Hepatitis C Diagnosis: Simplified Solutions, Predictive Barriers, and Future Promises
Abstract
1. Introduction
2. Why Are We in Need of Simplified HCV Diagnostic Algorithms?
2.1. To Achieve WHO Elimination Goals for Hepatitis C by 2030
2.2. To Find the Missing Millions Living with Undiagnosed HCV Infection
2.2.1. Vulnerable HCV Populations—A Major Threat for New HCV Outbreaks
2.2.2. Correctional Populations—A Potential Cause of New HCV Incidence in Inmates and Infection Transmission to the General Population
2.3. To Minimize the Steps to HCV Diagnosis
2.3.1. Limitations of the Traditional HCV Diagnostic Algorithms
2.3.2. Predisposing Factors to Patient Drop-Off with Traditional HCV Diagnostics
2.4. To Facilitate Accurate and Rapid HCV Diagnosis within Minutes
2.5. To Facilitate HCV Diagnosis in LMICs
2.6. To Improve Linkage to HCV Care
3. The Existing and Current Paradigms of HCV Diagnostics
3.1. The Classical HCV Screening and Diagnosis Approach
3.2. The Current and Emerging HCV Diagnostic Platforms Involving RDTs, POCT, DBS, and HCV Self-Test Approaches
3.2.1. Immunological Point of Care (POC) Rapid Diagnostic Tests (RDTs)
Pros and Cons of Immunological HCV POC RDTs
3.2.2. HCV Core Antigen (cAg) Detection as an Alternate to HCV RNA Testing
3.2.3. Non-Immunological Point of Care (POC) Rapid Diagnostic Tests (RDTs)
Advantages and Limitations of HCV RNA POC RDTs
3.2.4. Nanocomposites as HCV Diagnostic Approach
3.2.5. Multi-Disease Analyzers
3.2.6. DBS Sample Testing for HCV Screening and Diagnosis
Advantages and Disadvantages of DBS Sample Testing
Clinical Performance of DBS Sample Testing
3.2.7. HCV Diagnostic Platforms in the Pipeline or Late-Stage Development
HCV Self-Test Diagnostics
Anti-HCV Abs Fourth-Generation EIAs
HCV RNA Confirmation Assays and Tests for the Infection Cure
Plasma Separation Card
One-Step HCV cAg Diagnosis Platform for Therapeutic Monitoring and HCV Cure
3.2.8. HCV Screening and Genotype Identification Assays
4. The Real-World Clinical Performance of Current HCV Diagnostic Algorithms
4.1. HCV Micro-Elimination Approaches in the Context of HCV Diagnosis
4.1.1. Real-World Outcomes of HCV RNA POC Tests and DBS Sample Analysis in Vulnerable HCV Populations
4.1.2. Real-World Performance of HCV cAg Assay in High Risks HCV Populations
4.1.3. Real-World Performance of HCV cAg Assay in HCV Populations with Concomitant HIV or HBV Infection
4.2. HCV Macro-Elimination Approaches in the Context of HCV Diagnosis
4.2.1. The Georgian Experience by Initiating the World’s First HCV Macro-Elimination Program
4.2.2. Egypt HCV Macro-Elimination Approach While Initiating “Educate, Test and Treat HCV Program” and Launching the “100 Million Healthy Lives” Campaign
4.2.3. The US HCV Elimination Approaches in the Context of HCV Diagnosis
5. Real-World Challenges to HCV Diagnosis
5.1. Diagnostic Barriers at the Patient Level
5.1.1. Social Determinants of Health
5.1.2. Plausible Solutions to Overcome Social Determinants of Health during HCV Diagnosis
By Raising Awareness about Social Determinants of Health
By Introducing Simplified HCV Diagnosis in Stigmatized HCV Populations
By Implementing Decentralized HCV Screening Policies for the General Population
5.2. Diagnostic and Healthcare System Barriers
5.2.1. Advantages and Limitations of Centralized and Decentralized HCV Diagnostic Platforms/Settings
5.2.2. Potential Ways to Overcome Health System-Related Obstacles to Simplify HCV Diagnostics
Shifting Centralized Diagnostic Approaches to Complete or Partly POC Testing Approaches for Vulnerable HCV Populations
Minimizing Patient’s Visits up to Four or Less in HCV Care Pathway from Diagnosis to Cure in LMICs
One-Stop Shops for HCV Diagnosis and Treatment Rather Than Decentralized Testing Approaches
Telemedicine and ECHO Models to Expand Decentralized HCV Testing
One-Sample, One-Test, and One-Step HCV Diagnostic Approach
Task-Shifting Rather Than a Specialty in HCV Diagnosis and Treatment
5.3. Regulatory and Licensing Barriers
5.3.1. Advantages and Limitations of the WHO PQ and CE Marking for HCV Diagnostic Platforms
5.3.2. Designing of Simplified Regulatory Procedures for the Approvals of HCV Diagnostic Platforms in LMICs
5.3.3. Expediting the Review Process for the Approval of Current HCV Diagnostic Platforms in LMICs
5.3.4. Open Licensing Agreement for the Utilization of Current HCV Diagnostic Platforms
5.3.5. Enhancing Funding for HCV Diagnostic Research and Development (R&D)
5.4. Cost and Pricing Barriers
5.4.1. Potential Causes of High Costs for Current HCV Diagnostic Algorithms
5.4.2. Cost-Effectiveness of Simplified HCV Diagnostic Pathway in LMICs
5.4.3. Cost-Effectiveness of Simplified HCV Diagnostic Pathway to Launch National Test-and-Treat Programs
5.5. n-SARS-CoV-2 and the COVID-19 Pandemic
5.5.1. The Virus That Shook the World Has Also Halted Hepatitis C Diagnosis
5.5.2. Plausible Solutions to Continue HCV Screening during the COVID-19 Pandemic Period
6. Future Promises
“Everything should be made as simple as possible, but no simpler” (Albert Einstein 1879–1955).
6.1. The Next 5-Year Perspective
6.2. The Next 10 Years
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blach, S.; Zeuzem, S.; Manns, M.; Altraif, I.; Duberg, A.S.; Muljono, D.H.; Waked, I.; Alavian, M.S.; Lee, M.H.; Negro, F.; et al. Global prevalence and geno-type distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Hepatitis Report 2017 (Internet). Available online: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ (accessed on 1 March 2021).
- World Health Organization. Hepatitis C Fact Sheet; WHO: Geneva, Switzerland, 2019; Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 10 September 2019).
- hepCoalition. Sofosbuvir Turns 5 Years Old: The Vast Majority of People with Chronic Hepatitis C still Have not Been Treated. 2018. Available online: https://hepcoalition.org/IMG/pdf/factsheet_sofosbuvir_5_anniversary-2.pdf (accessed on 6 March 2021).
- Cooke, G.; Andrieux-Meyer, I.; Applegate, T.L.; Atun, R.; Burry, J.R.; Cheinquer, H.; Dusheiko, G.; Feld, J.J.; Gore, C.; Griswold, M.; et al. Lancet commission: Accelerating the elimination of viral hepatitis. Lancet Gastroenterol. Hepatol. 2019, 4, 135–184. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Hepatitis C. 2018. Available online: https://ecdc.europa.eu/en/hepatitis-c (accessed on 9 March 2021).
- Association Française pour l’Etude du Foie. Recommandations AFEF sur la Prise en Charge de L’hepatite Virale C. 2017. Available online: http://www.afef.asso.fr/ckfinder/userfiles/files/recommandationstextesofficiels/recommandations/RecommandationsAFEFMars2017.pdf (accessed on 10 March 2021).
- Cox, A.L.; El-Sayed, M.H.; Kao, J.-H.; Lazarus, J.V.; Lemoine, M.; Lok, A.S.; Zoulim, F. Progress towards elimination goals for viral hepatitis. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 533–542. [Google Scholar] [CrossRef]
- Hill, A.M.; Nath, S.; Simmons, B. The road to elimination of hepatitis C: Analysis of cures versus new infec-tions in 91 countries. J. Virus Erad. 2017, 3, 117–123. [Google Scholar] [CrossRef]
- Patel, A.A.; Bui, A.; Prohl, E.; Bhattacharya, D.; Wang, S.; Branch, A.D.; Perumalswami, P.V. Innovations in Hepatitis C Screening and Treatment. Hepatol. Commun. 2021, 5, 371–386. [Google Scholar] [CrossRef]
- Fourati, S.; Feld, J.J.; Chevaliez, S.; Luhmann, N. Approaches for simplified HCV diagnostic algorithms. J. Int. AIDS Soc. 2018, 21, e25058. [Google Scholar] [CrossRef]
- Chevaliez, S. Strategies for the improvement of HCV testing and diagnosis. Expert Rev. Anti-Infect. Ther. 2019, 17, 341–347. [Google Scholar] [CrossRef]
- Grebely, J.; Applegate, T.; Cunningham, P.; Feld, J.J. Hepatitis C point-of-care diagnostics: In search of a single visit diagnosis. Expert Rev. Mol. Diagn. 2017, 17, 1109–1115. [Google Scholar] [CrossRef]
- Hepatitis C Cure, Sofosbuvir, Turns 5 Years Old: The Vast Majority of People Still Nave Not Been Treated. Available online: https://hepcoalition.org/news/press-releases/article/hepatitis-c-cure-sofosbuvir-turns-5-years-old-the-vast-majority-of-people-still (accessed on 1 January 2021).
- Pawlotsky, J.-M.; Ramers, C.B.; Dillon, J.; Feld, J.J.; Lazarus, J. Simplification of Care for Chronic Hepatitis C Virus Infection. Semin. Liver Dis. 2020, 40, 392–402. [Google Scholar] [CrossRef]
- Duffell, E.F.; Hedrich, D.; Mardh, O.; Mozalevskis, A. Towards elimination of hepatitis B and C in European Union and European Economic Area countries: Monitoring the World Health Organization’s global health sector strategy core indicators and scaling up key interventions. Eurosurveillance 2017, 22. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Systematic Review on Hepatitis B and C Prevalence in the EU/EEA.; ECDC: Stockholm, Sweden, 2016. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Hepatitis C among Drug users in Europe. Epidemiology, Treatment and Prevention; EMCDDA: Lisbon, Portugal, 2016; Available online: http://www.emcdda.europa.eu/system/files/publications/2953/TDXD16002ENN_final_web.pdf (accessed on 16 March 2021).
