Diagnosis of Mucopolysaccharidoses and Mucolipidosis by Assaying Multiplex Enzymes and Glycosaminoglycans
Abstract
1. Introduction
2. Materials and Methods
2.1. DBS Samples
2.2. GAG Assay
2.3. Multiplex Enzyme Assay
2.4. Statistical Analysis
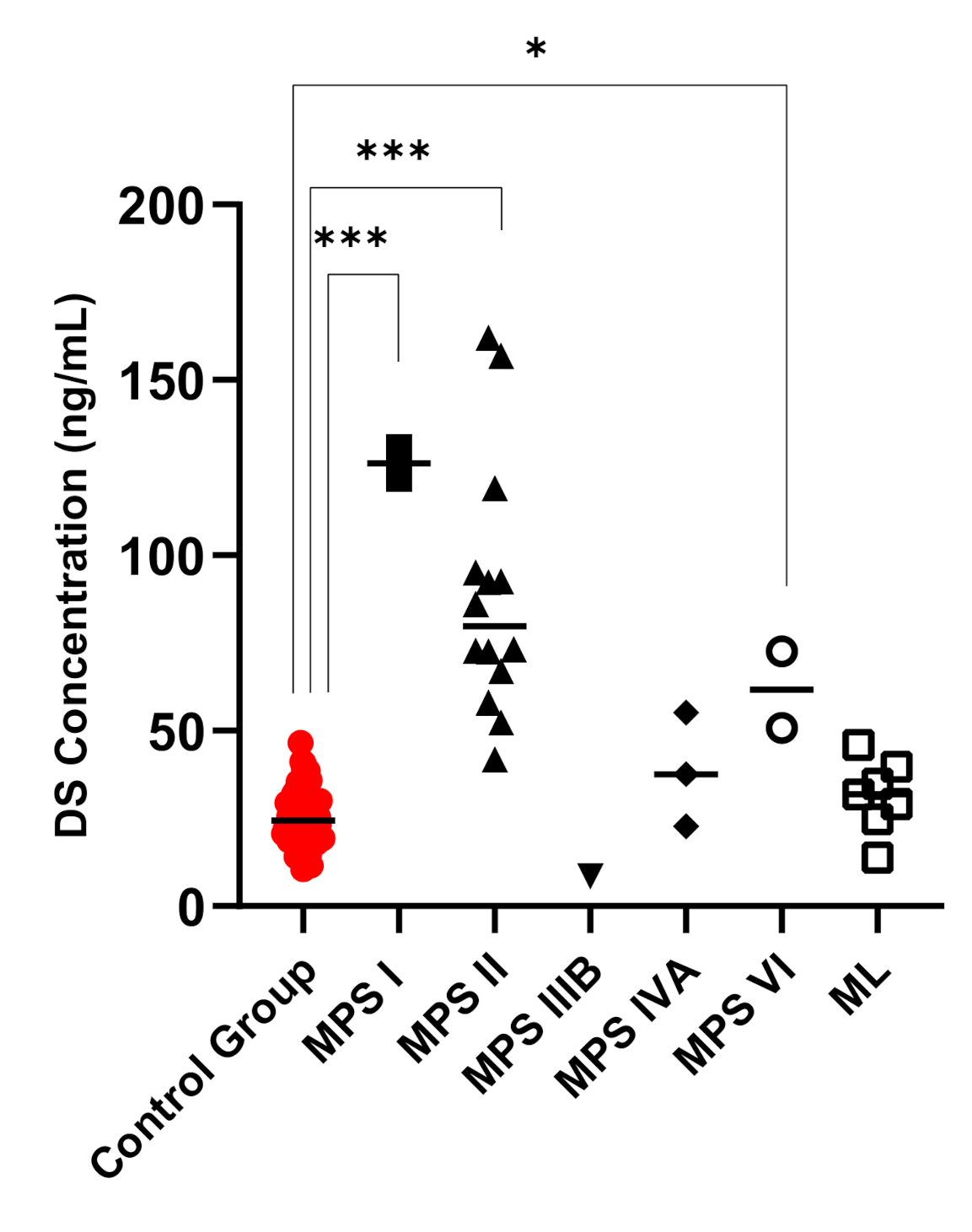
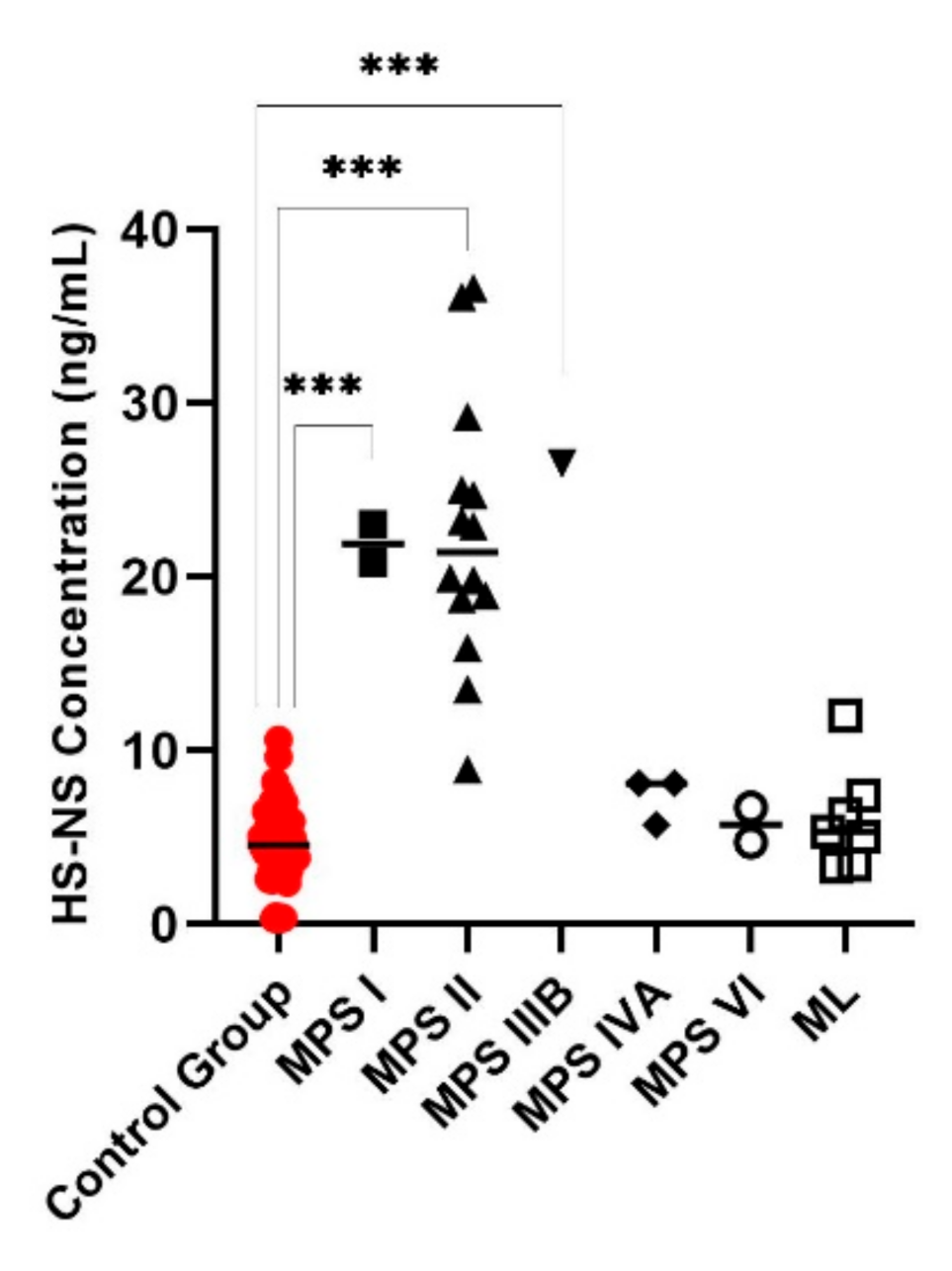
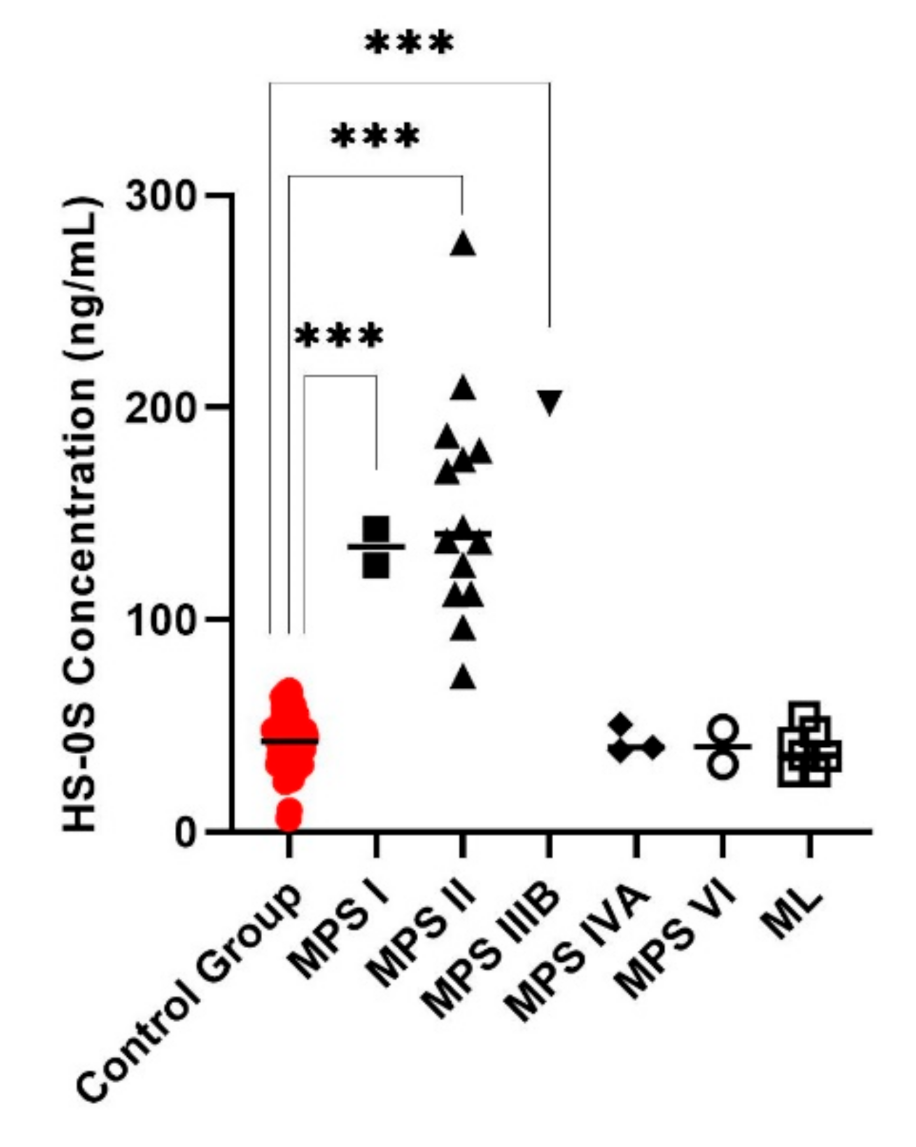
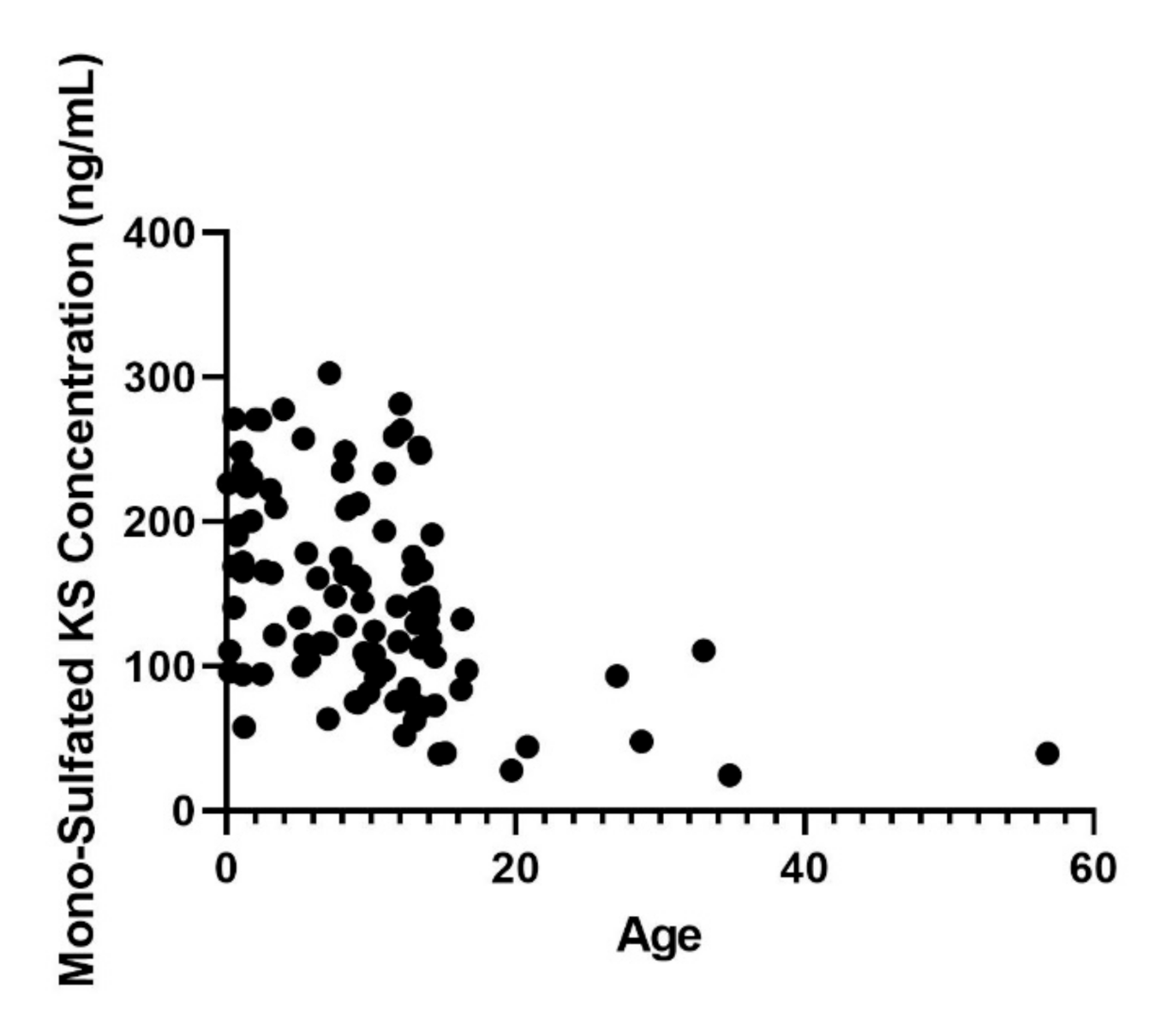
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tomatsu, S.; Averill, L.W.; Sawamoto, K.; Mackenzie, W.G.; Bober, M.B.; Pizarro, C.; Goff, C.J.; Xie, L.; Orii, T.; Theroux, M. Obstructive airway in Morquio A syndrome, the past, the present and the future. Mol. Genet. Metab. 2016, 117, 150–156. [Google Scholar] [CrossRef]
- Montaño, A.M.; Tomatsu, S.; Gottesman, G.S.; Smith, M.; Orii, T. International Morquio A Registry: Clinical manifestation and natural course of Morquio A disease. J. Inherit. Metab. Dis. 2007, 30, 165–174. [Google Scholar] [CrossRef]
- Tomatsu, S.; Montaño, A.M.; Oikawa, H.; Smith, M.; Barrera, L.; Chinen, Y.; Thacker, M.M.; Mackenzie, W.G.; Suzuki, Y.; Orii, T. Mucopolysaccharidosis type IVA (Morquio A disease): Clinical review and current treatment. Curr. Pharm. Biotechnol. 2011, 12, 931–945. [Google Scholar] [CrossRef]
- Tomatsu, S.; Mackenzie, W.G.; Theroux, M.C.; Mason, R.W.; Thacker, M.M.; Shaffer, T.H.; Montaño, A.M.; Rowan, D.; Sly, W.; Alméciga-Díaz, C.J.; et al. Current and emerging treatments and surgical interventions for Morquio A Syndrome: A review. Res. Rep. Endocr. Disord. 2012, 2012, 65–77. [Google Scholar] [CrossRef]
- Kubaski, F.; Yabe, H.; Suzuki, Y.; Seto, T.; Hamazaki, T.; Mason, R.W.; Xie, L.; Onsten, T.G.H.; Leistner-Segal, S.; Giugliani, R. Hematopoietic stem cell transplantation for patients with mucopolysaccharidosis II. Biol. Blood Marrow Transplant 2017, 23, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- McGill, J.J.; Inwood, A.C.; Coman, D.J.; Lipke, M.L.; De Lore, D.; Swiedler, S.J.; Hopwood, J.J. Enzyme replacement therapy for mucopolysaccharidosis VI from 8 weeks of age–a sibling control study. Clin. Genet. 2010, 77, 492–498. [Google Scholar] [CrossRef]
- Patel, P.; Suzuki, Y.; Tanaka, A.; Yabe, H.; Kato, S.; Shimada, T.; Mason, R.W.; Orii, K.E.; Fukao, T.; Orii, T. Impact of enzyme replacement therapy and hematopoietic stem cell therapy on growth in patients with Hunter syndrome. Mol. Genet. Metab. Rep. 2014, 1, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, S.; Azario, I.; Sawamoto, K.; Pievani, A.S.; Biondi, A.; Serafini, M. Neonatal cellular and gene therapies for mucopolysaccharidoses: The earlier the better? J. Inherit. Metab. Dis. 2016, 39, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Iwata, S.; Sukegawa, K.; Kokuryu, M.I.E.; Tomatsu, S.; Kondo, N.; Iwasa, S.; Orii, T. Glycosaminoglycans in neonatal urine. Arch. Dis. Child. Fetal Neonat. Ed. 2000, 82, F77. [Google Scholar] [CrossRef]
- Khan, S.; Alméciga-Díaz, C.J.; Sawamoto, K.; Mackenzie, W.G.; Theroux, M.C.; Pizarro, C.; Mason, R.W.; Orii, T.; Tomatsu, S. Mucopolysaccharidosis IVA and glycosaminoglycans. Mol. Genet. Metab. 2017, 120, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Kubaski, F.; Mason, R.W.; Nakatomi, A.; Shintaku, H.; Xie, L.; van Vlies, N.N.; Church, H.; Giugliani, R.; Kobayashi, H.; Yamaguchi, S. Newborn screening for mucopolysaccharidoses: A pilot study of measurement of glycosaminoglycans by tandem mass spectrometry. J. Inherit. Metab. Dis. 2017, 40, 151–158. [Google Scholar] [CrossRef]
