Surgically Induced Contrast Enhancements on Intraoperative and Early Postoperative MRI Following High-Grade Glioma Surgery: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Study Characteristics
3.2. Results of Individual Studies
3.2.1. Studies on Intraoperative MRI
3.2.2. Studies on Early Postoperative MRI within 72 h
3.2.3. Studies on Postoperative MRI beyond 72 h
3.3. Bias Assessment
4. Discussion
4.1. Limitations of Included Studies
4.2. Limitations of This Review
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Author | Reason for Exclusion |
|---|---|
| Aprile et al., (2008) [37] | Only perfusion MRI |
| Belhawi et al., (2010) [8] | Only T2-weighted/FLAIR MRI |
| Boyett et al., (2019) [38] | Conference abstract |
| Brochado et al., (2012) [39] | Conference abstract |
| Colen et al., (2012) [40] | Abstract only |
| Fei et al., (2020) [41] | Only diffusion/perfusion MRI |
| Finck et al., (2020) [42] | Only black-blood MRI sequence |
| Florez et al., (2020) [43] | Conference abstract |
| Garcia-Ruiz et al., (2021) [32] | No data for patterns of contrast enhancements |
| Heßelmann et al., (2017) [44] | No data for patterns of contrast enhancements |
| Lescher et al., (2016) [45] | Only FLAIR MRI |
| Lescher et al., (2014) [46] | Conference abstract |
| Majos et al., (2016) [5] | No data for patterns of contrast enhancements |
| Martin et al., (2000) [47] | No data for patterns of contrast enhancements |
| Özduman et al., (2014) [48] | Only dynamic contrast enhanced (DCE)-MRI |
Appendix B
| Authors | Enhancement Patterns | |||||
|---|---|---|---|---|---|---|
| Meningeal | Increased Choroid Plexus | Intraparenchymal | Enhancement Ring | Nodular | Frayed | |
| Wirtz et al. [29] | X | X | X | |||
| Knauth et al. [30] | X | X | X | |||
| Smets et al. [26] | X | X | ||||
| Ekinci et al. [15] | X | |||||
| Bette et al. [6] | X | X | ||||
| Lescher et al. [16] | X | |||||
| Sui et al. [27] | X | X | ||||
| Forsyth et al. [31] | X | |||||
References
- Gandhi, S.; Tayebi Meybodi, A.; Belykh, E.; Cavallo, C.; Zhao, X.; Syed, M.P.; Borba Moreira, L.; Lawton, M.T.; Nakaji, P.; Preul, M.C. Survival Outcomes Among Patients with High-Grade Glioma Treated with 5-Aminolevulinic Acid-Guided Surgery: A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and Molecular Prognostic Review of Glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef]
- Stummer, W.; Reulen, H.-J.; Meinel, T.; Pichlmeier, U.; Schumacher, W.; Tonn, J.-C.; Rohde, V.; Oppel, F.; Turowski, B.; Woiciechowsky, C.; et al. Extent of Resection and Survival in Glioblastoma Multiforme: Identification of and Adjustment for Bias. Neurosurgery 2008, 62, 564–576. [Google Scholar] [CrossRef]