- Wiessing, L.; Ferri, M.; Grady, B.; Kantzanou, M.; Sperle, I.; Cullen, K.J.; EMCDDA DRID Group; Hatzakis, A.; Prins, M.; Vickerman, P.; et al. Hepatitis C Virus Infection Epidemiology among People Who Inject Drugs in Europe: A Systematic Review of Data for Scaling Up Treatment and Prevention. PLoS ONE 2014, 9, e103345. [Google Scholar] [CrossRef]
- Hofmeister, M.G.; Rosenthal, E.; Barker, L.K.; Rosenberg, E.S.; Barranco, M.A.; Hall, E.W.; Edlin, B.R.; Mermin, J.; Ward, J.W.; Ryerson, A.B. Estimating Prevalence of Hepatitis C Virus Infection in the United States, 2013–2016. Hepatology 2019, 69, 1020–1031. [Google Scholar] [CrossRef]
- Rich, J.D.; Beckwith, C.G.; Macmadu, A.; Marshall, B.D.L.; Brinkley-Rubinstein, L.; Amon, J.J.; Milloy, M.J.; King, M.; Sanchez, J.; Atwoli, L.; et al. Clinical care of incarcerated people with HIV, viral hepatitis, or tuberculosis. Lancet 2016, 388, 1103–1114. [Google Scholar] [CrossRef]
- Beckwith, C.G.; Kurth, A.E.; Bazerman, L.; Solomon, L.; Patry, E.; Rich, J.D.; Kuo, I. Survey of US Correctional Institutions for Routine HCV Testing. Am. J. Public Health 2015, 105, 68–71. [Google Scholar] [CrossRef]
- Emory Center for the Health of Incarcerated Persons and MGH Institute for Technology Assessment. Available online: http://www.hepcorrections.org/ (accessed on 30 May 2020).
- Nijmeijer, B.M.; Koopsen, J.; Schinkel, J.; Prins, M.; Geijtenbeek, T.B. Sexually transmitted hepatitis C virus infections: Current trends, and recent advances in understanding the spread in men who have sex with men. J. Int. AIDS Soc. 2019, 22, e25348. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Epidemiological Assessment of Hepatitis B and C Prevalence among Migrants in the EU/EEA; ECDC: Stockholm, Sweden, 2016; Available online: http://ecdc.europa.eu/en/publications/Publications/epidemiological-assessment-hepatitis-B-and-C (accessed on 26 April 2021).
- Dibba, P.; Cholankeril, R.; Li, A.A.; Patel, M.; Fayek, M.; Dibble, C.; Okpara, N.; Hines, A.; Ahmed, A. Hepatitis C in Pregnancy. Diseases 2018, 6, 31. [Google Scholar] [CrossRef]
- Indolfi, G.; Easterbrook, P.; Dusheiko, G.; El-Sayed, M.H.; Jonas, M.M.; Thorne, C.; Bulterys, M.; Siberry, G.; Walsh, N.; Chang, M.; et al. Hepatitis C virus infection in children and adolescents. Lancet Gastroenterol. Hepatol. 2019, 4, 477–487. [Google Scholar] [CrossRef]
- Ly, K.N.; Jiles, R.B.; Teshale, E.H.; Foster, M.A.; Pesano, R.L.; Holmberg, S.D. Hepatitis C Virus Infection Among Reproductive-Aged Women and Children in the United States, 2006 to 2014. Ann. Intern. Med. 2017, 166, 775–782. [Google Scholar] [CrossRef]
- Ward, C.; Tudor-Williams, G.; Cotzias, T.; Hargreaves, S.; Regan, L.; Foster, G.R. Prevalence of hepatitis C among pregnant women attending an inner London obstetric department: Uptake and acceptability of named antenatal testing. Gut 2000, 47, 277–280. [Google Scholar] [CrossRef]
- Lambert, J.; Jackson, V.; Coulter-Smith, S.; Brennan, M.; Geary, M.; Kelleher, T.B.; O’Reilly, M.; Grundy, K.; Sammon, N.; Cafferkey, M. Universal antenatal screening for Hepatitis C at an Irish maternity hospital. Am. J. Obstet. Gynecol. 2008, 199, S144. [Google Scholar] [CrossRef]
- Diab-Elschahawi, M.; Dosch, V.; Honsig, C.; Jatzko, B.; Segagni, L.; Assadian, O.; Presterl, E. Evaluation of a uni-versal vs a targeted hepatitis C virus screening strategy among pregnant women at the Vienna University Hospital. Am. J. Infect. Control 2013, 41, 459–460. [Google Scholar] [CrossRef]
- Jhaveri, R.; Broder, T.; Bhattacharya, D.; Peters, M.G.; Kim, A.Y.; Jonas, M.M. Universal screening of preg-nant women for hepatitis C: The time is now. Clin. Infect. Dis. 2018, 67, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Schillie, S.; Wester, C.; Osborne, M.; Wesolowski, L.; Ryerson, A.B. CDC Recommendations for Hepatitis C Screening Among Adults—United States, 2020. MMWR Recomm. Rep. 2020, 69, 1–17. [Google Scholar] [CrossRef]
- Chaillon, A.; Rand, E.B.; Reau, N.; Martin, N.K. Cost-effectiveness of Universal Hepatitis C Virus Screening of Pregnant Women in the United States. Clin. Infect. Dis. 2019, 69, 1888–1895. [Google Scholar] [CrossRef]
- Rose, M.M.J.; Evans, J.; Prince, A.; Espinosa, C. Hepatitis C risk based vs. universal screening among preg-nant women: Implementation and cost-effectiveness analysis. In Proceedings of the Liver Meeting November, San Francisco, CA, USA, 9–13 November 2018. [Google Scholar]
- Oliver, C.; Black, J.; De Pont, S.; Sizemore, L.; Wester, C. Pregnancy Status, Risk Factors, and Opportunities for Referral to Care Among Reproductive-Aged Women with Newly Reported Chronic Hepatitis C Virus Infection in Tennessee. Public Health Rep. 2019, 135, 90–96. [Google Scholar] [CrossRef]
- Saab, S.; Kullar, R.; Gounder, P. The urgent need for hepatitis C screening in pregnant women: A call to action. Obstet. Gynecol. 2020, 135, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Modin, L.; Arshad, A.; Wilkes, B.; Benselin, J.; Lloyd, C.; Irving, W.L.; Kelly, D.A. Epidemiology and natural history of hepatitis C virus infection among children and young people. J. Hepatol. 2020, 70, 371–378. [Google Scholar]
- Smith, B.D.; Morgan, R.L.; Beckett, G.A.; Falck-Ytter, Y.; Holtzman, D.; Ward, J.W. Hepatitis C Virus Testing of Persons Born During 1945–1965: Recommendations from the Centers for Disease Control and Prevention. Ann. Intern. Med. 2012, 157, 817–822. [Google Scholar] [CrossRef]
- The European Union HCV Collaborators. Hepatitis C virus prevalence and level of intervention required to achieve the WHO tar-gets for elimination in the European Union by 2030: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 325–336. [Google Scholar] [CrossRef]
- Negro, F. Epidemiology of hepatitis C in Europe. Dig. Liver Dis. 2014, 46, S158–S164. [Google Scholar] [CrossRef] [PubMed]
- Yehia, B.R.; Schranz, A.; Umscheid, C.A.; Re, V.L. The Treatment Cascade for Chronic Hepatitis C Virus Infection in the United States: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e101554. [Google Scholar] [CrossRef] [PubMed]
- American Association for the Study of Liver Diseases (AASLD); Infectious Diseases Society of America (IDSA). HCV Guidance: Recommendations for Testing, Managing and Treating Hepatitis C. 2017. Available online: www.hcvguidelines.org (accessed on 27 September 2017).
- World Health Organization (WHO). WHO Guidelines on Hepatitis B and C Testing. 2016. Available online: http://apps.who.int/iris/handle/10665/ (accessed on 20 May 2017).
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2016. J. Hepatol. 2016, 66, 153–194. [Google Scholar]
- Udompap, P.; Mannalithara, A.; Heo, N.-Y.; Kim, D.; Kim, W.R. Increasing prevalence of cirrhosis among U.S. adults aware or unaware of their chronic hepatitis C virus infection. J. Hepatol. 2016, 64, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Spradling, P.R.; Tong, X.; Rupp, L.B.; Moorman, A.C.; Lu, M.; Teshale, E.H.; Gordon, S.C.; Vijayadeva, V.; Boscarino, J.A.; Schmidt, M.A. Trends in HCV RNA testing among HCV antibody-positive per-sons in care, 2003–2010. Clin. Infect. Dis. 2014, 59, 976–981. [Google Scholar] [CrossRef]
- Activist Guide to Hepatitis C Virus Diagnostics. Available online: https://www.hepcoalition.org/advocate/advocacy-tools/article/activist-guide-to-hepatitis-c-virus-diagnostics (accessed on 2 April 2021).