- Kubaski, F.; Osago, H.; Mason, R.W.; Yamaguchi, S.; Kobayashi, H.; Tsuchiya, M.; Orii, T.; Tomatsu, S. Glycosaminoglycans detection methods: Applications of mass spectrometry. Mol. Genet. Metab. 2017, 120, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Langan, T.J.; Jalal, K.; Barczykowski, A.L.; Carter, R.L.; Stapleton, M.; Orii, K.; Fukao, T.; Kobayashi, H.; Yamaguchi, S.; Tomatsu, S. Development of a newborn screening tool for mucopolysaccharidosis type I based on bivariate normal limits: Using glycosaminoglycan and alpha-L-iduronidase determinations on dried blood spots to predict symptoms. JIMD Rep. 2020, 52, 35–42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Y.; Brockmann, K.; Turecek, F.; Scott, C.R.; Gelb, M.H. Tandem mass spectrometry for the direct assay of enzymes in dried blood spots: Application to newborn screening for Krabbe disease. Clin. Chem. 2004, 50, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, M.; Arunkumar, N.; Kubaski, F.; Mason, R.W.; Tadao, O.; Tomatsu, S. Clinical presentation and diagnosis of mucopolysaccharidoses. Mol. Genet. Metab. 2018, 125, 4–17. [Google Scholar] [CrossRef]
- Stapleton, M.; Kubaski, F.; Mason, R.W.; Shintaku, H.; Kobayashi, H.; Yamaguchi, S.; Taketani, T.; Suzuki, Y.; Orii, K.; Orii, T. Newborn screening for mucopolysaccharidoses: Measurement of glycosaminoglycans by LC-MS/MS. Mol. Genet. Metab. Rep. 2020, 22, 100563. [Google Scholar] [CrossRef]
- Tomatsu, S.; Shimada, T.; Mason, R.W.; Montaño, A.M.; Kelly, J.; La Marr, W.A.; Kubaski, F.; Giugliani, R.; Guha, A.; Yasuda, E. Establishment of glycosaminoglycan assays for mucopolysaccharidoses. Metabolites 2014, 4, 655–679. [Google Scholar] [CrossRef]
- de Ruijter, J.; de Ru, M.H.; Wagemans, T.; IJlst, L.; Lund, A.M.; Orchard, P.J.; Schaefer, G.B.; Wijburg, F.A.; van Vlies, N. Heparan sulfate and dermatan sulfate derived disaccharides are sensitive markers for newborn screening for mucopolysaccharidoses types I.; II and III. Mol. Genet. Metab. 2012, 107, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Gelb, M.H.; Scott, C.R.; Turecek, F. Newborn screening for lysosomal storage diseases. Clin. Chem. 2015, 61, 335–346. [Google Scholar] [CrossRef]
- Kellar-Guenther, Y.; McKasson, S.; Hale, K.; Singh, S.; Sontag, M.K.; Ojodu, J. Implementing Statewide Newborn Screening for New Disorders: US Program Experiences. Int. J. Neonatal Screen. 2020, 6, 35. [Google Scholar] [CrossRef]
- Tomatsu, S.; Fujii, T.; Fukushi, M.; Oguma, T.; Shimada, T.; Maeda, M.; Kida, K.; Shibata, Y.; Futatsumori, H.; Montaño, A.M.; et al. Newborn screening and diagnosis of mucopolysaccharidoses. Mol. Genet. Metab. 2013, 110, 42–53. [Google Scholar] [CrossRef]
- Parini, R.; Broomfield, A.; Cleary, M.A.; De Meirleir, L.; Di Rocco, M.; Fathalla, W.M.; Guffon, N.; Lampe, C.; Lund, A.M.; Scarpa, M. International working group identifies need for newborn screening for mucopolysaccharidosis type I but states that existing hurdles must be overcome. Acta Paediatr. 2018, 107, 2059–2065. [Google Scholar] [CrossRef]
- Kubaski, F.; Sousa, I.; Amorim, T.; Pereira, D.; Trometer, J.; Souza, A.; Ranieri, E.; Polo, G.; Burlina, A.; Brusius-Facchin, A.C.; et al. Neonatal Screening for MPS Disorders in Latin America: A Survey of Pilot Initiatives. Int. J. Neonatal Screen. 2020, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Çelik, B.; Tomatsu, S.C.; Tomatsu, S.; Khan, S.A. Epidemiology of Mucopolysaccharidoses Update. Diagnostics 2020, 10, 264. [Google Scholar]
