A Proposed Dedicated Breast PET Lexicon: Standardization of Description and Reporting of Radiotracer Uptake in the Breast
Abstract
:1. Introduction
2. Methods
2.1. dbPET Imaging Protocols and Display
2.1.1. Study Protocols and Parameters
2.1.2. dbPET Image Display
2.2. dbPET Lexicon, Version 1.0
2.2.1. Image Quality
2.2.2. Background Fibroglandular Uptake (bFGU)
2.2.3. Breast Lesion
Focus
Mass Uptake (MU)
Non-Mass Uptake (NMU)
Associated Findings
Special Findings
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Image quality | |||
| Noise |
| Absence of noise or almost no noise | |
| Mild noise or noise localized only to the near FOV edge | ||
| Moderate noise | ||
| Significant noise, which can mask lesions | ||
| FOV |
| The whole radiotracer-avid fibroglandular gland is completely contained within the FOV. The entire retromammary space is identified. | |
| Most of the radiotracer-avid fibroglandular gland is included. The retromammary space is partially interrupted. | ||
| The radiotracer-avid fibroglandular gland is partly out of FOV. The retromammary space is partly or poorly visualized. | ||
| The breast in the FOV is visualized very limitedly. | ||
| Notes | If there are special notes (poor positioning, body movement, artifacts, and change of protocols (radiotracer dose, waiting time, emission scan duration, reconstruction parameters, etc.)), describe. | ||
| Background fibroglandular uptake (bFGU) | |||
| Extent |
| No radiotracer-avid fibroglandular tissue. When this category is chosen, subsequent evaluation of intensity and homogeneity of FGU will be skipped. | |
| A small amount of radiotracer-avid fibroglandular tissue, occupying approximately >0% to ≤25% of the assumed fibroglandular tissue in the breast by volume | ||
| A relatively small amount of radiotracer-avid fibroglandular tissue, occupying approximately >25% to ≤50% of the assumed fibroglandular tissue in the breast by volume | ||
| A relatively large amount of radiotracer-avid fibroglandular tissue, occupying approximately >50% to ≤75% of the assumed fibroglandular tissue in the breast by volume | ||
| A large amount of radiotracer-avid fibroglandular tissue, occupying approximately >75% to ≤100% of the assumed fibroglandular tissue in the breast by volume | ||
| Intensity |
| Very low intensity (Approximately SUV <1 for 18F-FDG). | |
| Low intensity (Approximately SUV ≥1 to <2 for 18F-FDG) | ||
| Mildly increased uptake. (Approximately SUV ≥2 to <3 for 18F-FDG) | ||
| Extremely increased uptake (Approximately SUV ≥3 for 18F-FDG) | ||
| Distribution |
| Almost uniform and confluent uptake of the radiotracer-avid fibroglandular tissue | |
| Heterogeneous fibroglandular tissue uptake, due to the mixture of fat (i.e., heterogenous, scattered, island-like or patchy) or uneven uptake level in the fibroglandular tissue itself. | ||
| Symmetry |
| Almost symmetrical pattern between the right and left breasts | |
| Asymmetrical pattern between the right and left breasts | ||
| Notes | If there are special notes such as post-mastectomy, post-reconstruction, describe. | ||
| Lesion type | |||
| Focus; Dot-like accumulation. Size threshold is not defined as a criterion, but focus is roughly smaller than 5 mm as a guide. | |||