- Reipold, E.I.; Easterbrook, P.; Trianni, A.; Panneer, N.; Krakower, D.; Ongarello, S.; Roberts, T.; Miller, V.; Denkinger, C. Optimising diagnosis of viraemic hepatitis C infection: The development of a target product profile. BMC Infect. Dis. 2017, 17, 707. [Google Scholar] [CrossRef]
- Zarei, M. Advances in point-of-care technologies for molecular diagnostics. Biosens. Bioelectron. 2017, 98, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Radin, J.M.; Topol, E.J.; Andersen, K.G.; Steinhubl, S.R. A laboratory in your pocket. Lancet 2016, 388, 1875. [Google Scholar] [CrossRef]
- Romao, V.C.; Martins, S.A.M.; Germano, J.; Cardoso, F.A.; Cardoso, S.; Freitas, P.P. Lab-on-Chip Devices: Gaining Ground Losing Size. ACS Nano 2017, 11, 10659–10664. [Google Scholar] [CrossRef]
- Gupta, E.; Agarwala, P.; Kumar, G.; Maiwall, R.; Sarin, S.K. Point-of-care testing (POCT) in molecular diagnostics: Performance evaluation of GeneXpert HCV RNA test in diagnosing and monitoring of HCV infection. J. Clin. Virol. 2017, 88, 46–51. [Google Scholar] [CrossRef]
- Grebely, J.L.; Lamoury, F.M.J.; Hajarizadeh, B.; Mowat, Y.; Marshall, A.D.; Bajis, S.; Marks, P.; Amin, J.; Smith, J.; Edwards, M.; et al. Evaluation of the Xpert HCV Viral Load point-of-care assay from venipuncture-collected and finger-stick capillary whole-blood samples: A cohort study. Lancet Gastroenterol. Hepatol. 2017, 2, 514–520. [Google Scholar] [CrossRef]
- Lamoury, F.M.J.; Bajis, S.; Hajarizadeh, B.; Marshall, A.D.; Martinello, M.; Ivanova, E.; Catlett, B.; Mowat, Y.; Marks, P.; Amin, J.; et al. Evaluation of the XpertHCV Viral Load Fingerstick point-of-care assay. J. Infect. Dis. 2018, 217, 1889–1896. [Google Scholar] [CrossRef]
- Duchesne, L.; Lacombe, K. Innovative technologies for point-of-care testing of viral hepatitis in low-resource and decentralized settings. J. Viral Hepat. 2017, 25, 108–117. [Google Scholar] [CrossRef]
- World Health Organization. Essential Medicines and Health Products: In the Lead-up to Paris AIDS Conference, WHO Prequalifies First Generic Hepatitis C Medicine and First HIV Self-Test. 2017. Available online: http://www.who.int/medicines/news/2017/1st_generic-hepC_1stHIVself-test-prequalified/en/ (accessed on 21 March 2021).
- Nasrullah, M.; Sergeenko, D.; Gvinjilia, L.; Gamkrelidze, A.; Tsertsvadze, T.; Butsashvili, M.; Metreveli, D.; Sharvadze, L.; Alkhazashvili, M.; Shadaker, S.; et al. The role of screening and treatment in national progress to-ward hepatitis C elimination—Georgia, 2015–2016. MMWR Morb. Mortal Wkly. Rep. 2017, 66, 773–776. [Google Scholar] [CrossRef]
- Mitruka, K.; Tsertsvadze, T.; Butsashvili, M.; Gamkrelidze, A.; Sabelashvili, P.; Adamia, E.; Chokheli, M.; Drobeniuc, J.; Hagan, L.; Harris, A.M.; et al. Launch of a Nationwide Hepatitis C Elimination Program-Georgia, April 2015. MMWR Morb. Mortal Wkly. Rep. 2015, 64, 753–757. [Google Scholar] [CrossRef]
- Gvinjilia, L.; Nasrullah, M.; Sergeenko, D.; Tsertsvadze, T.; Kamkamidze, G.; Butsashvili, M.; Gamkrelidze, A.; Imnadze, P.; Kvaratskhelia, V.; Chkhartishvili, N.; et al. National Progress Toward Hepatitis C Elimination—Georgia, 2015–2016. MMWR Morb. Mortal Wkly. Rep. 2016, 65, 1132–1135. [Google Scholar] [CrossRef]
- Kim, D.D.; Hutton, D.W.; Raouf, A.A.; Salama, M.; Hablas, A.; Seifeldin, I.A.; Soliman, A.S. Cost-effectiveness model for hepatitis C screening and treatment: Implications for Egypt and other countries with high prevalence. Glob. Public Health 2015, 10, 296–317. [Google Scholar] [CrossRef]
- WHO. Global Health Sector Strategy on Viral Hepatitis 2016—Towards Ending Viral Hepatitis; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Warkad, S.D.; Nimse, S.B.; Song, K.-S.; Kim, T. HCV Detection, Discrimination, and Genotyping Technologies. Sensors 2018, 18, 3423. [Google Scholar] [CrossRef]
- EASL. Recommendations on treatment of hepatitis C 2018. J. Hepatol. 2018, 69, 461–511. [Google Scholar] [CrossRef] [PubMed]
- EASL. Recommendations on Treatment of Hepatitis C 2016. J Hepatol. 2017, 66, 153–194. [Google Scholar] [CrossRef]
- Applegate, T.L.; Fajardo, E.; Sacks, J.A. Hepatitis C Virus Diagnosis and the Holy Grail. Infect. Dis. Clin. N. Am. 2018, 32, 425–445. [Google Scholar] [CrossRef]
- Warkad, S.D.; Song, K.-S.; Pal, D.; Nimse, S.B. Developments in the HCV Screening Technologies Based on the Detection of Antigens and Antibodies. Sensors 2019, 19, 4257. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.O.; Carman, W.F. The use of the OraSure® collection device for hepatitis virus testing in health care settings. J. Clin. Virol. 2005, 34, S22–S28. [Google Scholar] [CrossRef]
- Shivkumar, S.; Peeling, R.; Jafari, Y.; Joseph, L.; Pant, P.N. Accuracy of rapid and point-of-care screening tests for hepatitis C: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 157, 558–566. [Google Scholar] [CrossRef]
- Chevaliez, S.; Poiteau, L.; Rosa, I.; Soulier, A.; Roudot-Thoraval, F.; Laperche, S.; Hézode, C.; Pawlotsky, J.M. Prospective assessment of rapid diagnostic tests for the detection of antibodies to hepatitis C virus, a tool for improving access to care. Clin. Microbiol. Infect. 2016, 22, 459.e1–459.e6. [Google Scholar] [CrossRef]
- Greenman, J.; Roberts, T.; Cohn, J.; Messac, L. Dried blood spot in the genotyping, quantification and storage of HCV RNA: A systematic literature review. J. Viral Hepat. 2015, 22, 353–361. [Google Scholar] [CrossRef]
- Scalioni, L.P.; Cruz, H.M.; de Paula, V.S.; Miguel, J.C.; Marques, V.A.; Villela-Nogueira, C.A.; Milagres, F.A.; Cruz, M.S.; Bastos, F.I.; Andrade, T.M.; et al. Performance of rapid hepatitis C virus antibody assays among high- and low-risk populations. J. Clin. Virol. 2014, 60, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.J.; Park, Q.; Kang, E.S.; Yoo, B.C.; Park, K.U.; Kim, J.W.; Hwang, Y.S.; Kim, M.H. Performance evaluation of the OraQuick hepatitis C virus rapid antibody test. Ann. Lab. Med. 2013, 33, 184–189. [Google Scholar] [CrossRef]
- Pallarés, C.; Carvalho-Gomes, Â.; Hontangas, V.; Conde, I.; Di Maira, T.; Aguilera, V.; Benlloch, S.; Berenguer, M.; López-Labrador, F.X. Performance of the OraQuick Hepatitis C virus antibody test in oral fluid and fingerstick blood before and after treat-ment-induced viral clearance. J. Clin. Virol. 2018, 102, 77–83. [Google Scholar] [CrossRef]
- Khan, H.; Hill, A.; Main, J.; Brown, A.; Cooke, G. Can Hepatitis C Virus Antigen Testing Replace Ribonucleic Acid Polymearse Chain Reaction Analysis for Detecting Hepatitis C Virus? A Systematic Review. Open Forum Infect. Dis. 2017, 4, 252. [Google Scholar] [CrossRef] [PubMed]
- Duchesne, L.; Njouom, R.; Lissock, F.; Tamko-Mella, G.F.; Rallier, S.; Poiteau, L.; Soulier, A.; Chevaliez, S.; Vernet, G.; Rouveau, N.; et al. HCV Ag quantification as a one-step procedure in diagnosing chronic hepatitis C infection in Cameroon: The ANRS 12336 study. J. Int. AIDS Soc. 2017, 20, 21446. [Google Scholar] [CrossRef]
- Cresswell, F.V.; Fisher, M.; Hughes, D.J.; Shaw, S.G.; Homer, G.; Hassan-Ibrahim, M.O. Hepatitis C core anti-gen testing: A reliable, quick, and potentially cost effective alternative to hepatitis C polymerase chain reac-tion in diagnosing acute hepatitis C virus infection. Clin. Infect. Dis. 2015, 60, 263–266. [Google Scholar] [CrossRef][Green Version]
- Kamal, S.M.; Kassim, S.; El Gohary, E.; Fouad, A.; Nabegh, L.; Hafez, T.; Bahnasy, K.; Hassan, H.; Ghoraba, D. The accuracy and cost-effectiveness of hepatitis C core antigen assay in the monitoring of anti-viral therapy in patients with chronic hepatitis C genotype Aliment. Pharmacol. Ther. 2015, 42, 307–318. [Google Scholar] [CrossRef]
- Cohn, J.; Roberts, T.; Amorosa, V.; Lemoine, M.; Hill, A. Simplified diagnostic monitoring for hepatitis C, in the new era of direct-acting antiviral treatment. Curr. Opin. HIV AIDS 2015, 10, 369–373. [Google Scholar] [CrossRef]
- Abdollahi-Aghdam, A.; Majidi, M.R.; Omidi, Y. Microfluidic paper-based analytical devices (µPADs) for fast and ultrasensitive sensing of biomarkers and monitoring of diseases. BioImpacts BI 2018, 8, 237. [Google Scholar] [CrossRef]
- Eletxigerra, U.J.; Martinez-Perdiguero, S.; Merino, R.; Villalonga, J.; Pingarrón, J.M.; Campuzano, S. Amperometric magnetoimmunoassay for the direct detection of tumor necrosis factor alpha biomarker in human serum. Anal. Chim. Acta 2014, 838, 37–44. [Google Scholar] [CrossRef]
- Ghanbari, K.; Roushani, M.; Azadbakht, A. Ultra-sensitive aptasensor based on a GQD nanocomposite for detection of hepatitis C virus core antigen. Anal. Biochem. 2017, 534, 64–69. [Google Scholar] [CrossRef]
- Roh, C.; Lee, H.Y.; Kim, S.E.; Jo, S.K. Quantum-dots-based detection of hepatitis C virus (HCV) NS3 using RNA aptamer on chip. J. Chem. Technol. Biotechnol. 2010, 85, 1130–1134. [Google Scholar] [CrossRef]
- Easterbrook, P.J.; Roberts, T.; Sands, A.; Peeling, R. Diagnosis of viral hepatitis. Curr. Opin. HIV AIDS 2017, 12, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Lange, B.; Cohn, J.; Roberts, T.; Camp, J.; Chauffour, J.; Gummadi, N.; Ishizaki, A.; Nagarathnam, A.; Tuaillon, E.; van de Perre, P.; et al. Diagnostic accuracy of serological diagnosis of hepatitis C and B using dried blood spot samples (DBS): Two systematic reviews and meta-analyses. BMC Infect. Dis. 2017, 17, 700. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Morón, S.; Jiménez, B.A.; Jiménez-Sousa, M.A.; Bellón, J.M.; Ryan, P.; Resino, S. Evaluation of the diagnostic accuracy of laboratory-based screening for hepatitis C in dried blood spot samples: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Lange, B.; Roberts, T.; Cohn, J.; Greenman, J.; Camp, J.; Ishizaki, A.; Messac, L.; Tuaillon, E.; van de Perre, P.; Pichler, C.; et al. Diagnostic accuracy of detection and quantification of HBV-DNA and HCV-RNA using dried blood spot (DBS) samples—A systematic review and meta-analysis. BMC Infect. Dis. 2017, 17, 71–85. [Google Scholar] [CrossRef]
- Catlett, B.; Bajis, S.; Starr, M.; Dore, G.J.; Hajarizadeh, B.; Cunningham, P.H.; Applegate, T.L.; Grebely, J. Evaluation of the Aptima HCV Quant Dx Assay for HCV RNA detection from finger-stick capillary dried blood spot and venepuncture-collected samples. J. Infect. Dis. 2020, 223, 818–826. [Google Scholar] [CrossRef]
- 2020 Pipeline Report. Available online: https://www.treatmentactiongroup.org/resources/pipeline-report/2020-pipeline-report/last (accessed on 23 March 2021).