- Burton, B.K.; Charrow, J.; Hoganson, G.E.; Waggoner, D.; Tinkle, B.; Braddock, S.R.; Schneider, M.; Grange, D.K.; Nash, C.; Shryock, H. Newborn screening for lysosomal storage disorders in Illinois: The initial 15-month experience. J. Pediatr. 2017, 190, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.; Buroker, N.; Cournoyer, J.J.; Potier, A.M.; Trometer, J.D.; Elbin, C.; Schermer, M.J.; Kantola, J.; Boyce, A.; Turecek, F. Pilot study of newborn screening for six lysosomal storage diseases using Tandem Mass Spectrometry. Mol. Genet. Metab. 2016, 118, 304–309. [Google Scholar] [CrossRef]
- Hopkins, P.V.; Klug, T.; Vermette, L.; Raburn-Miller, J.; Kiesling, J.; Rogers, S. Incidence of 4 lysosomal storage disorders from 4 years of newborn screening. JAMA Pediatr. 2018, 172, 696–697. [Google Scholar] [CrossRef]
- Wasserstein, M.P.; Caggana, M.; Bailey, S.M.; Desnick, R.J.; Edelmann, L.; Estrella, L.; Holzman, I.; Kelly, N.R.; Kornreich, R.; Kupchik, S.G. The New York pilot newborn screening program for lysosomal storage diseases: Report of the First 65,000 Infants. Genet. Med. 2019, 21, 631–640. [Google Scholar] [CrossRef]
- Arunkumar, N.; Langan, T.J.; Stapleton, M.; Kubaski, F.; Mason, R.W.; Singh, R.; Kobayashi, H.; Yamaguchi, S.; Suzuki, Y.; Orii, K. Newborn screening of mucopolysaccharidoses: Past, present, and future. J. Hum. Genet. 2020, 65, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, P.V.; Campbell, C.; Klug, T.; Rogers, S.; Raburn-Miller, J.; Kiesling, J. Lysosomal storage disorder screening implementation: Findings from the first six months of full population pilot testing in Missouri. J. Pediatr. 2015, 166, 172–177. [Google Scholar] [CrossRef]
- Liu, Y.; Yi, F.; Kumar, A.B.; Kumar Chennamaneni, N.; Hong, X.; Scott, C.R.; Gelb, M.H.; Turecek, F. Multiplex tandem mass spectrometry enzymatic activity assay for newborn screening of the mucopolysaccharidoses and type 2 neuronal ceroid lipofuscinosis. Clin. Chem. 2017, 63, 1118–1126. [Google Scholar] [CrossRef]
- Mashima, R.; Ohira, M.; Okuyama, T.; Tatsumi, A. Quantification of the enzyme activities of iduronate-2-sulfatase, N-acetylgalactosamine-6-sulfatase and N-acetylgalactosamine-4-sulfatase using liquid chromatography-tandem mass spectrometry. Mol. Genet. Metab. Rep. 2018, 14, 36–40. [Google Scholar] [CrossRef]
- Oguni, T.; Tomatsu, S.; Tanaka, M.; Orii, K.; Fukao, T.; Watanabe, J.; Fukuda, S.; Notsu, Y.; Vu, D.C.; Can, T.B.N. Validation of Liquid Chromatography-Tandem Mass Spectrometry-Based 5-Plex Assay for Mucopolysaccharidoses. Int. J. Mol. Sci. 2020, 21, 2025. [Google Scholar] [CrossRef]
- Pollard, L.; Wood, T. Multiplex DBS enzyme assay for MPS II, IIIB, IVA, VI, VII and CLN2 via LC-MS/MS expands clinical utility of DBS enzyme testing. Mol. Genet. Metab. 2019, 126, S119. [Google Scholar] [CrossRef]
- Sista, R.S.; Wang, T.; Wu, N.; Graham, C.; Eckhardt, A.; Winger, T.; Srinivasan, V.; Bali, D.; Millington, D.S.; Pamula, V.K. Multiplex newborn screening for Pompe, Fabry, Hunter, Gaucher, and Hurler diseases using a digital microfluidic platform. Clin. Chim. Acta 2013, 424, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Mason, R.W.; Kobayashi, H.; Yamaguchi, S.; Tomatsu, S. Advances in glycosaminoglycan detection. Mol. Genet. Metab. 2020, 130, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Oguma, T.; Tomatsu, S.; Okazaki, O. Analytical method for determination of disaccharides derived from keratan sulfates in human serum and plasma by high-performance liquid chromatography/turbo-ionspray ionization tandem mass spectrometry. Biomed. Chromatogr. 2007, 21, 356–362. [Google Scholar] [CrossRef]
