Anaplastic Lymphoma Kinase- and CD30-Positive Anaplastic Large-Cell Lymphoma of the External Auditory Canal
Abstract
:1. Introduction
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALCL | anaplastic large-cell lymphoma |
| CT | computed tomography |
| ALK | anaplastic lymphoma kinase |
| RT | radiotherapy |
| CCRT | concurrent chemoradiotherapy |
| EAC | external auditory canal |
References
- Stein, H.; Mason, D.Y.; Gerdes, J.; O’Connor, N.; Wainscoat, J.; Pallesen, G.; Gatter, K.; Falini, B.; Delsol, G.; Lemke, H.; et al. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: Evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood 1985, 66, 848–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beljaards, R.C.; Kaudewitz, P.; Berti, E.; Gianotti, R.; Neumann, C.; Rosso, R.; Paulli, M.; Meijer, C.J.; Willemze, R. Primary cutaneous CD30-positive large cell lymphoma: Definition of a new type of cutaneous lymphoma with a favorable prognosis. A European Multicenter Study of 47 patients. Cancer 1993, 71, 2097–2104. [Google Scholar] [CrossRef]
- Rodriguez, V.; Perolada, J.M.; Ibanez, I.; Fernandez, A. Anaplastic large T-cell lymphoma of the external ear in childhood. Acta Otorrinolaringol. 2013, 64, 230–232. [Google Scholar] [CrossRef]