- Nguyen, L.; Nguyen, V.; Le Ai, K.; Truong, M.; Tran, T.; Jamil, M.; Johnson, C.; Reipold, E.; Easterbrook, P.; Park, K. Acceptability and Usability of HCV Self-Testing in High Risk Populations in Vietnam. Diagnostics 2021, 11, 377. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, M.; Hang, L.; Kong, F.; Yan, H.; Zhang, Y.; Feng, X.; Gao, Y.; Wang, C.; Ma, H.; et al. Evaluation of a new point-of-care oral anti-HCV test for screening of hepatitis C virus infection. Virol. J. 2020, 17, 14. [Google Scholar] [CrossRef]
- Kimble, M.M.; Stafylis, C.; Treut, P.; Saab, S.; Klausner, J.D. Clinical evaluation of a hepatitis C antibody rapid immunoassay on self-collected oral fluid specimens. Diagn. Microbiol. Infect. Dis. 2019, 95, 149–151. [Google Scholar] [CrossRef]
- Vetter, B.N.; Reipold, E.I.; Ongarello, S.; Fajardo, E.; Tyshkovskiy, A.; Ben, I.; Vasylyev, M. Prospective evaluation of hepatitis C virus antibody detection in whole blood collected on dried blood spots with the INNOTEST® HCV Ab IV enzyme immunoassay. J. Clin. Virol. 2021, 137, 104783. [Google Scholar] [CrossRef]
- Padhi, A.; Gupta, E.; Singh, G.; Agarwal, R.; Sharma, M.K.; Sarin, S.K. Evaluation of the Point of Care Molecular Diagnostic Genedrive HCV ID Kit for the detection of HCV RNA in clinical samples. Epidemiol. Infect. 2020, 1–23. [Google Scholar] [CrossRef]
- Marins, E.G.; Krey, N.; Becker, A.; Melzer, S.; Hoppler, M. Evaluation of the cobas® HCV test for quantifying HCV RNA in dried plasma spots collected using the cobas® Plasma Separation Card. J. Virol. Methods 2020, 278, 113820. [Google Scholar] [CrossRef] [PubMed]
- Prinsenberg, T.; Rebers, S.; Boyd, A.; Zuure, F.; Prins, M.; Van Der Valk, M.; Schinkel, J. Dried blood spot self-sampling at home is a feasible technique for hepatitis C RNA detection. PLoS ONE 2020, 14, e0231385. [Google Scholar] [CrossRef]
- Van Tilborg, M.; Al Marzooqi, S.H.; Wong, W.W.; Maan, R.; Vermehren, J.; Maasoumy, B.; Mazzulli, T.; Bolotin, S.; Garber, G.; Guerra, F.; et al. HCV core antigen as an alternative to HCV RNA testing in the era of direct-acting antivirals: Retrospective screening and diagnostic cohort studies. Lancet Gastroenterol. Hepatol. 2018, 3, 856–864. [Google Scholar] [CrossRef]
- Biondi, M.J.; Van Tilborg, M.; Smookler, D.; Heymann, G.; Aquino, A.; Perusini, S.; Mandel, E.; Kozak, R.A.; Cherepanov, V.; Kowgier, M.; et al. Hepatitis C Core-Antigen Testing from Dried Blood Spots. Viruses 2019, 11, 830. [Google Scholar] [CrossRef]
- Evaluation of Dried Blood Spot for HCV RNA Testing. Evaluation of Dried Blood Spot for HCV RNA Testing—Full Text View. Available online: ClinicalTrials.gov (accessed on 31 March 2021).
- Chantratita, W.; Song, K.-S.; Nimse, S.B.; Pongthanapisith, V.; Thongbaiphet, N.; Wongtabtim, G.; Pasomsub, E.; Angkanavin, K.; Sonawane, M.D.; Warkad, S.D.; et al. 6 HCV Genotyping 9G test for HCV 1a, 1b, 2, 3, 4 and 6 (6a, 6f, 6i and 6n) with high accuracy. J. Virol. Methods 2017, 246, 95–99. [Google Scholar] [CrossRef]
- Warkad, S.D.; Nimse, S.B.; Song, K.-S.; Chantratita, W.; Pongthanapisith, V.; Nawale, L.U.; Kim, T. Performance of 6 HCV genotyping 9G test for HCV genotyping in clinical samples. Virol. J. 2018, 15, 107. [Google Scholar] [CrossRef]
- Warkad, S.D.; Nimse, S.B.; Song, K.-S.; Kim, T. Development of a Method for Screening and Genotyping of HCV 1a, 1b, 2, 3, 4, and 6 Genotypes. ACS Omega 2020, 8, 10794–10799. [Google Scholar] [CrossRef]
- Poljak, M. Simplification of hepatitis C testing: A time to act. Acta Dermatovenerol. Alp. Pannonica Adriat. 2020, 29. [Google Scholar] [CrossRef]
- Saludes, V.; Antuori, A.; Lazarus, J.; Folch, C.; González-Gómez, S.; González, N.; Ibáñez, N.; Colom, J.; Matas, L.; Casabona, J.; et al. Evaluation of the Xpert HCV VL Fingerstick point-of-care assay and dried blood spot HCV-RNA testing as simplified diagnostic strategies among people who inject drugs in Catalonia, Spain. Int. J. Drug Policy 2020, 80, 102734. [Google Scholar] [CrossRef]
- Velásquez-Orozco, F.; Rando-Segura, A.; Martínez-Camprecios, J.; Salmeron, P.; Najarro-Centeno, A.; Esteban, À.; Quer, J.; Buti, M.; Pumarola-Suñe, T.; Rodríguez-Frías, F. Utility of the Cobas® Plasma Separation Card as a Sample Collection Device for Serological and Virological Diagnosis of Hepatitis C Virus Infection. Diagnostics 2021, 11, 473. [Google Scholar] [CrossRef]
- Antuori, A.; Montoya, V.; Piñeyro, D.; Sumoy, L.; Joy, J.; Krajden, M.; González-Gómez, S.; Folch, C.; Casabona, J.; Matas, L.; et al. Characterization of acute HCV infection and transmission networks in people who currently inject drugs in Catalonia: Usefulness of dried blood spots. Hepatology 2021. [Google Scholar] [CrossRef] [PubMed]
- Saludes, V.; Antuori, A.; Folch, C.; González, N.; Ibáñez, N.; Majó, X.; Colom, J.; Matas, L.; Casabona, J.; Martró, E.; et al. Utility of a one-step screening and diagnosis strategy for viremic HCV infection among people who inject drugs in Catalonia. Int. J. Drug Policy 2019, 74, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Saludes, V.; Folch, C.; Morales-Carmona, A.; Ferrer, L.; Fernàndez-López, L.; Muñoz, R.; Jiménez, M.; Loureiro, E.; Fernández-Dávila, P.; Bascuñana, E.; et al. Community-based screening of hepatitis C with a one-step RNA detection algorithm from dried-blood spots: Analysis of key populations in Barcelona, Spain. J. Viral Hepat. 2017, 25, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Valerio, H.; Alavi, M.; Silk, D.; Treloar, C.; Martinello, M.; Milat, A.; Dunlop, A.; Holden, J.; Henderson, C.; Amin, J.; et al. Progress Towards Elimination of Hepatitis C Infection Among People Who Inject Drugs in Australia: The ETHOS Engage Study. Clin. Infect. Dis. 2021, 73, e69–e78. [Google Scholar] [CrossRef] [PubMed]
- Catlett, B.; Carrera, A.; Starr, M.; Applegate, T.L.; Lowe, P.; Grebely, J.; Cunningham, H.P. Performance evaluation of the Hologic Aptima HCV Quant Dx assay for detection of HCV RNA from dried blood spots. J. Clin. Virol. 2019, 112, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Bregenzer, A.; Warmann, N.; Ottiger, C.; Fux, C.A. Rapid point-of-care HCV RNA quantification in capillary whole blood for diagnosing chronic HCV infection, monitoring treatment and detecting reinfection. Swiss Med. Wkly. 2019, 149, w20137. [Google Scholar] [CrossRef] [PubMed]