- Oguma, T.; Tomatsu, S.; Montano, A.M.; Okazaki, O. Analytical method for the determination of disaccharides derived from keratan, heparan, and dermatan sulfates in human serum and plasma by high-performance liquid chromatography/turbo ionspray ionization tandem mass spectrometry. Anal. Biochem. 2007, 368, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Herbst, Z.M.; Urdaneta, L.; Klein, T.; Fuller, M.; Gelb, M.H. Evaluation of Multiple Methods for Quantification of Glycosaminoaminoglycan Biomarkers in Newborn Dried Blood Spots from Patients with Severe and Attenuated Mucopolysaccharidosis-I. Int. J. Neonat. Screen. 2020, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Auray-Blais, C.; Lavoie, P.; Zhang, H.; Gagnon, R.; Clarke, J.T.R.; Maranda, B.; Young, S.P.; An, Y.; Millington, D.S. An improved method for glycosaminoglycan analysis by LC–MS/MS of urine samples collected on filter paper. Clin. Chim. Acta 2012, 413, 771–778. [Google Scholar] [CrossRef]
- Tanaka, N.; Kida, S.; Kinoshita, M.; Morimoto, H.; Shibasaki, T.; Tachibana, K.; Yamamoto, R. Evaluation of cerebrospinal fluid heparan sulfate as a biomarker of neuropathology in a murine model of mucopolysaccharidosis type II using high-sensitivity LC/MS/MS. Mol. Genet. Metab. 2018, 125, 53–58. [Google Scholar] [CrossRef]
- Trim, P.J.; Lau, A.A.; Hopwood, J.J.; Snel, M.F. A simple method for early age phenotype confirmation using toe tissue from a mouse model of MPS IIIA. Rapid Commun. Mass Spectrom. 2014, 28, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wood, T.; Young, S.P.; Millington, D.S. A straightforward, quantitative ultra-performance liquid chromatography-tandem mass spectrometric method for heparan sulfate, dermatan sulfate and chondroitin sulfate in urine: An improved clinical screening test for the mucopolysaccharidoses. Mol. Genet. Metab. 2015, 114, 123–128. [Google Scholar] [CrossRef]
- Zhang, H.; Young, S.P.; Millington, D.S. Quantification of glycosaminoglycans in urine by isotope-dilution liquid chromatography-electrospray ionization tandem mass spectrometry. Curr. Protoc. Hum. Genet. 2013, 76, 12–17. [Google Scholar] [CrossRef]
- Zhang, H.; Young, S.P.; Auray-Blais, C.; Orchard, P.J.; Tolar, J.; Millington, D.S. Analysis of glycosaminoglycans in cerebrospinal fluid from patients with mucopolysaccharidoses by isotope-dilution ultra-performance liquid chromatography–tandem mass spectrometry. Clin. Chem. 2011, 57, 1005–1012. [Google Scholar] [CrossRef]
- Forni, G.; Malvagia, S.; Funghini, S.; Scolamiero, E.; Mura, M.; Della Bona, M.; Villanelli, F.; Damiano, R.; la Marca, G. LC-MS/MS method for simultaneous quantification of heparan sulfate and dermatan sulfate in urine by butanolysis derivatization. Clin. Chim. Acta 2019, 488, 98–103. [Google Scholar] [CrossRef]
- Trim, P.J.; Hopwood, J.J.; Snel, M.F. Butanolysis derivatization: Improved sensitivity in LC-MS/MS quantitation of heparan sulfate in urine from mucopolysaccharidosis patients. Anal. Chem. 2015, 87, 9243–9250. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.; Brown, J.R.; Al-Mafraji, K.; Lamanna, W.C.; Beitel, J.R.; Boons, G.-J.; Esko, J.D.; Crawford, B.E. Disease-specific non–reducing end carbohydrate biomarkers for mucopolysaccharidoses. Nat. Chem. Biol. 2012, 8, 197–204. [Google Scholar] [CrossRef]
