2019, 29, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Pschadka, G.; Engelhardt, M.; Niehoff, C.; Toub, D. Term Delivery in an Infertile Patient after Transcervical Radiofrequency Fibroid Ablation and Assisted Reproductive Technology. J. Gynecol. Surg. 2019, 35, 253–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rattray, D.D.; Weins, L.; Regush, L.C.; Bowen, J.M.; O’Reilly, D.; Thiel, J.A. Clinical outcomes and health care utilization pre- and post-laparoscopic radiofrequency ablation of symptomatic fibroids and laparoscopic myomectomy: A randomized trial of uterine-sparing techniques (TRUST) in Canada. Clin. Outcomes Res. 2018, 10, 201–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsen, K.; Hrobjartsson, A.; Korsholm, M.; Mogensen, O.; Humaidan, P.; Ravn, P. Fertility after uterine artery embolization of fibroids: A systematic review. Arch. Gynecol. Obstet. 2018, 297, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.; Khalaf, Y.; Yeong, C.T.; Seed, P.; Taylor, A.; Braude, P. A prospective controlled study of the effect of intramural uterine fibroids on the outcome of assisted conception. Hum. Reprod. 2001, 16, 2411–2417. [Google Scholar] [CrossRef] [Green Version]
- Metwally, M.; Raybould, G.; Cheong, Y.C.; Horne, A.W. Surgical treatment of fibroids for subfertility. Cochrane Database Syst. Rev. 2020, 1, CD003857. [Google Scholar] [CrossRef]
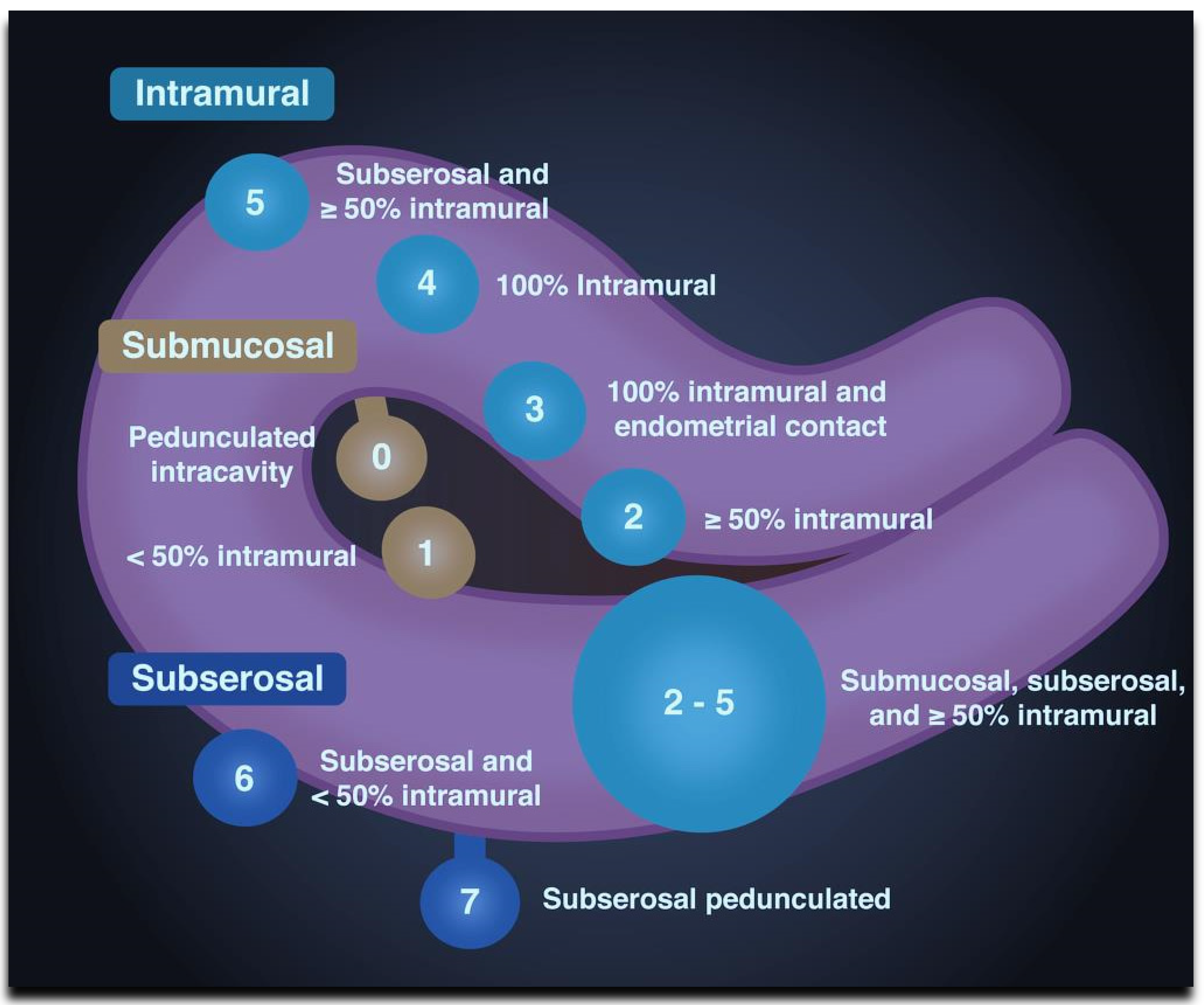

| Type | Location | |
|---|---|---|
| Submucosal | 0 | Pedunculated intracavitary |
| 1 | <50% intramural | |
| Intramural | 2 | ≥50% intramural |
| 3 | Contact with the endometrium, 100% intramural | |
| 4 | ||
| 5 | Intramural Subserosal ≥50% intramural | |
| Subserosal | 6 | Subserosal <50% intramural |
| 7 | Subserosal pedunculated | |
| 8 | Other (e.g., cervical, intraligamentous). | |
| Hybrid (having contact with both the endometrium and the serosal layer). The numbers are listed separately with a hyphen. The first refers to the relationship with the endometrium, and the second refers to the relationship with the serosa. | 2–5 | Submucosal and subserosal, each with less than half the diameter in the endometrial and peritoneal cavities, respectively. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freytag, D.; Günther, V.; Maass, N.; Alkatout, I. Uterine Fibroids and Infertility. Diagnostics 2021, 11, 1455. https://doi.org/10.3390/diagnostics11081455
Freytag D, Günther V, Maass N, Alkatout I. Uterine Fibroids and Infertility. Diagnostics. 2021; 11(8):1455. https://doi.org/10.3390/diagnostics11081455
Chicago/Turabian StyleFreytag, Damaris, Veronika Günther, Nicolai Maass, and Ibrahim Alkatout. 2021. "Uterine Fibroids and Infertility" Diagnostics 11, no. 8: 1455. https://doi.org/10.3390/diagnostics11081455
APA StyleFreytag, D., Günther, V., Maass, N., & Alkatout, I. (2021). Uterine Fibroids and Infertility. Diagnostics, 11(8), 1455. https://doi.org/10.3390/diagnostics11081455