| Single or multiple |
| Single focus | |
| Three of more foci, scattered in the breast | ||
| Mass uptake (MU); Three-dimensionally mass-like uptake | |||
| Shape |
| Elliptical or egg-shaped uptake (may have up to 2 mild undulations with a dull angle) | |
| Spherical, circular, round uptake (may have up to 2 mild undulations with a dull angle) | ||
| Lobulated uptake with clear undulations with sharp angles, or 3 or more undulations | ||
| Uptake with irregular margins (sawtooth, spiculated, angular, or indistinct) or lesions that develop irregular extension | ||
| Internal pattern |
| Almost homogeneous accumulation | |
| Heterogeneous accumulation | ||
| Rim-shaped accumulation with a loss or marked reduction of accumulation in the central part. The rim includes full rim and partial rim. | ||
| Non-mass uptake (NMU); A pattern different from that of the background fibroglandular tissue, and has a shape that cannot be called a mass or focus | |||
| Distribution |
| Less than 25% of quadrant or less than 2 cm in diameter | |
| Linear toward the nipple. It may be tubular or branched. | ||
| Distribution of triangles with one apex towards the nipple side, suggesting distribution of glandular lobes and ducts | ||
| 25% or more of quadrant or 2 cm or more in diameter | ||
| Multiple uptake areas in 3 or more locations, independent from the distribution of glandular lobes. Focus can be included, but uptake pattern at least one location should be NMU. | ||
| Diffuse accumulation | ||
| Internal pattern |
| Almost homogeneous | |
| Heterogeneous | ||
| Common assessment for lesion | |||
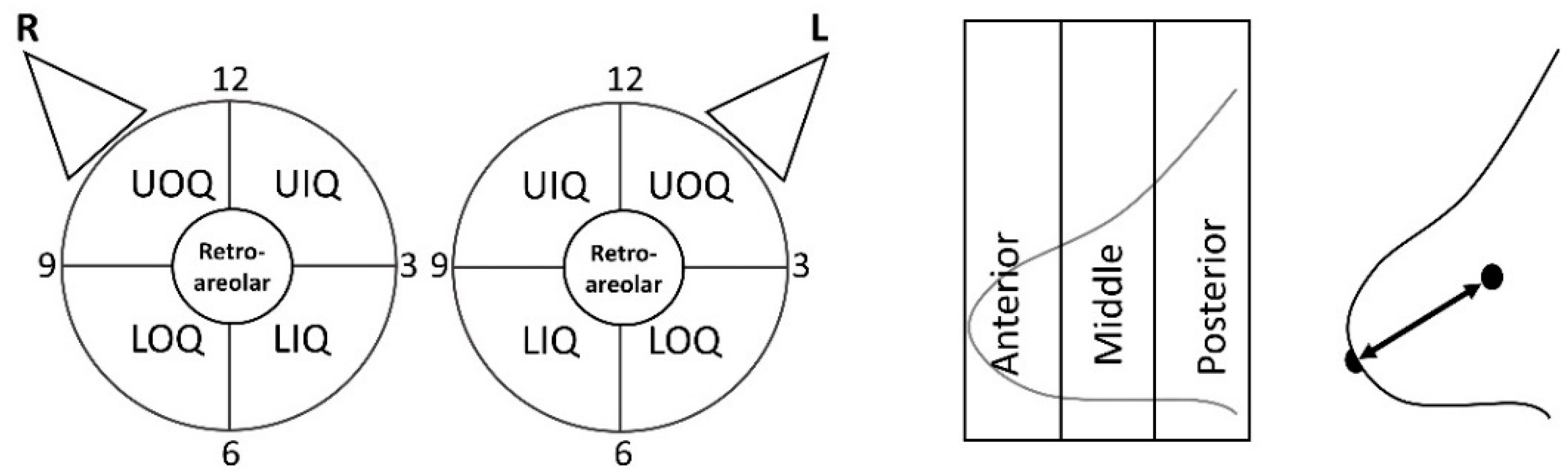
| Location | Laterality | Right | |
| Left | |||
| Quadrant | UOQ | Upper outer quadrant | |
| LOQ | Lower outer quadrant | ||
| UIQ | Upper inner quadrant | ||
| LIQ | Lower inner quadrant | ||
| Retroareolar | Around or posterior to the nipple | ||
| Clock face | Clock-face position | ||
| Depth | Anterior | 1/3 anterior of the breast within FOV | |
| Middle | 1/3 middle of the breast within FOV | ||
| Posterior | 1/3 posterior of the breast within FOV | ||
| Distance from the nipple | Distance of the lesion from the nipple | ||
| Qualitative uptake intensity |
| No abnormal accumulation | |
| Low intensity (Approximately SUV < 2 for 18F-FDG). | ||
| Moderate uptake (Approximately SUV ≥ 2, <3 for 18F-FDG) | ||
| Increased uptake (Approximately SUV ≥ 3 for 18F-FDG) | ||
| Quantitative uptake intensity | Qualitative radiotracer uptake values, i.e., SUV | ||
| Associated findings | Nipple retraction | ||
| Skin retraction | |||
| Nipple uptake | |||
| Skin uptake | |||
| Pectoral muscle/chest wall uptake | |||
| Special findings | |||
| Cyst-like uptake | A clear circular uptake defect with or without surrounding uptake. This category is selected when cyst or fat necrosis is strongly suspected or known. | ||
| Breast implant | Accumulation defect due to implant | ||
References
- Satoh, Y.; Kawamoto, M.; Kubota, K.; Murakami, K.; Hosono, M.; Senda, M.; Sasaki, M.; Momose, T.; Ito, K.; Okamura, T.; et al. Clinical practice guidelines for high-resolution breast PET, 2019 Edition. Ann. Nucl. Med. 2021, 35, 406–414. [Google Scholar] [CrossRef]