- Kubaski, F.; Suzuki, Y.; Orii, K.; Giugliani, R.; Church, H.J.; Mason, R.W.; Dũng, V.C.; Ngoc, C.T.B.; Yamaguchi, S.; Kobayashi, H. Glycosaminoglycan levels in dried blood spots of patients with mucopolysaccharidoses and mucolipidoses. Mol. Genet. Metab. 2017, 120, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, S.; Shimada, T.; Mason, R.W.; Kelly, J.; LaMarr, W.A.; Yasuda, E.; Shibata, Y.; Futatsumori, H.; Montaño, A.M.; Yamaguchi, S. Assay for glycosaminoglycans by tandem mass spectrometry and its applications. J. Anal. Bioanal. Tech. 2014, 2014, 6. [Google Scholar]
- Hintze, J.P.; Tomatsu, S.; Fujii, T.; Montaño, A.M.; Yamaguchi, S.; Suzuki, Y.; Fukushi, M.; Ishimaru, T.; Orii, T. Comparison of liquid chromatography-tandem mass spectrometry and sandwich ELISA for determination of keratan sulfate in plasma and urine. Biomark Insights 2011, 6, 69–78. [Google Scholar] [CrossRef]
- Burin, M.G.; Scholz, A.P.; Gus, R.; Sanseverino, M.T.V.; Fritsh, A.; Magalhaes, J.A.; Timm, F.; Barrios, P.; Chesky, M.; Coelho, J.C. Investigation of lysosomal storage diseases in nonimmune hydrops fetalis. Prenat. Diagn. 2004, 24, 653–657. [Google Scholar] [CrossRef]
- Kubaski, F.; Brusius-Facchin, A.C.; Mason, R.W.; Patel, P.; Burin, M.G.; Michelin-Tirelli, K.; Kessler, R.G.; Bender, F.; Leistner-Segal, S.; Moreno, C.A. Elevation of glycosaminoglycans in the amniotic fluid of a fetus with mucopolysaccharidosis VII. Prenat. Diagn. 2017, 37, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.; Braun, S.; Coerdt, W.; Merz, E.; Young, E.; Sewell, A.C. Fetal presentation of Morquio disease type A. Prenat Diagn. 1992, 12, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.B.; Masi, S.; Ghomashchi, F.; Chennamaneni, N.K.; Ito, M.; Scott, C.R.; Turecek, F.; Gelb, M.H.; Spacil, Z. Tandem mass spectrometry has a larger analytical range than fluorescence assays of lysosomal enzymes: Application to newborn screening and diagnosis of mucopolysaccharidoses types II, IVA, and VI. Clin. Chem. 2015, 61, 1363–1371. [Google Scholar] [CrossRef]
- Tomatsu, S.; Okamura, K.; Maeda, H.; Taketani, T.; Castrillon, S.V.; Gutierrez, M.A.; Nishioka, T.; Fachel, A.A.; Orii, K.O.; Grubb, J.H. Keratan sulphate levels in mucopolysaccharidoses and mucolipidoses. J. Inherit. Metab. Dis. 2005, 28, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, S.; Montaño, A.M.; Oguma, T.; Dung, V.C.; Oikawa, H.; Gutiérrez, M.L.; Yamaguchi, S.; Suzuki, Y.; Fukushi, M.; Barrera, L.A. Validation of disaccharide compositions derived from dermatan sulfate and heparan sulfate in mucopolysaccharidoses and mucolipidoses II and III by tandem mass spectrometry. Mol. Genet. Metab. 2010, 99, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Velho, R.V.; Harms, F.L.; Danyukova, T.; Ludwig, N.F.; Friez, M.J.; Cathey, S.S.; Filocamo, M.; Tappino, B.; Güneş, N.; Tüysüz, B. The lysosomal storage disorders mucolipidosis type II, type III alpha/beta, and type III gamma: Update on GNPTAB and GNPTG mutations. Hum. Mutat. 2019, 40, 842–864. [Google Scholar]