- Prabdial-Sing, N.; Gaelejwe, L.; Makhathini, L.; Thaver, J.; Manamela, M.J.; Malfeld, S.; Spearman, C.W.; Sonderup, M.; Scheibe, A.; Young, K.; et al. The performance of hepatitis C virus ( HCV ) antibody point-of-care tests on oral fluid or whole blood and dried blood spot testing for HCV serology and viral load among individuals at higher risk for HCV in South Africa. Health Sci. Rep. 2021, 4, e229. [Google Scholar] [CrossRef]
- Pollock, K.G.; McDonald, S.A.; Gunson, R.; McLeod, A.; Went, A.; Goldberg, D.J.; Hutchinson, S.J.; Barclay, S.T. Real-world utility of HCV core antigen as an alternative to HCV RNA testing: Implications for viral load and genotype. J. Viral Hepat. 2020, 27, 996–1002. [Google Scholar] [CrossRef]
- Lamoury, F.M.; Soker, A.; Martinez, D.; Hajarizadeh, B.; Cunningham, E.B.; Cunningham, P.; Bruggmann, P.; Foster, G.R.; Dalgard, O.; Backmund, M.; et al. Hepatitis C virus core antigen: A simplified treatment monitoring tool, including for post-treatment relapse. J. Clin. Virol. 2017, 92, 32–38. [Google Scholar] [CrossRef]
- Wong, X.Z.; Gan, C.C.; Mohamed, R.; Yahya, R.; Ganapathy, S.; Tan, S.S.; Lim, S.K. Hepatitis C core antigen testing to diagnose active hepatitis C infection among haemodialysis patients. BMC Nephrol. 2020, 21, 480. [Google Scholar] [CrossRef]
- Vetter, B.N.; Reipold, E.I.; Ongarello, S.; Audu, R.; Ige, F.A.; Alkhazashvili, M.; Chitadze, N.; Vanroye, F.; De Weggheleire, A.; An, S.; et al. Sensitivity and Specificity of Rapid Diagnostic Tests for Hepatitis C Virus With or Without HIV Coinfection: A Multicentre Laboratory Evaluation Study. J. Infect. Dis. 2020, 2, 289. [Google Scholar] [CrossRef]
- Tsertsvadze, T.; Gamkrelidze, A.; Chkhartishvili, N.; Abutidze, A.; Sharvadze, L.; Kerashvili, V.; Butsashvili, M.; Metreveli, D.; Gvinjilia, L.; Shadaker, S.; et al. Three years of progress towards achieving hepatitis C elimination in the country of Georgia, April 2015–March 2018. Clin. Infect. Dis. 2019, 71, ciz956. [Google Scholar] [CrossRef]
- Averhoff, F.; Shadaker, S.; Gamkrelidze, A.; Kuchuloria, T.; Gvinjilia, L.; Getia, V.; Sergeenko, D.; Butsashvili, M.; Tsertsvadze, T.; Sharvadze, L.; et al. Progress and challenges of a pioneering hepatitis C elimination program in the country of Georgia. J. Hepatol. 2020, 72, 680–687. [Google Scholar] [CrossRef]
- Shiha, G.; Soliman, R.; Mikhail, N.N.; Easterbrook, P. An educate, test and treat model towards elimination of hepatitis C infection in Egypt: Feasibility and effectiveness in 73 villages. J. Hepatol. 2020, 72, 658–669. [Google Scholar] [CrossRef]
- Waked, I.; Esmat, G.; Elsharkawy, A.; El-Serafy, M.; Abdel-Razek, W.; Ghalab, R.; Elshishiney, G.; Salah, A.; Abdel Megid, S.; Kabil, K.; et al. Screening and Treatment Program to Eliminate Hepatitis C in Egypt. N. Engl. J. Med. 2020, 382, 1166–1174. [Google Scholar] [CrossRef]
- Konerman, M.A.; Thomson, M.; Gray, K.; Moore, M.; Choxi, H.; Seif, E.; Lok, A.S. Impact of an electronic health record alert in primary care on increasing hepatitis c screening and curative treatment for baby boomers. Hepatology 2017, 66, 1805–1813. [Google Scholar] [CrossRef]
- International Treatment Preparedness Coalition-Global. Activist Toolkit on PrEP; ITPC Global: Johannesburg, South Africa, 2018; Available online: http://itpcglobal.org/wp-content/uploads/2018/02/ITPC-PrEP-Toolkit-English.pdf (accessed on 1 April 2021).
- Degenhardt, L.; Peacock, A.; College, S.; Leung, J.; Grebely, J.; Vickerman, P.; Stone, J.; Cunningham, E.B.; Trickey, A.; Dumchev, K.; et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: A multistage systematic review. Lancet Glob. Health 2017, 5, 1192–1207. [Google Scholar] [CrossRef]
- Annual Meeting of the Canadian Association for the Study of the Liver (CASL), the Canadian Network on Hepatitis C (CANHEPC) and the Canadian Association of Hepatology Nurses (CAHN) 2020 Abstracts. Available online: https://canlivj.utpjournals.press/doi/pdf/10.3138/canlivj.3.1.abst (accessed on 3 April 2021).
- Collaborative Registration Procedure (CRP) for In Vitro Diagnostics (IVDs)—Information Note. Available online: https://www.who.int/diagnostics_laboratory/191111_crp_ivd_information_note.pdf (accessed on 4 April 2021).
- Solomon, S.S.; Quinn, T.C.; Solomon, S.; McFall, A.M.; Srikrishnan, A.K.; Verma, V.; Kumar, M.S.; Laeyendecker, O.; Celentano, D.D.; Iqbal, S.H.; et al. Integrating HCV testing with HIV programs improves hepatitis C outcomes in people who inject drugs: A cluster-randomized trial. J. Hepatol. 2020, 72, 67–74. [Google Scholar] [CrossRef]
- Walker, J.G.; Mafirakureva, N.; Iwamoto, M.; Campbell, L.; Kim, C.S.; Hastings, R.; Doussett, J.; Le Paih, M.; Balkan, S.; Marquardt, T.; et al. Cost and cost-effectiveness of a simplified treatment model with direct-acting antivirals for chronic hepatitis C in Cambodia. Liver Int. 2020, 40, 2356–2366. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Liu, H.; Li, W.; Lin, F.; Jiang, L.; Li, X.; Xu, P.; Zhang, L.; Zhao, L.; et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J. Hepatol. 2020, 73, 807–816. [Google Scholar] [CrossRef]
 = central lab,
= central lab,  = phlebotomy,
= phlebotomy,  = sample,
= sample,  = testing,
= testing,  = hepatic disease assessment,
= hepatic disease assessment,  = diagnosis,
= diagnosis,  = treatment,
= treatment,  = linkage to care,
= linkage to care,  = mortality,
= mortality,  = Egypt. PWIDs = Patients who inject drugs, RDT = rapid diagnostic test, DBS = dried blood spot, DAAs = direct-acting antivirals, GT = genotyping, WORA = women of reproductive age, POCT = point-of-care testing.
= Egypt. PWIDs = Patients who inject drugs, RDT = rapid diagnostic test, DBS = dried blood spot, DAAs = direct-acting antivirals, GT = genotyping, WORA = women of reproductive age, POCT = point-of-care testing.
 = central lab,
= central lab,  = phlebotomy,
= phlebotomy,  = sample,
= sample,  = testing,
= testing,  = hepatic disease assessment,
= hepatic disease assessment,  = diagnosis,
= diagnosis,  = treatment,
= treatment,  = linkage to care,
= linkage to care,  = mortality,
= mortality,  = Egypt. PWIDs = Patients who inject drugs, RDT = rapid diagnostic test, DBS = dried blood spot, DAAs = direct-acting antivirals, GT = genotyping, WORA = women of reproductive age, POCT = point-of-care testing.