- Grabowski, M.M.; Recinos, P.F.; Nowacki, A.S.; Schroeder, J.L.; Angelov, L.; Barnett, G.H.; Vogelbaum, M.A. Residual Tumor Volume versus Extent of Resection: Predictors of Survival after Surgery for Glioblastoma. J. Neurosurg. 2014, 121, 1115–1123. [Google Scholar] [CrossRef]
- Majos, C.; Cos, M.; Castaner, S.; Gil, M.; Plans, G.; Lucas, A.; Bruna, J.; Aguilera, C. Early Post-Operative Magnetic Resonance Imaging in Glioblastoma: Correlation among Radiological Findings and Overall Survival in 60 Patients. Eur. Radiol. 2016, 26, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Bette, S.; Gempt, J.; Huber, T.; Boeckh-Behrens, T.; Ringel, F.; Meyer, B.; Zimmer, C.; Kirschke, J.S. Patterns and Time Dependence of Unspecific Enhancement in Postoperative Magnetic Resonance Imaging After Glioblastoma Resection. World Neurosurg. 2016, 90, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, S.; Braga, T.A.; Barker, F.G.; Lev, M.H.; Gonzalez, R.G.; Henson, J.W. Clinical and Radiographic Features of Peritumoral Infarction Following Resection of Glioblastoma. Neurology 2006, 67, 1668–1670. [Google Scholar] [CrossRef] [PubMed]
- Belhawi, S.M.K.; Hoefnagels, F.W.A.; Baaijen, J.C.; Aliaga, E.S.; Reijneveld, J.C.; Heimans, J.J.; Barkhof, F.; Vandertop, W.P.; Hamer, P.C.D.W. Early Postoperative MRI Overestimates Residual Tumour after Resection of Gliomas with No or Minimal Enhancement. Eur. Radiol. 2011, 21, 1526–1534. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Jost, S.; Aghi, M.K.; Heimberger, A.B.; Sampson, J.H.; Wen, P.Y.; Macdonald, D.R.; Van den Bent, M.J.; Chang, S.M. Application of Novel Response/Progression Measures for Surgically Delivered Therapies for Gliomas: Response Assessment in Neuro-Oncology (RANO) Working Group. Neurosurgery 2012, 70, 234–243. [Google Scholar] [CrossRef]
- Thust, S.C.; Heiland, S.; Falini, A.; Jäger, H.R.; Waldman, A.D.; Sundgren, P.C.; Godi, C.; Katsaros, V.K.; Ramos, A.; Bargallo, N.; et al. Glioma Imaging in Europe: A Survey of 220 Centres and Recommendations for Best Clinical Practice. Eur. Radiol. 2018, 28, 3306–3317. [Google Scholar] [CrossRef]
- Albert, F.K.; Forsting, M.; Sartor, K.; Adams, H.P.; Kunze, S. Early Postoperative Magnetic Resonance Imaging after Resection of Malignant Glioma: Objective Evaluation of Residual Tumor and Its Influence on Regrowth and Prognosis. Neurosurgery 1994, 34, 45–60. [Google Scholar] [CrossRef]
- Forsting, M.; Albert, F.K.; Kunze, S.; Adams, H.P.; Zenner, D.; Sartor, K. Extirpation of Glioblastomas: MR and CT Follow-up of Residual Tumor and Regrowth Patterns. AJNR Am. J. Neuroradiol. 1993, 14, 77–87. [Google Scholar]
- Sato, N.; Bronen, R.A.; Sze, G.; Kawamura, Y.; Coughlin, W.; Putman, C.M.; Spencer, D.D. Postoperative Changes in the Brain: MR Imaging Findings in Patients without Neoplasms. Radiology 1997, 204, 839–846. [Google Scholar] [CrossRef]