- Miyake, K.K.; Nakamoto, Y.; Togashi, K. Current Status of Dedicated Breast PET Imaging. Curr. Radiol. Rep. 2016, 4, 16. [Google Scholar] [CrossRef]
- Thompson, C.J.; Murthy, K.; Picard, Y.; Weinberg, I.N.; Mako, R. Positron emission mammography (PEM): A promising technique for detecting breast cancer. IEEE Trans. Nucl. Sci. 1995, 42, 1012–1017. [Google Scholar] [CrossRef] [Green Version]
- Miyake, K.K.; Matsumoto, K.; Inoue, M.; Nakamoto, Y.; Kanao, S.; Oishi, T.; Kawase, S.; Kitamura, K.; Yamakawa, Y.; Akazawa, A.; et al. Performance Evaluation of a New Dedicated Breast PET Scanner Using NEMA NU4-2008 Standards. J. Nucl. Med. 2014, 55, 1198–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moliner, L.; González, A.J.; Soriano, A.; Sánchez, F.; Correcher, C.; Orero, A.; Carles, M.; Vidal, L.F.; Barberá, J.; Caballero, L.; et al. Design and evaluation of the MAMMI dedicated breast PET. Med. Phys. 2012, 39, 5393–5404. [Google Scholar] [CrossRef]
- Eo, J.S.; Chun, I.K.; Paeng, J.C.; Kang, K.W.; Lee, S.M.; Han, W.; Noh, D.Y.; Chung, J.K.; Lee, D.S. Imaging sensitivity of dedicated positron emission mammography in relation to tumor size. Breast 2012, 21, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Kalinyak, J.E.; Berg, W.A.; Schilling, K.; Madsen, K.S.; Narayanan, D.; Tartar, M. Breast cancer detection using high-resolution breast PET compared to whole-body PET or PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Ozawa, Y.; Kubouchi, K.; Nakamura, S.; Nakajima, Y.; Inoue, T. Comparative analysis of imaging sensitivity of positron emission mammography and whole-body PET in relation to tumor size. Clin. Nucl. Med. 2015, 40, 21–25. [Google Scholar] [CrossRef]
- Yano, F.; Itoh, M.; Hirakawa, H.; Yamamoto, S.; Yoshikawa, A.; Hatazawa, J. Diagnostic Accuracy of Positron Emission Mammography with 18F-Fluorodeoxyglucose in Breast Cancer Tumor of Less than 20 mm in Size. Asia Ocean J. Nucl. Med. Biol. 2019, 7, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Sueoka, S.; Sasada, S.; Masumoto, N.; Emi, A.; Kadoya, T.; Okada, M. Performance of dedicated breast positron emission tomography in the detection of small and low-grade breast cancer. Breast Cancer Res. Treat. 2021, 187, 125–133. [Google Scholar] [CrossRef]
- D’Orsi, C.; Sickles, E.; Mendelson, E.; Morris, E. Breast Imaging Reporting and Data System: ACR BI-RADS—Breast Imaging Atlas, 5th ed.; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Narayanan, D.; Madsen, K.S.; Kalinyak, J.E.; Berg, W.A. Interpretation of positron emission mammography: Feature analysis and rates of malignancy. AJR Am. J. Roentgenol. 2011, 196, 956–970. [Google Scholar] [CrossRef] [PubMed]
- Satoh, Y.; Motosugi, U.; Omiya, Y.; Onishi, H. Unexpected Abnormal Uptake in the Breasts at Dedicated Breast PET: Incidentally Detected Small Cancers or Nonmalignant Features? AJR Am. J. Roentgenol. 2019, 212, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Sasada, S.; Masumoto, N.; Kimura, Y.; Emi, A.; Kadoya, T.; Okada, M. Classification of Abnormal Findings on Ring-type Dedicated Breast PET for the Detection of Breast Cancer. Anticancer Res. 2020, 40, 3491–3497. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, N.; Kadoya, T.; Sasada, S.; Emi, A.; Arihiro, K.; Okada, M. Intratumoral heterogeneity on dedicated breast positron emission tomography predicts malignancy grade of breast cancer. Breast Cancer Res. Treat. 2018, 171, 315–323. [Google Scholar] [CrossRef]