= Egypt. PWIDs = Patients who inject drugs, RDT = rapid diagnostic test, DBS = dried blood spot, DAAs = direct-acting antivirals, GT = genotyping, WORA = women of reproductive age, POCT = point-of-care testing.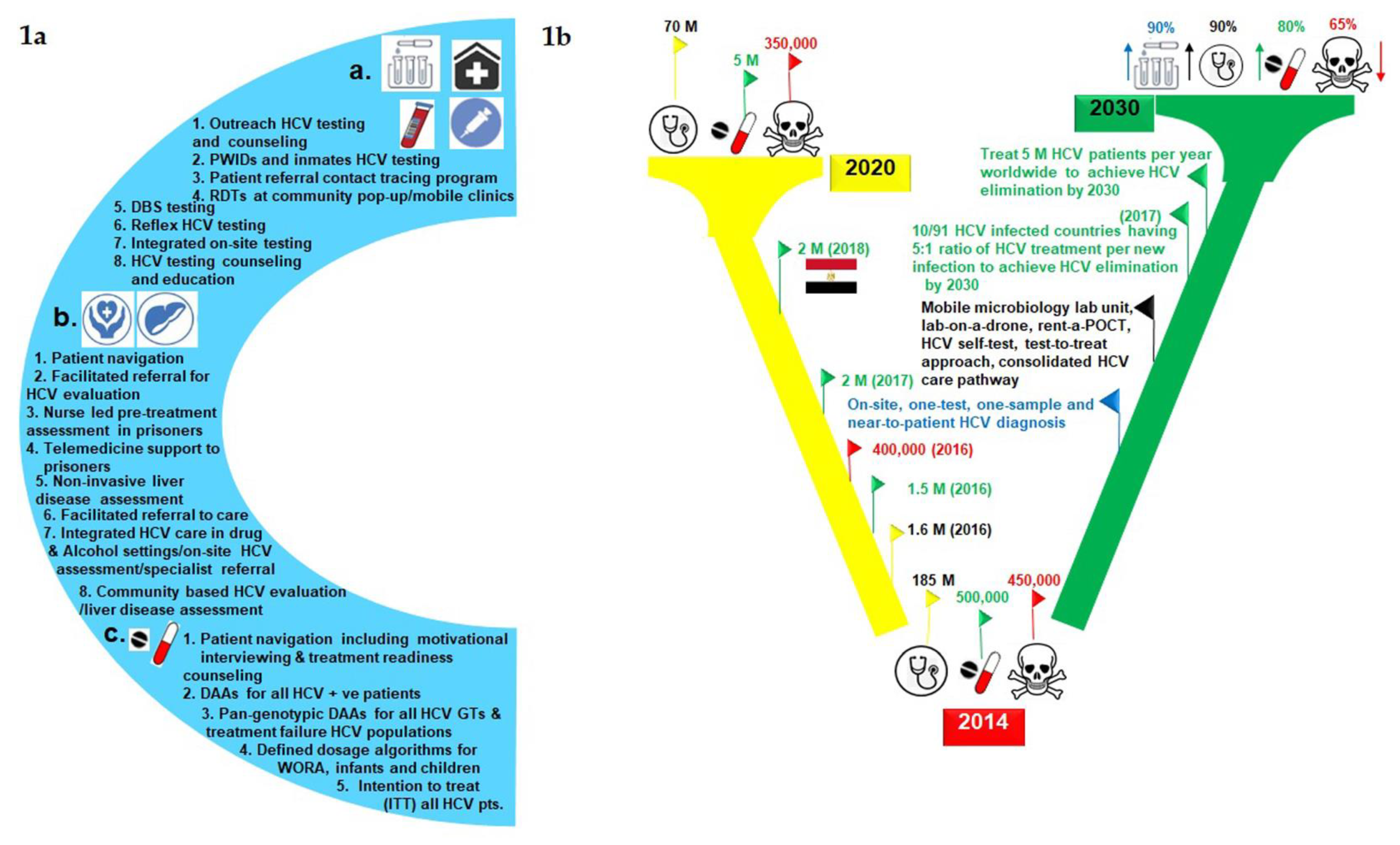
 = central lab,
= central lab,  = phlebotomy,
= phlebotomy,  = sample,
= sample,  = HCV RNA testing,
= HCV RNA testing,  = hepatic disease assessment,
= hepatic disease assessment,  = diagnosis,
= diagnosis,  = treatment,
= treatment,  = RDT,
= RDT,  = DBS,
= DBS,  = RDT + HCV RNA + diagnosis,
= RDT + HCV RNA + diagnosis,  = treatment week,
= treatment week,  = end of treatment,
= end of treatment,  = HCV cure,
= HCV cure,  = linkage to care,
= linkage to care,  = testing
= testing  = mortality
= mortality  = HCV genotyping. RDT = rapid diagnostic tests, Ab = Antibody, DBS = dried blood spot, POC = point-of-care, RBV = ribavirin, Phleb = phlebotomy.
= HCV genotyping. RDT = rapid diagnostic tests, Ab = Antibody, DBS = dried blood spot, POC = point-of-care, RBV = ribavirin, Phleb = phlebotomy.
 = central lab,
= central lab,  = phlebotomy,
= phlebotomy,  = sample,
= sample,  = HCV RNA testing,
= HCV RNA testing,  = hepatic disease assessment,
= hepatic disease assessment,  = diagnosis,
= diagnosis,  = treatment,
= treatment,  = RDT,
= RDT,  = DBS,
= DBS,  = RDT + HCV RNA + diagnosis,
= RDT + HCV RNA + diagnosis,  = treatment week,
= treatment week,  = end of treatment,
= end of treatment,  = HCV cure,
= HCV cure,  = linkage to care,
= linkage to care,  = testing
= testing  = mortality
= mortality  = HCV genotyping. RDT = rapid diagnostic tests, Ab = Antibody, DBS = dried blood spot, POC = point-of-care, RBV = ribavirin, Phleb = phlebotomy.
= HCV genotyping. RDT = rapid diagnostic tests, Ab = Antibody, DBS = dried blood spot, POC = point-of-care, RBV = ribavirin, Phleb = phlebotomy.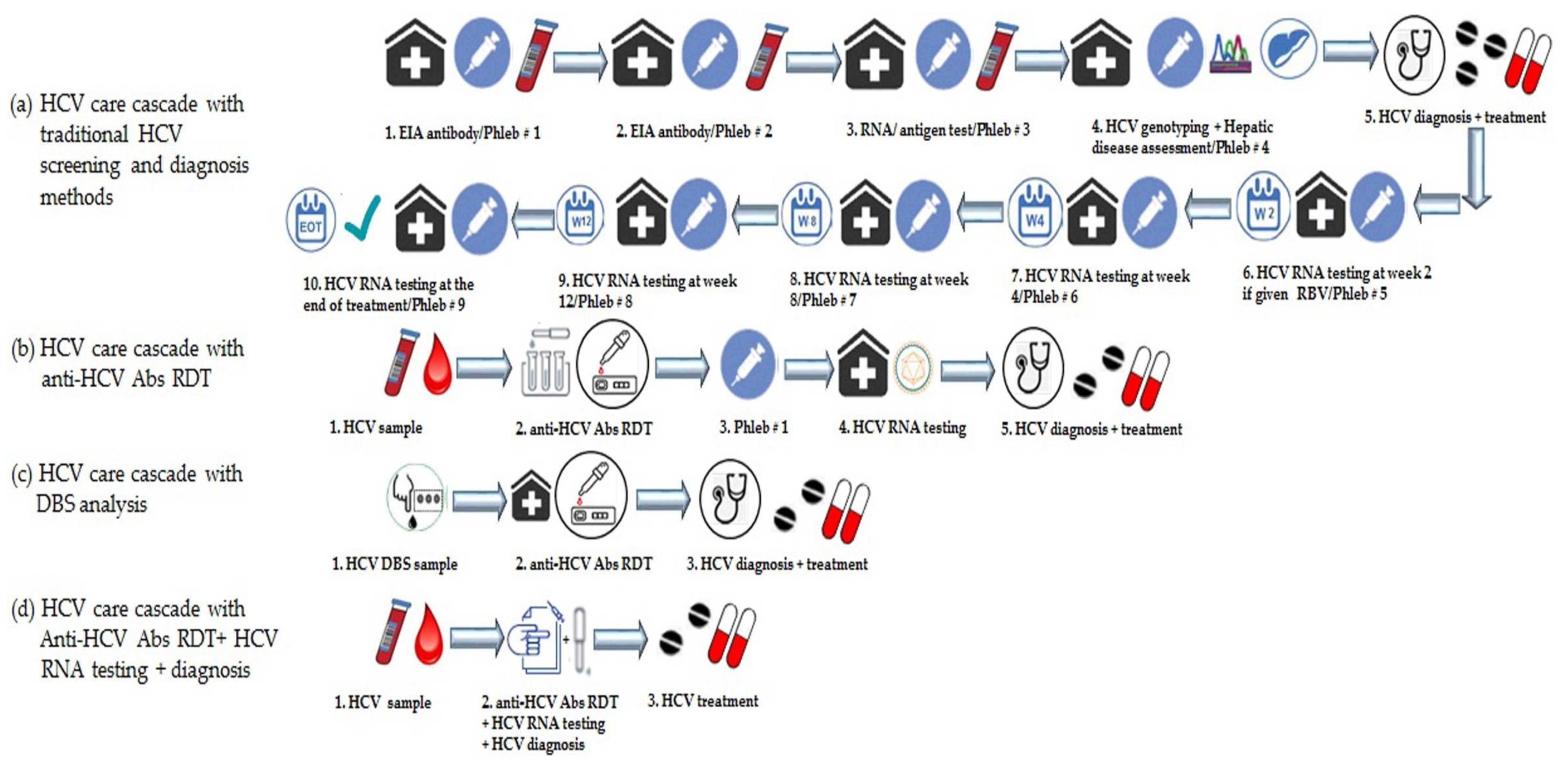
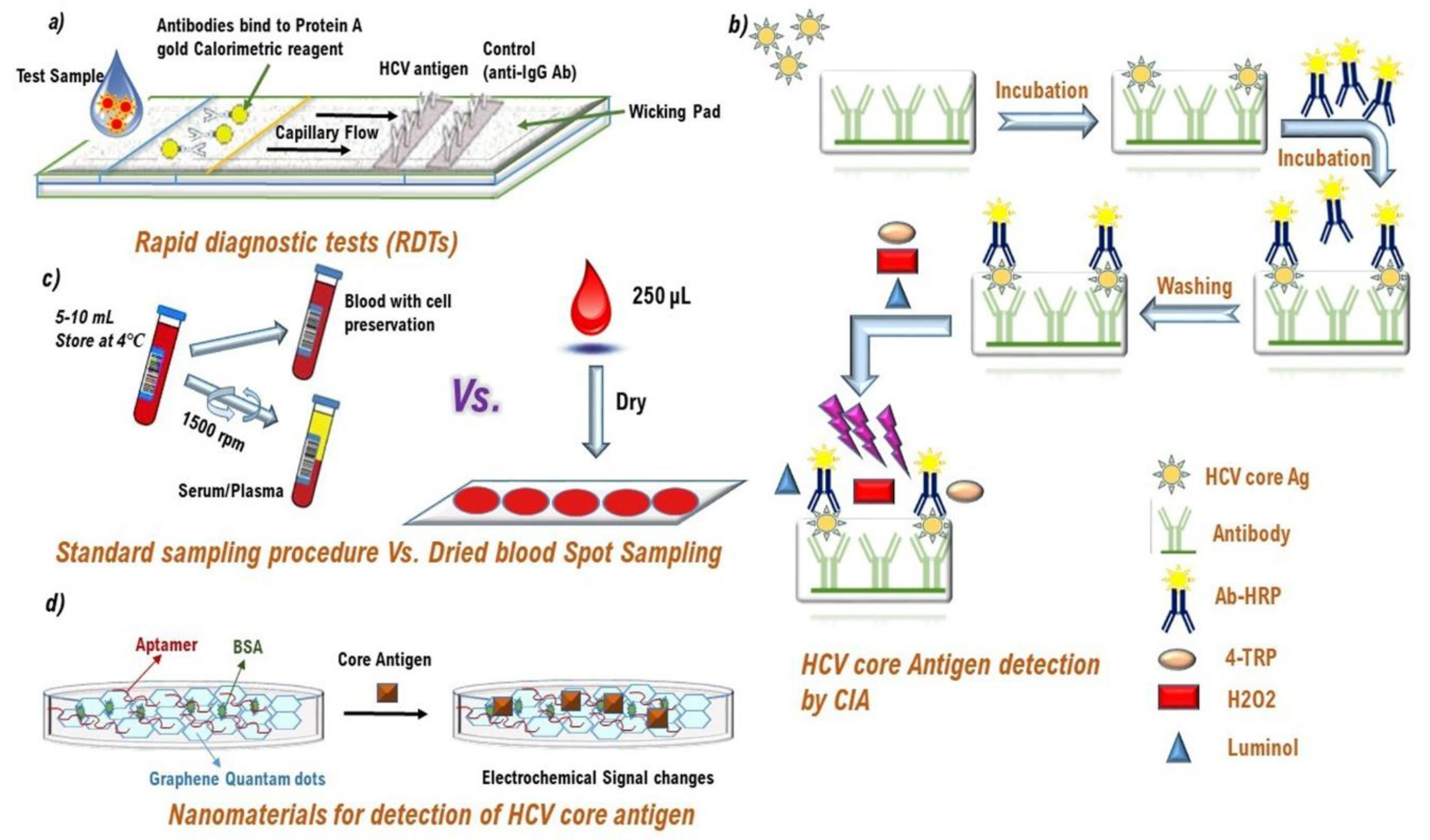
| Anti-HCV Abs Tests | HCV EIA Tests | ||
|---|---|---|---|
| Advantages | Disadvantages | Advantages | Disadvantages |
| Easy to use (i.e., better for task shifting) | Low throughput | High sensitivity and high specificity (approx. 100%) | Effective specimen transport system required |
| Suitable for oral fluid, plasma, serum, saliva, finger prick, and whole blood | Inadequate for patients with low Ab titer or with the compromised immune system | High sample throughput (good for large volumes) | A cold chain environment from 2 °C to 8 °C is required for reagents and not always possible in LMICs |
| Feasible for decentralization HCV testing | Subjective interpretation (i.e., operator dependent) | Low cost if used for high volume samples | Additional precise equipment requires (e.g., micropipette, centrifuges) |
| Equipment-free and suitable for POC analysis with small lab infrastructure | Possible higher cost based on volume | Data processing-based results | Possible higher cost based on volume |
| Good sensitivity and specificity | Compromised quality control | Cost-effective for a large number of samples | Turnaround time usually longer (one batch runs 96 samples), 12 or 24 samples test configuration required for different volume |
| Qualitative results interpretation (i.e., yes/no) | Easier oversight quality control | Interference from different sample matrices | |
| Fast turnaround time (i.e., ~20 min) | Polyvalency for serological screening of HIV, HBV, etc. | Requires technically skilled staff | |
| 1–2 years shelf life if stored properly at room temperature | 1–2 years shelf life if stored properly at room temperature | Equipment calibration, maintenance, and service required | |
| HCV cAg tests | HCV RNA confirmatory test platform (Abbott Realtime®) | ||
| Good to conduct HCV cAg tests for large sample settings (i.e., 100 samples/h) | Cannot use without an effective specimen transport system | Good to conduct HCV RNA tests for high volume settings (i.e., 93 samples/h) | An effective specimen transport system required |
| The testing platform also suitable for HIV screening based on licensing in different countries (i.e., polyvalency) | Not licensed for use with DBS samples | Also suitable for multiple disease screening (e.g., HIV, HCV genotyping) based on licensing in different countries | Not licensed for use with DBS samples |
| Advantageous to detect cAg in the bloodstream (i.e., 2 weeks after infection) than anti-HCV ab tests (i.e., 10 weeks after infection) | Only used for diagnostic confirmation; cannot confirm HCV cure | Low cost for high volume samples | HCV test cost: USD 11–23 (GF price) which may vary depends on volume and term commitment |
| Low cost if used for high volume samplesCost-effective for a large number of samples | Less sensitive with chances of miss infections | Easier oversight quality control | Centralized lab settings are required to handle large volume specimens |
| Easier oversight quality control | Centralized lab settings are required to handle large volume specimens | Turnaround time usually longer | |
| Turnaround time usually longer | Skilled technical staff requires for instrument handling | ||
| Skilled technical staff requires for instrument handling | Equipment calibration, maintenance, and service required | ||
| Equipment calibration, maintenance, and service required | |||
| HCV test cost: USD 8–23 | |||