- Yang, M.; Cho, S.Y.; Park, H.-D.; Choi, R.; Kim, Y.-E.; Kim, J.; Lee, S.-Y.; Ki, C.-S.; Kim, J.-W.; Sohn, Y.B. Clinical, biochemical and molecular characterization of Korean patients with mucolipidosis II/III and successful prenatal diagnosis. Orphanet J. Rare Dis. 2017, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, M.F.; Lacerda, L.; Alves, S. Glycosaminoglycan storage disorders: A review. Biochem. Res. Int. 2012, 2012, 471325. [Google Scholar] [CrossRef]
- Lehman, T.J.A.; Miller, N.; Norquist, B.; Underhill, L.; Keutzer, J. Diagnosis of the mucopolysaccharidoses. Rheumatology 2011, 50, v41–v48. [Google Scholar] [CrossRef]
- Osago, H.; Shibata, T.; Hara, N.; Kuwata, S.; Kono, M.; Uchio, Y.; Tsuchiya, M. Quantitative analysis of glycosaminoglycans, chondroitin/dermatan sulfate, hyaluronic acid, heparan sulfate, and keratan sulfate by liquid chromatography–electrospray ionization–tandem mass spectrometry. Anal. Biochem. 2014, 467, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Rowan, D.J.; Tomatsu, S.; Grubb, J.H.; Montaño, A.M.; Sly, W.S. Assessment of bone dysplasia by micro-CT and glycosaminoglycan levels in mouse models for mucopolysaccharidosis type I.; IIIA, IVA, and VII. J. Inherit. Metab. Dis. 2013, 36, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, K.A.; Pollard, L.M.; Cathey, S.; Wood, T. Measurement of elevated concentrations of urine Keratan sulfate by UPLC-MSMS in lysosomal storage disorders (LSDs): Comparison of urine Keratan sulfate levels in MPS IVA versus other LSDs. JIMD Rep. 2016, 34, 11–18. [Google Scholar] [PubMed]
- Fujitsuka, H.; Sawamoto, K.; Peracha, H.; Mason, R.W.; Mackenzie, W.; Kobayashi, H.; Yamaguchi, S.; Suzuki, Y.; Orii, K.; Orii, T. Biomarkers in patients with mucopolysaccharidosis type II and IV. Mol. Genet. Metab. Rep. 2019, 19, 100455. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Mason, R.W.; Giugliani, R.; Orii, K.; Fukao, T.; Suzuki, Y.; Yamaguchi, S.; Kobayashi, H.; Orii, T.; Tomatsu, S. Glycosaminoglycans analysis in blood and urine of patients with mucopolysaccharidosis. Mol. Genet. Metab. 2018, 125, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Tomatsu, S.; Mason, R.W.; Yasuda, E.; Mackenzie, W.G.; Hossain, J.; Shibata, Y.; Montaño, A.M.; Kubaski, F.; Giugliani, R.; et al. Di-sulfated Keratan Sulfate as a Novel Biomarker for Mucopolysaccharidosis II, IVA, and IVB. JIMD Rep. 2011, 21, 1–13. [Google Scholar]
- Burlina, A.B.; Polo, G.; Rubert, L.; Gueraldi, D.; Cazzorla, C.; Duro, G.; Salviati, L.; Burlina, A.P. Implementation of second-tier tests in newborn screening for lysosomal disorders in North Eastern Italy. Int. J. Neonatal. Screen. 2019, 5, 24. [Google Scholar] [CrossRef]
- Hall, P.L.; Sanchez, R.; Hagar, A.F.; Jerris, S.C.; Wittenauer, A.; Wilcox, W.R. Two-tiered newborn screening with post-analytical tools for Pompe disease and mucopolysaccharidosis type I results in performance improvement and future direction. Int. J. neonat. Screen. 2020, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-K.; Lin, H.-Y.; Wang, T.-J.; Huang, Y.-H.; Chan, M.-J.; Liao, H.-C.; Lo, Y.-T.; Wang, L.-Y.; Tu, R.-Y.; Fang, Y.-Y. Status of newborn screening and follow up investigations for Mucopolysaccharidoses I and II in Taiwan. Orphanet J. Rare Dis. 2018, 13, 1–14. [Google Scholar] [CrossRef]
- Burlina, A.B.; Polo, G.; Salviati, L.; Duro, G.; Zizzo, C.; Dardis, A.; Bembi, B.; Cazzorla, C.; Rubert, L.; Zordan, R. Newborn screening for lysosomal storage disorders by tandem mass spectrometry in North East Italy. J. Inherit. Metab. Dis. Off. J. Soc. Study Inborn Errors Metab. 2018, 41, 209–219. [Google Scholar] [CrossRef]