- Henegar, M.M.; Moran, C.J.; Silbergeld, D.L. Early Postoperative Magnetic Resonance Imaging Following Nonneoplastic Cortical Resection. J. Neurosurg. 1996, 84, 174–179. [Google Scholar] [CrossRef]
- Ekinci, G.; Akpinar, I.N.; Baltacioğlu, F.; Erzen, C.; Kiliç, T.; Elmaci, I.; Pamir, N. Early-Postoperative Magnetic Resonance Imaging in Glial Tumors: Prediction of Tumor Regrowth and Recurrence. Eur. J. Radiol. 2003, 45, 99–107. [Google Scholar] [CrossRef]
- Lescher, S.; Schniewindt, S.; Jurcoane, A.; Senft, C.; Hattingen, E. Time Window for Postoperative Reactive Enhancement after Resection of Brain Tumors: Less than 72 h. Neurosurg. Focus 2014, 37, E3. [Google Scholar] [CrossRef] [PubMed]
- Knauth, M.; Wirtz, C.R.; Tronnier, V.M.; Aras, N.; Kunze, S.; Sartor, K. Intraoperative MR Imaging Increases the Extent of Tumor Resection in Patients with High-Grade Gliomas. AJNR Am. J. Neuroradiol. 1999, 20, 1642–1646. [Google Scholar] [PubMed]
- Kuhnt, D.; Becker, A.; Ganslandt, O.; Bauer, M.; Buchfelder, M.; Nimsky, C. Correlation of the Extent of Tumor Volume Resection and Patient Survival in Surgery of Glioblastoma Multiforme with High-Field Intraoperative MRI Guidance. Neuro-Oncology 2011, 13, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.P.; Trantakis, C.; Rubach, M.; Schulz, T.; Dietrich, J.; Winkler, D.; Renner, C.; Schober, R.; Geiger, K.; Brosteanu, O.; et al. Intraoperative MRI to Guide the Resection of Primary Supratentorial Glioblastoma Multiforme—A Quantitative Radiological Analysis. Neuroradiology 2005, 47, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Senft, C.; Bink, A.; Franz, K.; Vatter, H.; Gasser, T.; Seifert, V. Intraoperative MRI Guidance and Extent of Resection in Glioma Surgery: A Randomised, Controlled Trial. Lancet Oncol. 2011, 12, 997–1003. [Google Scholar] [CrossRef]
- Miskin, N.; Unadkat, P.; Carlton, M.E.; Golby, A.J.; Young, G.S.; Huang, R.Y. Frequency and Evolution of New Postoperative Enhancement on 3 Tesla Intraoperative and Early Postoperative Magnetic Resonance Imaging. Neurosurgery 2019, 87, 238–246. [Google Scholar] [CrossRef]
- Zaidi, H.A.; Chowdhry, S.A.; Wilson, D.A.; Spetzler, R.F. The Dilemma of Early Postoperative Magnetic Resonance Imaging: When Efficiency Compromises Accuracy: Case Report. Neurosurgery 2014, 74, E335–E340. [Google Scholar] [CrossRef]
- Lara-Velazquez, M.; Al-Kharboosh, R.; Jeanneret, S.; Vazquez-Ramos, C.; Mahato, D.; Tavanaiepour, D.; Rahmathulla, G.; Quinones-Hinojosa, A. Advances in Brain Tumor Surgery for Glioblastoma in Adults. Brain Sci. 2017, 7, 166. [Google Scholar] [CrossRef]