| HCV RNA confirmatory test platform (Cepheid GeneXpert®) | HCV RNA confirmatory test platform (RocheCobas®Taqman) | ||
| Xpert VL has an HCV RNA quantitative assay for plasma samples and Xpert VL FS for fingerstick whole blood samples | Xpert VL and Xpert VL FS are not available in many countries and not approved in the USA | Suitable for both HCV RNA qualitative and quantitative RNA confirmation (93 tests/batch) | Phase-out for the Cobas® 4800 and 6800 systems |
| Xpert VL HCV RNA assay is CE marked and has WHO PQ certifications | High cartridge costs for Xpert VL in many countries | HCV RNA qualitative test is cheaper than quantitative | Effective specimen transport system required |
| Xpert VL FS is also CE-marked and WHO PQ | Biowaste incineration may not be available in decentralized settings for both Xpert VL and Xpert VL FS platforms | Low cost with reduced test pricing to 85 LMICs | Not licensed for use with DBS samples |
| Xpert VL FS is suitable for HCV-infected IDUs with poor vein access | Lab infrastructure is required for GeneXpert instrument and reagents | Efficient automated system | Turnaround time usually longer |
| Xpert VL FS cartridge is feasible for simplification in sample collection | Transport cost and centrifuges are required for plasma specimens used of Xpert VL platforms | Polyvalency for screening multiple diseases (e.g., TB, HIV, HPV, HBV, etc.) based on licensing in different countries | Lab infrastructure is required to house equipment |
| Xpert VL is very accurate, simple to operate, and good for task shifting to facilitate decentralized HCV testing | Distributor mark-ups, additional services, and maintenance costs required | Easier oversight quality control | Skilled technical staff requires for instrument handling despite fully automated |
| Xpert VL turnaround time is around 108–110 min on the same day while Xpert VL FS in 60 min | Fixed test price regardless of sample volumes | Equipment service and maintenance required | |
| Having an extensive test menu for Integration of testing across other disease programs (e.g., TB, HIV, HPV, HBV, etc.) | Preferential pricing is not publically available | ||
| Preferential pricing in 145 LMICs | |||
| HCV RNA confirmatory test platform (Genedrive®HCV) | Dried blood spot (DBS) samples | ||
| Suitable for HCV RNA POC testing | High cost per patient sample | An alternative to plasma samples | An organized, strong, and effective transport system for sample delivery |
| Small portable unit (weighs 1 kg) | Both device and test pricing are high | Suitable for small volume samples collected by fingerstick | Reduced analytical sensitivity for RNA analysis due to small sample volume |
| Qualitative HCV RNA analysis (+ve/−ve/undetected) | Sensitivity is less than lab-based methods (i.e., LLoD is 2362 IU/mL than the recommended 1000 IU/mL for other HCV RNA POC tests) | An alternative to RDTs, in harm reduction centers or prison settings | No immediate delivery of results to the patients |
| Turnaround time 90 min, suitable for same-day diagnosis | Low throughput per device | Suitable for HCV Ab testing, genotyping, and treatment resistance testing | Quality standards need to develop |
| Suitable for few numbers of patients/day | A large volume sample (i.e., 30 µL plasma) is required which could be challenging in POC settings | Useful for HCV vertical transmission detection in infants and children | Limited use for research purposes because of lack of SRA status for companies |
| Room temperature storage for reagents | Not fully automated, some steps require precise micro pipetting for sample/reagents | Inexpensive | Scale-up of DBS in LMICs requires additional studies and validation work |
| Requires uninterrupted power supply, which may not available in some LMICs | Suitable for mass/large HCV screening campaigns in vulnerable populations and highly prevalent HCV settings | Requires uninterrupted power supply, which may not available in some LMICs | |
| Only valid for HCV testing and not appropriate for multi-disease testing (i.e., polyvalency) | Appropriate for decentralized collection of samples to deliver by courier in central labs | Only valid for HCV testing and not appropriate for multi-disease testing | |
| No risk of biohazard because of nonhazardous sample interface | |||
| Room temperature transportation | |||
| Low level of training and suitable for task-shifting | |||
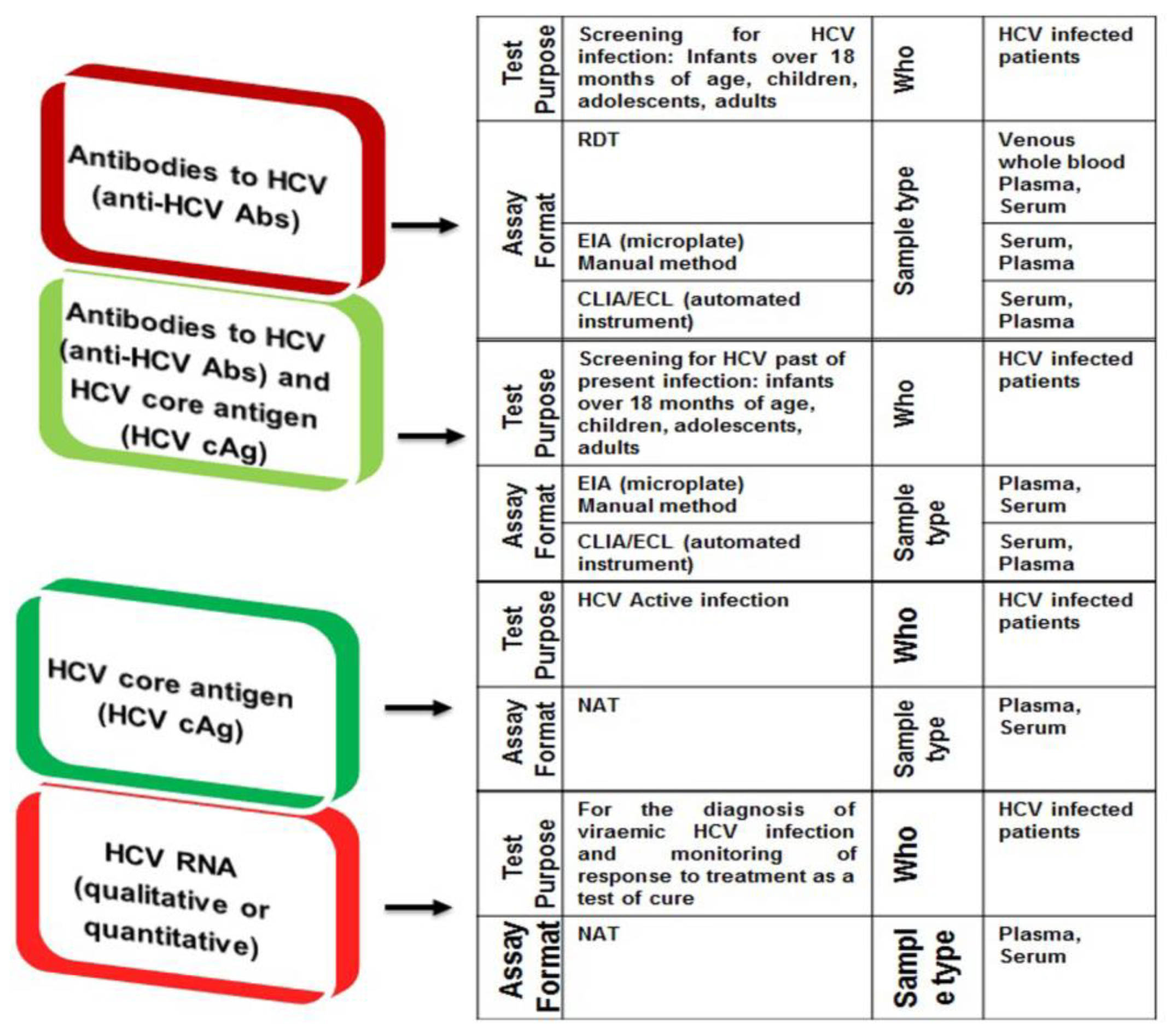
| Test Name | Sample Type/Setting | Turnaround Time | Multiplexing | Price | Regulatory Status | WHO PQ Review | System Suitability |
|---|---|---|---|---|---|---|---|
| 1. HCV Enzyme Immunoassay (HCV-EIA) tests | |||||||
| INNOTEST HCV Ab IV | Serum, plasma | 179 min | Yes (HIV & other markers) | ND | WHO PQ | - | Central labs |
| INNO-LIA HCV Score | Serum, plasma | 1 day | No | ND | WHO PQ CE-marked | - | Strip-based method but requires cold chain and other small equipment |
| Bioelisa HCV 4.0 | Serum, plasma | 150 min | Yes (HIV, HBV, HEV, among others) | ND | WHO PQ, CE-marked | - | Central labs |
| Murex Anti-HCV 4.0 | Serum, plasma | 120 min | Yes (HIV, HBV, HEV, among others) | ND | WHO PQ, CE-marked | - | Central labs |
| Enzygnost Anti-HCV 4.0 | Serum, plasma | 120 min | Yes (HBV, HAV) | ND | Did not receive WHO PQ approval | - | Central labs |
| 2. HCV core antigen (HCV cAg) tests | |||||||