- Sakaguchi, R.; Kataoka, M.; Kanao, S.; Miyake, K.K.; Nakamoto, Y.; Sugie, T.; Toi, M.; Mikami, Y.; Togashi, K. Distribution pattern of FDG uptake using ring-type dedicated breast PET in comparison to whole-body PET/CT scanning in invasive breast cancer. Ann. Nucl. Med. 2019, 33, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Delbeke, D.; Coleman, R.E.; Guiberteau, M.J.; Brown, M.L.; Royal, H.D.; Siegel, B.A.; Townsend, D.W.; Berland, L.L.; Parker, J.A.; Hubner, K.; et al. Procedure guideline for tumor imaging with 18 F-FDG PET/CT 1.0. J. Nucl. Med. 2006, 47, 885–895. [Google Scholar] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.A.; Madsen, K.S.; Schilling, K.; Tartar, M.; Pisano, E.D.; Larsen, L.H.; Narayanan, D.; Ozonoff, A.; Miller, J.P.; Kalinyak, J.E. Breast cancer: Comparative effectiveness of positron emission mammography and MR imaging in presurgical planning for the ipsilateral breast. Radiology 2011, 258, 59–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimatsu, K.; Nakamoto, Y.; Miyake, K.K.; Ishimori, T.; Kanao, S.; Toi, M.; Togashi, K. Higher breast cancer conspicuity on dbPET compared to WB-PET/CT. Eur. J. Radiol. 2017, 90, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conners, A.L.; Hruska, C.B.; Tortorelli, C.L.; Maxwell, R.W.; Rhodes, D.J.; Boughey, J.C.; Berg, W.A. Lexicon for standardized interpretation of gamma camera molecular breast imaging: Observer agreement and diagnostic accuracy. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 971–982. [Google Scholar] [CrossRef] [PubMed]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyake, K.K.; Kataoka, M.; Ishimori, T.; Matsumoto, Y.; Torii, M.; Takada, M.; Satoh, Y.; Kubota, K.; Satake, H.; Yakami, M.; et al. A Proposed Dedicated Breast PET Lexicon: Standardization of Description and Reporting of Radiotracer Uptake in the Breast. Diagnostics 2021, 11, 1267. https://doi.org/10.3390/diagnostics11071267
Miyake KK, Kataoka M, Ishimori T, Matsumoto Y, Torii M, Takada M, Satoh Y, Kubota K, Satake H, Yakami M, et al. A Proposed Dedicated Breast PET Lexicon: Standardization of Description and Reporting of Radiotracer Uptake in the Breast. Diagnostics. 2021; 11(7):1267. https://doi.org/10.3390/diagnostics11071267
Chicago/Turabian StyleMiyake, Kanae K., Masako Kataoka, Takayoshi Ishimori, Yoshiaki Matsumoto, Masae Torii, Masahiro Takada, Yoko Satoh, Kazunori Kubota, Hiroko Satake, Masahiro Yakami, and et al. 2021. "A Proposed Dedicated Breast PET Lexicon: Standardization of Description and Reporting of Radiotracer Uptake in the Breast" Diagnostics 11, no. 7: 1267. https://doi.org/10.3390/diagnostics11071267
APA StyleMiyake, K. K., Kataoka, M., Ishimori, T., Matsumoto, Y., Torii, M., Takada, M., Satoh, Y., Kubota, K., Satake, H., Yakami, M., Isoda, H., Ikeda, D. M., Toi, M., & Nakamoto, Y. (2021). A Proposed Dedicated Breast PET Lexicon: Standardization of Description and Reporting of Radiotracer Uptake in the Breast. Diagnostics, 11(7), 1267. https://doi.org/10.3390/diagnostics11071267