- Merkus, P.; Copper, M.P.; Van Oers, M.H.; Schouwenburg, P.F. Lymphoma in the ear. ORL J. Otorhinolaryngol. Relat. Spec. 2000, 62, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, O.; Serafini, A.N.; Franzmann, E.; Kittaneh, M.; Montague, N.; Kalkanis, D. F-18 FDG PET/CT in the assessment of recurrent anaplastic T-cell lymphoma of the external auditory canal. Clin. Nucl. Med. 2010, 35, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Marcal, N.; Campelos, S.; Dias, L.; Goncalves, M.; Pereira, G.; Godinho, T. Primary cutaneous CD30-positive anaplastic large-cell lymphoma of the external auditory canal. Ear Nose Throat J. 2012, 91, E10–E12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruschini, L.; De Vito, A.; Fortunato, S.; Pelosini, M.; Cervetti, G.; Petrini, M.; Berrettini, S. A Case of Primary Non-Hodgkin’s Lymphoma of the External Auditory Canal. Case Rep. Otolaryngol. 2013, 2013, 138397. [Google Scholar] [CrossRef]
- Zhang, T.; Dai, C.; Wang, Z. The misdiagnosis of external auditory canal carcinoma. Eur. Arch. Otorhinolaryngol. 2013, 270, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Nong, L.; Liang, L.; Zheng, Y.; Li, D.; Li, X.; Li, T. Extranodal NK/T-cell lymphoma, nasal type without evidence of EBV infection. Oncol. Lett. 2020, 20, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Rassidakis, G.Z.; Sarris, A.H.; Herling, M.; Ford, R.J.; Cabanillas, F.; McDonnell, T.J.; Medeiros, L.J. Differential expression of BCL-2 family proteins in ALK-positive and ALK-negative anaplastic large cell lymphoma of T/null-cell lineage. Am. J. Pathol. 