| Abbott ARCHITECT HCV Ag assay | Serum, plasma | ND | Yes (HIV, HBV, HAV, among others) | USD 8–23 (EUR 7–20) per test; USD 100,000 per instrument | CE-marked, WHO PQ | - | Central labs |
| Monolisa HCV Ag-Ab ULTRA V2 | Serum, plasma | 150–180 min | Yes (HIV, HBV, HAV, among others) | ND | CE-marked, | WHO PQ (Jan 2020) | Central labs |
| 3. HCV Rapid diagnostic tests (HCV-RDTs) | |||||||
| OraQuick HCV RDT | Oral, fingerstick, venous blood | 20 min | No | USD 8 (MSF price); USD 12 per test | FDA Approved CE-marked WHO PQ | - | Price remains too expensive for LMICs |
| SD Bioline | 10 μL whole blood, serum, plasma | 5–20 min | HIV | USD 1–2.40 per test | WHO PQ | - | - |
| Intec Rapid anti-HCV | 10 μL, whole blood, serum, plasma | 15–20 min | No | <USD 1–2.40 per test | WHO PQ, CE-marked | - | - |
| Standard Q HCV Ab Test | Whole blood, serum, plasma | 10–15 min | No | ND | CE-IVD | WHO PQ March 2020 | POC |
| Premier Medical Corporation First Response® HCV Card Test | Whole blood, serum, plasma | ND | No | USD 0.60–1 per test | CE-marked | WHO PQ under review | - |
| ABON HCV Rapid Test Device | Whole blood, serum, plasma | ND | No | ND | WHO PQ under review | - | |
| 4. HCV RNA viral load tests | |||||||
| Cepheid Xpert VL Assay (use with Cepheid GeneXpert) | Plasma samples, can be POC | Same-day results in 108–110 min | HIV, TB | USD 17,000 per instrument; USD 14.90 per test (for all virological tests in LMICs) | WHO PQ, CE-marked, not approved in United States | - | Can be used for all genotypes; need WHO recommendations for use in all genotypes |
| Cepheid Xpert VL Fingerstick | 100 μL, capillary blood, Fingerstick Tertiary PoC: harm reduction settings, maybe easier to give for some PWID with poor vein access | Within 60 min | HIV | ND | CE-IVD; Not FDA-approved | Modified version of the VL assay; CE-marked, not approved in United States | Tertiary POC, harm reduction settings |
| Genedrive HCV RNA | 30 μL plasma, serum | 90 min | No | USD 5000 per device; USD 25-35 per test USD 30–40/test in India USD 14–18/test for Africa | CE-IVD | WHO PQ (May 2020) | POC; Decentralized diagnosis |
| RealTime HCV Viral Load | 0.5 mL plasma, 0.2 mL serum, DBS (FS) | ND | HIV | USD 11–23 per test; Global Fund price varies according to test volume/term commitment | CE-IVD (for HIV DBS and HCV RNA) | WHO PQ | Lab HCV viral load, All six GTs detected |
| Abbott Alinity m HCV assay RNA | Plasma, serum | 135 min | No | USD 50 per test | FDA-approved in March 2020 | CE-IVD, WHO PQ (March 2020) | Lab, clinical. All HCV GTs detected |
| Hologic Aptima HCV Quant | Serum, plasma | ND | HIV | USD 10–15 per test; USD 12 (€12–15) all-inclusive price for HCV VL | FDA- approved | - | CE-marked; Lab-based |
| Biocentric Generic HCV PCR assay | Serum, plasma | ND | HIV | USD 23 per test, USD 13.50–17 per test | - | - | CE-marked |
| Roche Cobas 6800/8800 systems (HCV RNA) | Lab-based, High volume clinical settings | First 96 results <3.5 h, every 90 min for 96 more results (Cobas 6800 System); first 96 results <3.5 h, every 30 min for 96 more results (Cobas 8800 System) | HIV | USD 35–45 per test; USD 340,000-USD 475,000 per instrument (depending on instrument and volume) | - | - | CE-marked, FDA approved |
| 5. HCV diagnostics pipeline or late-stage development tests | |||||||
| ARCHITECT HCV cAg assay | Serum, DBS | ND | USD 8–23 | CE-IVD | WHO PQ | Lab | |
| HCV Self-Test | Oral fluid, capillary blood | - | - | <USD 4 per test | TK | - | PoC; sexual health clinics, harm reduction centers, at home |
| Well anti-HCV RDT; Self-Test | Oral | 15–20 min | TK | TK | - | POC; primary care, at-home settings | |
| TrueNAT HCV PCR | 250 µL whole blood, 500 µL plasma | 40 min | polyvalent | USD 10,000 (small); USD 14,000–18,000 (large) USD 18 per test, depending on volume | CDSCO-India approval in 2019; | CE-IVD and WHO PQ (Estimated in 2020) | POC; primary healthcare |
| COBAS® Plasma Separation Card | Whole blood, plasma, dried plasma spots | - | - | TK, GPRO range: USD 10–15 per test Egypt (high-volume): USD 7 per test | Not FDA approved | CE-IVD (for HIV only, in development for HCV in 2020/2021) | lab |
| DBS on various PCR platforms | 0.2 mL serum DBS (fngerstick) | - | - | TK | RealTime HCV Viral load, CE-IVD (for HIV DBS and HCV RNA); WHO PQ (Dec 2019) COBAS® AmpliPrep/COBAS TaqMan HCV Test CE-IVD (for HIV only) Aptima® HCV Quant Dx Assay (TK) | - | Lab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahid, I.; Alzahrani, A.R.; Al-Ghamdi, S.S.; Alanazi, I.M.; Rehman, S.; Hassan, S. Hepatitis C Diagnosis: Simplified Solutions, Predictive Barriers, and Future Promises. Diagnostics 2021, 11, 1253. https://doi.org/10.3390/diagnostics11071253
Shahid I, Alzahrani AR, Al-Ghamdi SS, Alanazi IM, Rehman S, Hassan S. Hepatitis C Diagnosis: Simplified Solutions, Predictive Barriers, and Future Promises. Diagnostics. 2021; 11(7):1253. https://doi.org/10.3390/diagnostics11071253
Chicago/Turabian StyleShahid, Imran, Abdullah R. Alzahrani, Saeed S. Al-Ghamdi, Ibrahim M. Alanazi, Sidra Rehman, and Sajida Hassan. 2021. "Hepatitis C Diagnosis: Simplified Solutions, Predictive Barriers, and Future Promises" Diagnostics 11, no. 7: 1253. https://doi.org/10.3390/diagnostics11071253
APA StyleShahid, I., Alzahrani, A. R., Al-Ghamdi, S. S., Alanazi, I. M., Rehman, S., & Hassan, S. (2021). Hepatitis C Diagnosis: Simplified Solutions, Predictive Barriers, and Future Promises. Diagnostics, 11(7), 1253. https://doi.org/10.3390/diagnostics11071253