- Eriksen, M.B.; Frandsen, T.F. The Impact of Patient, Intervention, Comparison, Outcome (PICO) as a Search Strategy Tool on Literature Search Quality: A Systematic Review. J. Med. Libr. Assoc. JMLA 2018, 106, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Smets, T.; Lawson, T.M.; Grandin, C.; Jankovski, A.; Raftopoulos, C. Immediate Post-Operative MRI Suggestive of the Site and Timing of Glioblastoma Recurrence after Gross Total Resection: A Retrospective Longitudinal Preliminary Study. Eur. Radiol. 2013, 23, 1467–1477. [Google Scholar] [CrossRef]
- Sui, Z.; Zhang, X.; Li, H.; Xu, D.; Li, G. Magnetic Resonance Imaging Evaluation of Brain Glioma before Postoperative Radiotherapy. Clin. Transl. Oncol. 2020. [Google Scholar] [CrossRef]
- Masuda, Y.; Akutsu, H.; Ishikawa, E.; Matsuda, M.; Masumoto, T.; Hiyama, T.; Yamamoto, T.; Kohzuki, H.; Takano, S.; Matsumura, A. Evaluation of the Extent of Resection and Detection of Ischemic Lesions with Intraoperative MRI in Glioma Surgery: Is Intraoperative MRI Superior to Early Postoperative MRI? J. Neurosurg. 2019, 131, 209–216. [Google Scholar] [CrossRef]
- Wirtz, C.R.; Knauth, M.; Staubert, A.; Bonsanto, M.M.; Sartor, K.; Kunze, S.; Tronnier, V.M. Clinical Evaluation and Follow-up Results for Intraoperative Magnetic Resonance Imaging in Neurosurgery. Neurosurgery 2000, 46, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Knauth, M.; Aras, N.; Wirtz, C.R.; Dörfler, A.; Engelhorn, T.; Sartor, K. Surgically Induced Intracranial Contrast Enhancement: Potential Source of Diagnostic Error in Intraoperative MR Imaging. AJNR Am. J. Neuroradiol. 1999, 20, 1547–1553. [Google Scholar] [PubMed]
- Forsyth, P.A.; Petrov, E.; Mahallati, H.; Cairncross, J.G.; Brasher, P.; MacRae, M.E.; Hagen, N.A.; Barnes, P.; Sevick, R.J. Prospective Study of Postoperative Magnetic Resonance Imaging in Patients with Malignant Gliomas. J. Clin. Oncol. 1997, 15, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, A.; Naval-Baudin, P.; Ligero, M.; Pons-Escoda, A.; Bruna, J.; Plans, G.; Calvo, N.; Cos, M.; Majós, C.; Perez-Lopez, R. Precise Enhancement Quantification in Post-Operative MRI as an Indicator of Residual Tumor Impact Is Associated with Survival in Patients with Glioblastoma. Sci. Rep. 2021, 11, 695. [Google Scholar] [CrossRef]
- De Barros, A.; Attal, J.; Roques, M.; Nicolau, J.; Sol, J.-C.; Charni, S.; Cohen-Jonathan-Moyal, E.; Roux, F.-E. Glioblastoma Survival Is Better Analyzed on Preradiotherapy MRI than on Postoperative MRI Residual Volumes: A Retrospective Observational Study. Clin. Neurol. Neurosurg. 2020, 196, 105972. [Google Scholar] [CrossRef] [PubMed]
- Booth, T.C.; Luis, A.; Brazil, L.; Thompson, G.; Daniel, R.A.; Shuaib, H.; Ashkan, K.; Pandey, A. Glioblastoma Post-Operative Imaging in Neuro-Oncology: Current UK Practice (GIN CUP Study). Eur. Radiol. 2020, 31, 2933–2943. [Google Scholar] [CrossRef] [PubMed]