- Baerg, M.M.M.; Stoway, S.D.; Hart, J.; Mott, L.; Peck, D.S.; Nett, S.L.; Eckerman, J.S.; Lacey, J.M.; Turgeon, C.T.; Gavrilov, D. Precision newborn screening for lysosomal disorders. Genet. Med. 2018, 20, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.R.; Elliott, S.; Hong, X.; Huang, J.-Y.; Kumar, A.B.; Yi, F.; Pendem, N.; Chennamaneni, N.K.; Gelb, M.H. Newborn screening for mucopolysaccharidoses: Results of a pilot study with 100,000 dried blood spots. J. Pediatr. 2020, 216, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Brusius-Facchin, A.C.; Siebert, M.; Leão, D.; Malaga, D.R.; Pasqualim, G.; Trapp, F.; Matte, U.; Giugliani, R.; Leistner-Segal, S. Phenotype-oriented NGS panels for mucopolysaccharidoses: Validation and potential use in the diagnostic flowchart. Genet. Mol. Biol. 2020, 42, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chopra, S.; Graham, C.; Nelson, L.; Ng, R.; Nuffer, M.; Vadlamani, P.; Norton, S.; Pamula, V. Demonstration of a digital microfluidic platform for the high throughput analysis of 12 discrete fluorimetric enzyme assays using a single newborn dried blood spot punch. Mol. Genet. Metab. 2018, 123, S132. [Google Scholar] [CrossRef]

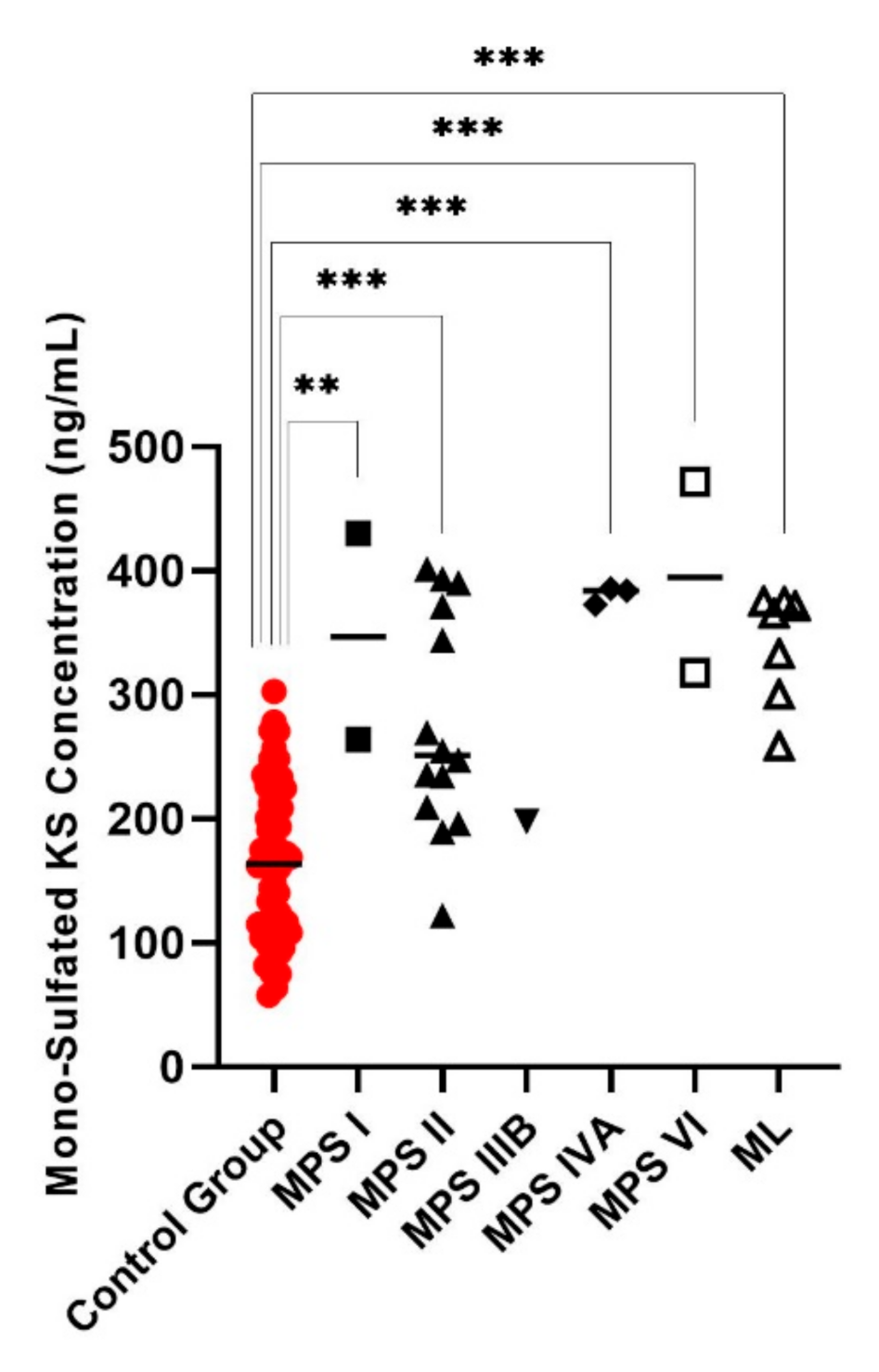
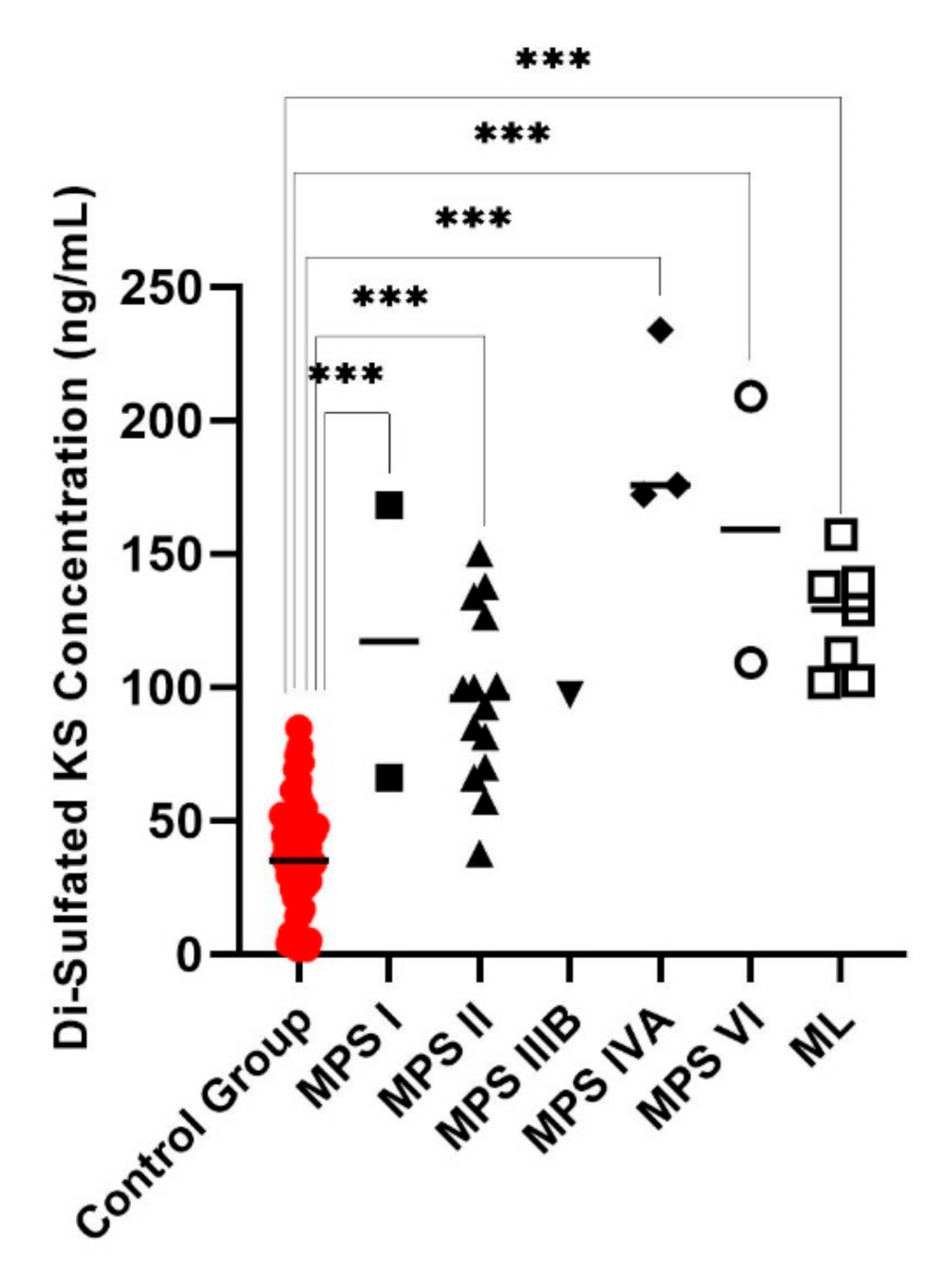
| Case | Sex | Age | GAG Concentration (ng/mL) | Enzyme Activity ( μmol/hr/L) | Diagnosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DS | HS0S | HSNS | monoKS | diKS | Di/total KS % | MPS-I | MPS-II | MPS-IIIB | MPS-IVA | MPS-VI | ||||
| 1 | 2 | 8.1 | 31.9 | 42.0 | 7.4 | 374.6 | 112.9 | 23.2 | 9.34 | 103.74 | 21.81 | 0.96 | 6.70 | ML, High KS and enzyme activities |
| 2 | 2 | 9.3 | 13.9 | 35.5 | 5.3 | 371.5 | 157.2 | 29.7 | 5.61 | 132.16 | 24.79 | 1.03 | 5.77 | ML, High KS and enzyme activities |
| 3 | 1 | 8.5 | 46.0 | 36.0 | 5.0 | 300.4 | 102.3 | 25.4 | 3.54 | 198.48 | 35.03 | 1.81 | 5.52 | ML, High KS and enzyme activities |
| 4 | 2 | 2.2 | 34.9 | 28.7 | 3.4 | 365.8 | 139.3 | 27.6 | 11.22 | 141.07 | 34.59 | 0.85 | 5.45 | ML, High KS and enzyme activities |
| 5 | 2 | 4.8 | 29.1 | 28.5 | 3.3 | 375.2 | 137.6 | 26.8 | 14.05 | 103.87 | 34.48 | 0.78 | 4.46 | ML, High KS and enzyme activities |
| 6 | 1 | 9.6 | 39.8 | 54.4 | 12.0 | 333.1 | 129.1 | 27.9 | 14.46 | 112.96 | 26.32 | 0.85 | 6.99 | ML, High KS and enzyme activities |
| 7 | 1 | 9.8 | 121.8 | 142.9 | 23.0 | 430.1 | 168.3 | 28.1 | 0.44 | 2.88 | 3.06 | 0.17 | 0.67 | MPS I |
| 8 | 1 | 0.9 | 130.8 | 125.6 | 20.8 | 263.9 | 66.2 | 20.1 | 0.41 | 5.44 | 2.57 | 0.43 | 1.36 | MPS I |
| 9 | 1 | 4 | 58.1 | 125.9 | 18.7 | 254.6 | 100.6 | 28.3 | 5.89 | 0.41 | 3.81 | 0.79 | 2.11 | MPS II |
| 10 | 1 | 2.5 | 119.2 | 112.2 | 15.9 | 247.3 | 70.0 | 22.1 | 5.91 | 0.08 | 4.40 | 0.66 | 2.50 | MPS II |
| 11 | 1 | 0.8 | 72.7 | 175.6 | 23.2 | 196.0 | 37.9 | 16.2 | 5.16 | 0.06 | 3.99 | 1.75 | 3.13 | MPS II |
| 12 | 1 | 5.1 | 41.9 | 96.6 | 13.5 | 235.6 | 99.4 | 29.7 | 3.72 | 0.11 | 3.70 | 0.29 | 1.08 | MPS II |
| 13 | 1 | 3.8 | 73.2 | 73.7 | 8.9 | 121.9 | 66.2 | 35.2 | 2.00 | 0.05 | 2.38 | 0.30 | 0.88 | MPS II |
| 14 | 1 | 2.5 | 162.1 | 143.5 | 22.9 | 234.3 | 85.2 | 26.7 | 3.51 | 0.05 | 5.12 | 0.35 | 1.06 | MPS II |
| 15 | 1 | 4.6 | 72.8 | 170.2 | 24.7 | 371.3 | 126.3 | 25.4 | 2.63 | 0.05 | 6.80 | 0.35 | 1.09 | MPS II |
| 16 | 1 | 5.5 | 92.6 | 179.9 | 29.2 | 344.0 | 99.9 | 22.5 | 3.28 | 0.06 | 2.22 | 0.29 | 0.90 | MPS II |