- Kubben, P.L.; ter Meulen, K.J.; Schijns, O.E.; ter Laak-Poort, M.P.; van Overbeeke, J.J.; van Santbrink, H. Intraoperative MRI-Guided Resection of Glioblastoma Multiforme: A Systematic Review. Lancet Oncol. 2011, 12, 1062–1070. [Google Scholar] [CrossRef]
- Bero, L.; Chartres, N.; Diong, J.; Fabbri, A.; Ghersi, D.; Lam, J.; Lau, A.; McDonald, S.; Mintzes, B.; Sutton, P.; et al. The Risk of Bias in Observational Studies of Exposures (ROBINS-E) Tool: Concerns Arising from Application to Observational Studies of Exposures. Syst. Rev. 2018, 7, 242. [Google Scholar] [CrossRef]
- Aprile, I.; Armadori, M.; Conti, G.; Ottaviano, I.; Ranaldi, A.; Ottaviano, P. MR Perfusion Imaging of Intracranial Tumors: A Retrospective Study of 218 Patients. Neuroradiol. J. 2008, 21, 472–489. [Google Scholar] [CrossRef]
- Boyett, D.; Englander, Z.; Zanazzi, Z.; Marie, T.; McKhann, G.; Sisti, M.; Grinband, J.; Canoll, P.; Bruce, J. MR Imaging Is Not Reliable for Tumor Presence in Post-Treatment Recurrent High-Grade Glioma. J. Neurosurg. 2019. [Google Scholar] [CrossRef]
- Brochado, A.T.V.H.S.R.; Reis, C.; Linhares, P.; Rocha, A.; Vaz, R. Early Postoperative Magnetic Resonance Imaging in Glioblastomas. Neuroradiology 2012. [Google Scholar] [CrossRef]
- Colen, R.; Kovacs, A.; Zinn, P.; Jolesz, F. MRI to Predict Surgical and Radiation Dosimetry Borders: A Methodology Feasibility Study. Neuro-Oncology 2012. [Google Scholar] [CrossRef]
- Fei, Q.; Qian, L.-X.; Zhang, Y.-J.; Guo, W.-J.; Bian, X.-H.; Yin, L.; Yan, P.-W.; Wang, T.-T.; Qian, P.-D.; Guo, Z.; et al. The Utility of Diffusion and Perfusion Magnetic Resonance Imaging in Target Delineation of High-Grade Gliomas. BioMed Res. Int. 2020, 2020, 8718097. [Google Scholar] [CrossRef]
- Finck, T.; Gempt, J.; Krieg, S.M.; Meyer, B.; Zimmer, C.; Wiestler, B.; Kirschke, J.S.; Sollmann, N. Assessment of the Extent of Resection in Surgery of High-Grade Glioma—Evaluation of Black Blood Sequences for Intraoperative Magnetic Resonance Imaging at 3 Tesla. Cancers 2020, 12, 1580. [Google Scholar] [CrossRef] [PubMed]
- Florez, E.; Hamidi, R.; Howard, C. Response Assessment in Recurrent Glioblastoma Based on Contrast-Enhanced T1-Weighted Subtraction Color Maps and Rano Criteria. J. Investig. Med. 2020, 68, 435–710. [Google Scholar] [CrossRef]
- Hesselmann, V.; Mager, A.-K.; Goetz, C.; Detsch, O.; Theisgen, H.-K.; Friese, M.; Schwindt, W.; Gottschalk, J.; Kremer, P. Accuracy of High-Field Intraoperative MRI in the Detectability of Residual Tumor in Glioma Grade IV Resections. Rofo 2017, 189, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Lescher, S.; Jurcoane, A.; Schniewindt, S.; Senft, C.; Hattingen, E. Misleading FLAIR Imaging Pattern after Glioma Surgery with Intraoperative MRI. Neurosurg. Rev. 2016, 39, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Lescher, S.; Schniewindt, S.; Jurcoane, A.; Senft, S.; Hattingen, E. SAH-like Pattern in Flair Imaging after Intraoperative MRI Guidance in Patients with Malignant Gliomas Surgery. Clin. Neuroradiol. 2014. [Google Scholar] [CrossRef]