| 17 | 1 | 1.6 | 95.1 | 277.8 | 36.6 | 269.5 | 92.8 | 25.6 | 4.75 | 0.08 | 8.20 | 0.31 | 1.01 | MPS II |
| 18 | 1 | 5 | 156.9 | 209.9 | 36.1 | 393.7 | 134.1 | 25.4 | 3.23 | 0.06 | 4.07 | 0.34 | 1.19 | MPS II |
| 19 | 1 | 5 | 92.3 | 112.4 | 19.8 | 390.2 | 137.9 | 26.1 | 3.69 | 0.06 | 5.63 | 0.42 | 1.19 | MPS II |
| 20 | 1 | 1.1 | 52.3 | 136.8 | 19.9 | 189.3 | 57.3 | 23.2 | 8.71 | 0.20 | 5.57 | 0.82 | 1.84 | MPS II |
| 21 | 1 | 2.7 | 67.1 | 186.8 | 25.0 | 209.3 | 81.5 | 28.0 | 7.69 | 0.19 | 8.56 | 1.02 | 2.03 | MPS II |
| 22 | 1 | 3.9 | 8.6 | 202.2 | 26.5 | 197.7 | 97.2 | 33.0 | 4.42 | 6.51 | 0.05 | 0.47 | 1.59 | MPS IIIB |
| 23 | 2 | 5.9 | 37.7 | 50.6 | 8.1 | 372.5 | 172.1 | 31.6 | 2.35 | 6.47 | 2.76 | 0.00 | 1.01 | MPS IVA |
| 24 | 2 | 11.9 | 55.3 | 38.4 | 5.7 | 385.6 | 175.7 | 31.3 | 1.36 | 4.13 | 2.00 | 0.00 | 0.65 | MPS IVA |
| 25 | 1 | 6.4 | 22.8 | 39.9 | 8.1 | 383.9 | 233.9 | 37.9 | 2.30 | 3.74 | 2.26 | 0.00 | 1.17 | MPS IVA |
| 26 | 1 | 2.9 | 72.6 | 48.4 | 6.7 | 317.8 | 109.3 | 25.6 | 3.42 | 8.13 | 3.87 | 0.57 | 0.00 | MPS VI |
| 27 | 2 | 7.1 | 50.8 | 31.9 | 4.7 | 472.0 | 209.1 | 30.7 | 1.86 | 6.53 | 2.27 | 0.13 | 0.00 | MPS VI |
| Age (Years) | N | Mean (ng/mL) | SD (ng/mL) | Cut off (ng/mL) |
|---|---|---|---|---|
| 0–4.9 | 26 | 185.6 | 62.8 | 311.34 |
| 5.0–10.0 | 27 | 147.5 | 54.5 | 256.63 |
| 10.0–15.0 | 33 | 136.3 | 62.3 | 260.84 |
| 15.0–20.0 | 5 | 76.3 | 42.8 | 161.79 |
| over 20 | 6 | 60 | 34 | 127.99 |
| Age (Years) | N | Mean (ng/mL) | SD (ng/mL) | Cut off (ng/mL) |
|---|---|---|---|---|
| 0–4.9 | 26 | 28.8 | 18.9 | 66.6 |
| 5.0–10.0 | 27 | 41.5 | 17.8 | 77.1 |
| 10.0–15.0 | 33 | 39 | 16.1 | 71.3 |
| 15.0–20.0 | 5 | 18.7 | 10 | 38.7 |
| over 20 | 6 | 20.2 | 18 | 56.2 |
| Age (Years) | N | Mean (%) | SD (%) | Cut off (%) |
|---|---|---|---|---|
| 0–4.9 | 27 | 13.6 | 7.4 | 28.29 |
| 5.0–10.0 | 23 | 21.8 | 4.5 | 30.81 |
| 10.0–15.0 | 34 | 22.3 | 4.58 | 31.46 |
| 15.0–20.0 | 5 | 20.1 | 3.76 | 27.57 |
| over 20 | 6 | 23.9 | 9.74 | 43.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arunkumar, N.; Vu, D.C.; Khan, S.; Kobayashi, H.; Ngoc Can, T.B.; Oguni, T.; Watanabe, J.; Tanaka, M.; Yamaguchi, S.; Taketani, T.; et al. Diagnosis of Mucopolysaccharidoses and Mucolipidosis by Assaying Multiplex Enzymes and Glycosaminoglycans. Diagnostics 2021, 11, 1347. https://doi.org/10.3390/diagnostics11081347
Arunkumar N, Vu DC, Khan S, Kobayashi H, Ngoc Can TB, Oguni T, Watanabe J, Tanaka M, Yamaguchi S, Taketani T, et al. Diagnosis of Mucopolysaccharidoses and Mucolipidosis by Assaying Multiplex Enzymes and Glycosaminoglycans. Diagnostics. 2021; 11(8):1347. https://doi.org/10.3390/diagnostics11081347
Chicago/Turabian StyleArunkumar, Nivethitha, Dung Chi Vu, Shaukat Khan, Hironori Kobayashi, Thi Bich Ngoc Can, Tsubasa Oguni, Jun Watanabe, Misa Tanaka, Seiji Yamaguchi, Takeshi Taketani, and et al. 2021. "Diagnosis of Mucopolysaccharidoses and Mucolipidosis by Assaying Multiplex Enzymes and Glycosaminoglycans" Diagnostics 11, no. 8: 1347. https://doi.org/10.3390/diagnostics11081347
APA StyleArunkumar, N., Vu, D. C., Khan, S., Kobayashi, H., Ngoc Can, T. B., Oguni, T., Watanabe, J., Tanaka, M., Yamaguchi, S., Taketani, T., Ago, Y., Ohnishi, H., Saikia, S., Álvarez, J. V., & Tomatsu, S. (2021). Diagnosis of Mucopolysaccharidoses and Mucolipidosis by Assaying Multiplex Enzymes and Glycosaminoglycans. Diagnostics, 11(8), 1347. https://doi.org/10.3390/diagnostics11081347