- Martin, A.J.; Hall, W.A.; Liu, H.; Pozza, C.H.; Michel, E.; Casey, S.O.; Maxwell, R.E.; Truwit, C.L. Brain Tumor Resection: Intraoperative Monitoring with High-Field-Strength MR Imaging-Initial Results. Radiology 2000, 215, 221–228. [Google Scholar] [CrossRef]
- Ozduman, K.; Yildiz, E.; Dincer, A.; Sav, A.; Pamir, M.N. Using Intraoperative Dynamic Contrast-Enhanced T1-Weighted MRI to Identify Residual Tumor in Glioblastoma Surgery. J. Neurosurg. 2014, 120, 60–66. [Google Scholar] [CrossRef]

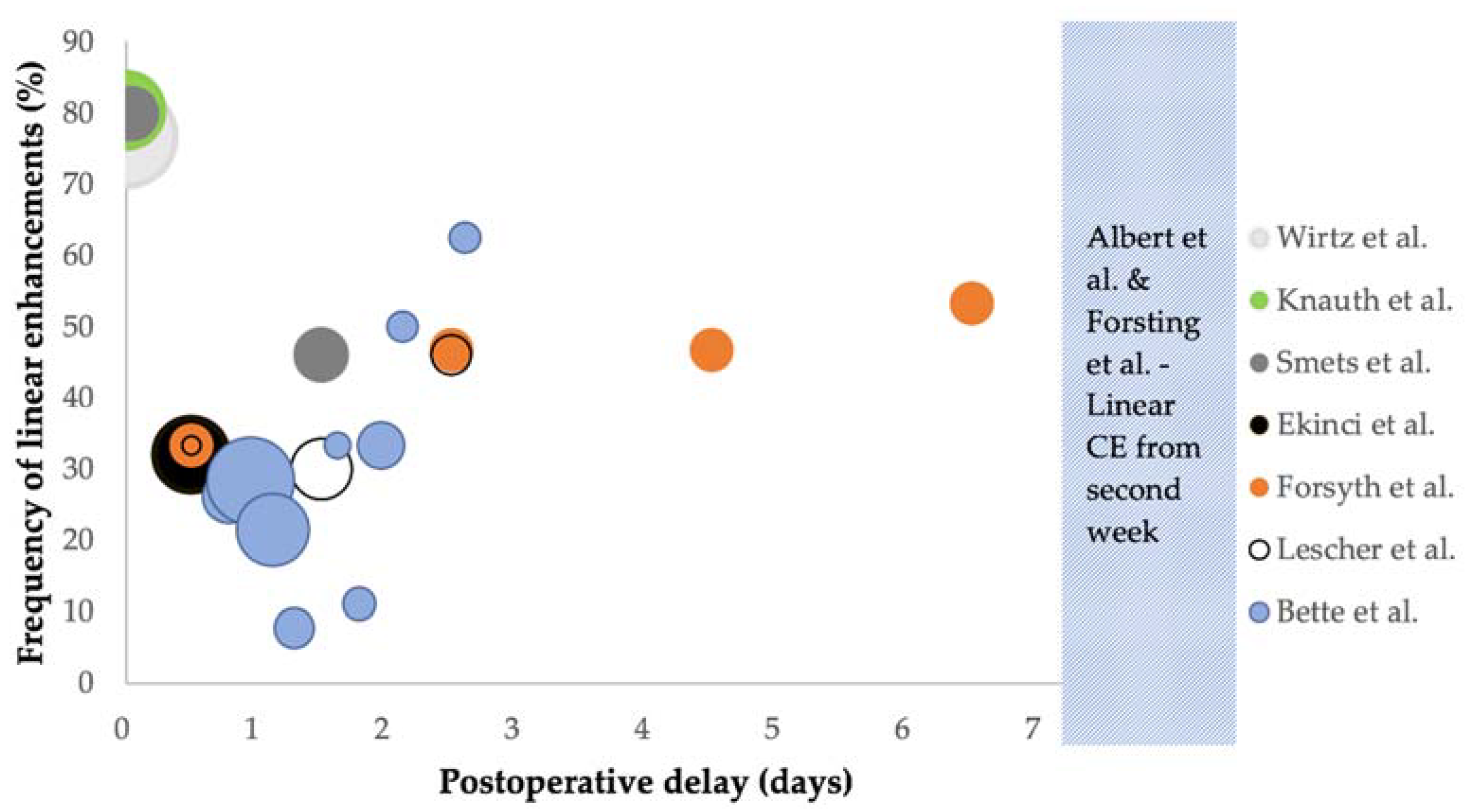
| Authors | Year | Design | Cases | Pathology (%) | Tesla | Sequences | epMRI Timing | CE Assessments | CE Comparison | |
|---|---|---|---|---|---|---|---|---|---|---|
| iMRI | Miskin et al. [21] | 2019 | R | 64 | HGG (69%) | 3 T iMRI 1.5/3 T epMRI | iMRI: T1 epMRI: NS | <72 h | New enhancements | iMRI and epMRI with preMRI epMRI with iMRI and follow-up |
| Masuda et al. [28] | 2018 | p | 22 | HGG (95%) | 1.5 T iMRI 1.5 T epMRI | T1, T2, DWI, MPRAGE | <24 h | New enhancements | PreMRI | |
| Wirtz et al. [29] | 2000 | p | 88 | HGG (70%) | 0.2 T | T1, T2, FLAIR | n/A | Surgically induced as linear, intraparenchymal | PreMRI | |
| Knauth et al. [30] | 1999 | p | 48 | HGG (71%) | 0.2 T | T1 | Day 1–3 | Surgically induced as linear, intraparenchymal | PreMRI and epMRI | |
| <72 h | Smets et al. [26] | 2013 | R | 24 | GBM (100%) | 3 T | T1, T2, DWI | <2 h and24–48 h | Linear, micronodular, frayed | Follow-up MRI |
| Ekinci et al. [15] | 2003 | R | 50 | HGG (78%) | 1.5 T | T1, T2 | <24 h | Thin linear, thick linear, thick linear-nodular | Follow-up MRI | |
| Bette et al. [6] | 2016 | R | 173 | GBM (100%) | 3 T | T1, FLAIR, MPRAGE | <17 to >72 h in 4-h intervals | Linear, nodular | Follow-up MRI | |
| Lescher et al. [16] | 2014 | R | 46 | HGG (100%) | 3 T | T1, T2, FLAIR, other | <24 to >48 h in 3 groups | Surgically induced if thin linear Tumoral if bulky/nodular | Follow-up MRI | |
| >72 h | Sui et al. [27] | 2020 | R | 106 | HGG (66%) | 3 T | T1, T2, FLAIR | <24 h to 30 days | Enhancement ring | Follow-up MRI |
| Forsyth et al. [31] | 1997 | p | 17 | HGG (100%) | 1.5 T (4 on 0.5 T) | T1, T2 | Day 1, 3, 5, 7, 14 and 21 | Surgically induced as linear. Subdivided by intensity. | Follow-up MRI | |
| Albert et al. [11] | 1994 | p | 60 | HGG (100%) | 1 T | T1 | Day 1–5, week 2, week 4–6, then bimonthly | Enhancement patterns evaluated over time. | Follow-up MRI | |
| Forsting et al. [12] | 1993 | p | 68 | GBM (100%) | 1 T | T1 | Day 1–5, week 2, week 4–6, then bimonthly | Enhancement patterns evaluated over time. | Follow-up MRI |
| Author | Timing | Frequency (%) | Surgically Induced (% of Linear CE) | ||
|---|---|---|---|---|---|
| iMRI | Wirtz et al. [29] | iMRI | 76.7% (66/86) | NS | |
| Knauth et al. [30] | iMRI | 80.4% (41/51) | NS | ||
| <72 h | Smets et al. [26] | <2 h 24–48 h | 80% 46% | NS | |
| Ekinci et al. [15] | <24 h | 32% (16/50) | 87.5% (14/16) * | ||
| Bette et al. [6] | <45 h >45 h | 24.1% (39/162) 45.5% (20/44) | 61.5% (24/39) 75% (15/20) | ||
| Lescher et al. [16] | <72 h | 28.3% (13/46) | NS | ||
| >72 h | Forsyth et al. [31] | Day 1 Day 3 Day 5 Day 7 Day 14 Day 21 | All grades 33.3% (5/15) * 46.7% (7/15) * 46.7% (7/15) * 53.3% (8/15) * 53.3% (8/15) * 40% (6/15) * | Grade 2–3 0% (0/15) * 20% (3/15) * 40% (6/15) * 40% (6/15) * 53.3% (8/15) * 27% (4/15) * | NS |
| Albert et al. [11] | Day 1–5, week 2, week 4–6, then bimonthly | Did not occur before day 4, developed in week 2 and had resolved after 2 months in most patients | NS | ||
| Forsting et al. [12] | Day 1–5, week 2, week 4–6, then bimonthly | Did not occur before day 4, developed in week 2 and had resolved after 2 months in most patients | NS | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rykkje, A.M.; Li, D.; Skjøth-Rasmussen, J.; Larsen, V.A.; Nielsen, M.B.; Hansen, A.E.; Carlsen, J.F. Surgically Induced Contrast Enhancements on Intraoperative and Early Postoperative MRI Following High-Grade Glioma Surgery: A Systematic Review. Diagnostics 2021, 11, 1344. https://doi.org/10.3390/diagnostics11081344
Rykkje AM, Li D, Skjøth-Rasmussen J, Larsen VA, Nielsen MB, Hansen AE, Carlsen JF. Surgically Induced Contrast Enhancements on Intraoperative and Early Postoperative MRI Following High-Grade Glioma Surgery: A Systematic Review. Diagnostics. 2021; 11(8):1344. https://doi.org/10.3390/diagnostics11081344
Chicago/Turabian StyleRykkje, Alexander Malcolm, Dana Li, Jane Skjøth-Rasmussen, Vibeke Andrée Larsen, Michael Bachmann Nielsen, Adam Espe Hansen, and Jonathan Frederik Carlsen. 2021. "Surgically Induced Contrast Enhancements on Intraoperative and Early Postoperative MRI Following High-Grade Glioma Surgery: A Systematic Review" Diagnostics 11, no. 8: 1344. https://doi.org/10.3390/diagnostics11081344
APA StyleRykkje, A. M., Li, D., Skjøth-Rasmussen, J., Larsen, V. A., Nielsen, M. B., Hansen, A. E., & Carlsen, J. F. (2021). Surgically Induced Contrast Enhancements on Intraoperative and Early Postoperative MRI Following High-Grade Glioma Surgery: A Systematic Review. Diagnostics, 11(8), 1344. https://doi.org/10.3390/diagnostics11081344