2001, 159, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.S.; Pandolfino, T.; Guitart, J.; Rosen, S.; Kuzel, T.M. Primary cutaneous T-cell lymphoma: Review and current concepts. J. Clin. Oncol. 2000, 18, 2908–2925. [Google Scholar] [CrossRef] [PubMed]
- Bekkenk, M.W.; Geelen, F.A.; van Voorst Vader, P.C.; Heule, F.; Geerts, M.L.; van Vloten, W.A.; Meijer, C.J.; Willemze, R. Primary and secondary cutaneous CD30(+) lymphoproliferative disorders: A report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood 2000, 95, 3653–3661. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Hoppe, R.T.; Kohler, S.; Harvell, J.D.; Reddy, S.; Kim, Y.H. CD30+ cutaneous lymphoproliferative disorders: The Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J. Am. Acad. Dermatol. 2003, 49, 1049–1058. [Google Scholar] [CrossRef]
- Geller, S.; Canavan, T.N.; Pulitzer, M.; Moskowitz, A.J.; Myskowski, P.L. ALK-positive primary cutaneous anaplastic large cell lymphoma: A case report and review of the literature. Int. J. Dermatol. 2018, 57, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Foyil, K.V.; Bartlett, N.L. Brentuximab vedotin and crizotinib in anaplastic large-cell lymphoma. Cancer J. 2012, 18, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Pro, B.; Advani, R.; Brice, P.; Bartlett, N.L.; Rosenblatt, J.D.; Illidge, T.; Matous, J.; Ramchandren, R.; Fanale, M.; Connors, J.M.; et al. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood 2017, 130, 2709–2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Donk, N.W.; Dhimolea, E. Brentuximab vedotin. MAbs 2012, 4, 458–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Illidge, T.; Fanale, M.; Advani, R.; Bartlett, N.L.; Christensen, J.H.; Morschhauser, F.; Domingo-Domenech, E.; et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global, double-blind, randomised, phase 3 trial. Lancet 2019, 393, 229–240. [Google Scholar] [CrossRef] [Green Version]
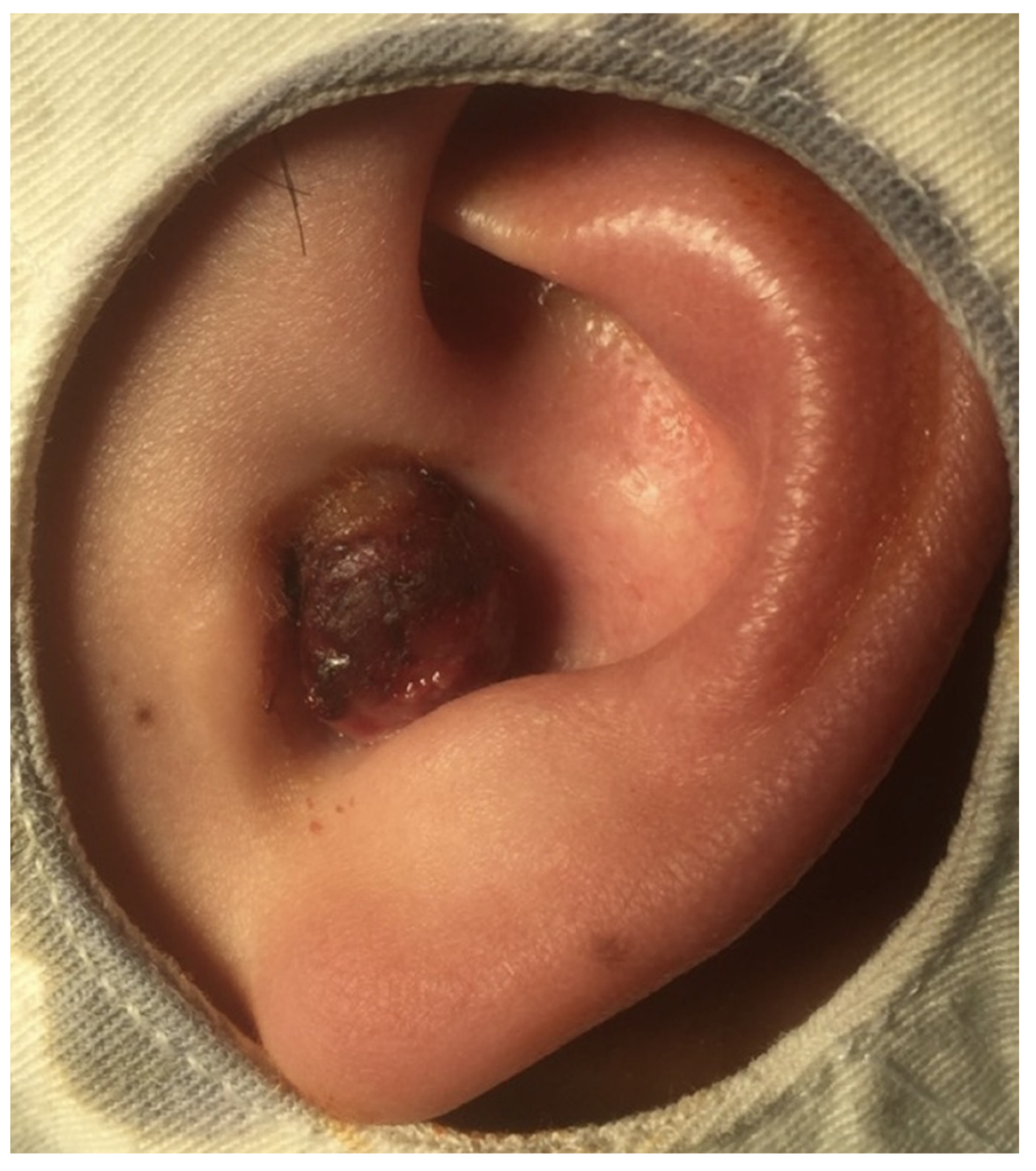
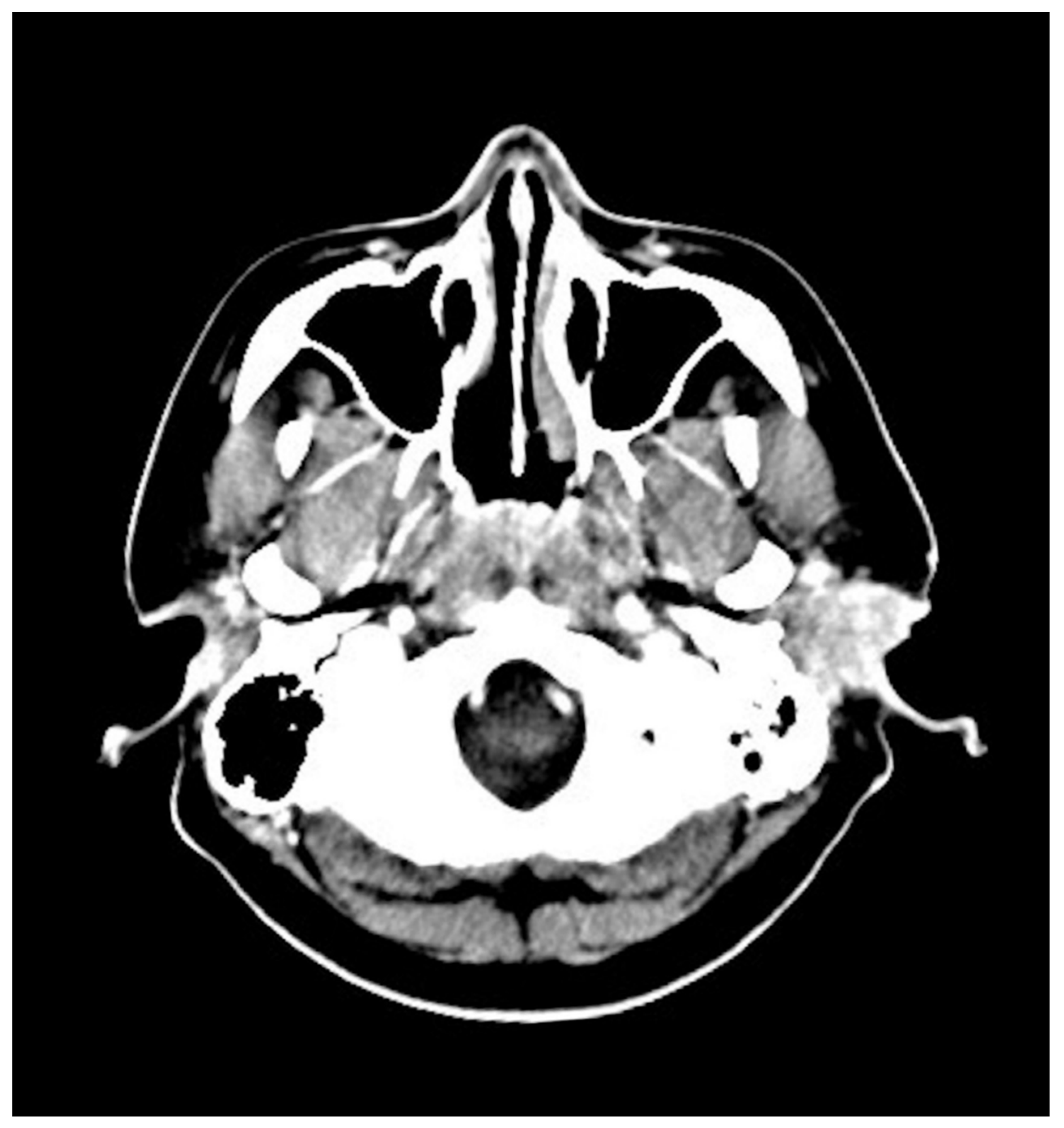
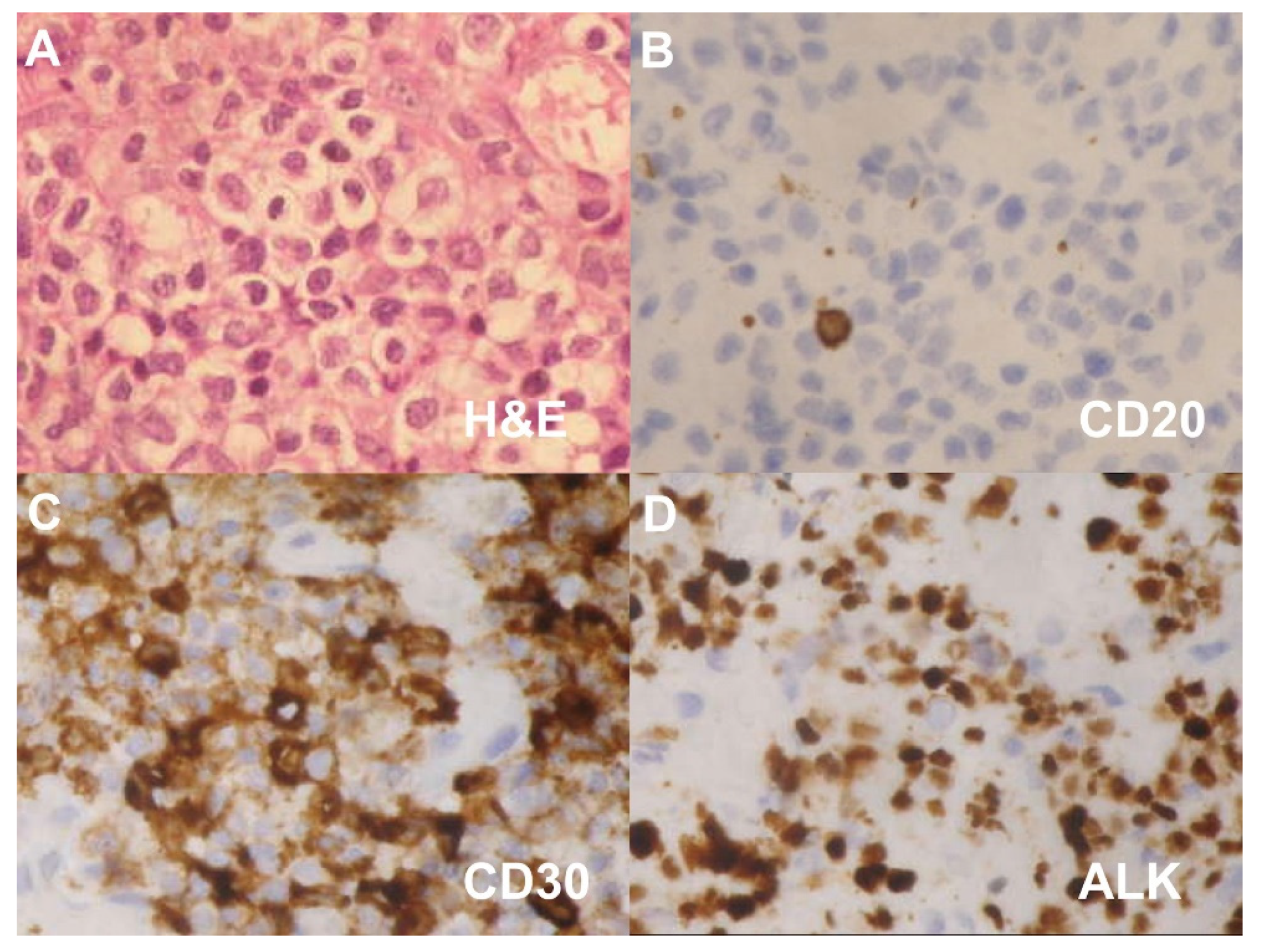

| Authors (Year) | Gender | Age (Y) | Initial Symptoms | CT | PET | IHC Positive | ALK | Treatment |
|---|---|---|---|---|---|---|---|---|
| Merkus et al., 2000 | Female | 83 | Otalgia, otorrhea | Bony destruction of EAC | NA | CD30 | NA | RT |
| Gomaa et al., 2010 | Female | 47 | Otalgia, EAC swelling | No osseous erosion | Radio-uptake | CD4, CD5, CD30 | NA | RT |
| Marcal et al., 2012 | Female | 68 | Otalgia, hypoacusis | No osseous erosion | NA | CD3, CD5, CD30 | Negative | RT |
| Present case, 2021 | Male | 34 | Painless mass | No osseous erosion | Radio-uptake | CD3, CD30 | Positive | CCRT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-L.; Chan, K.-C. Anaplastic Lymphoma Kinase- and CD30-Positive Anaplastic Large-Cell Lymphoma of the External Auditory Canal. Diagnostics 2021, 11, 1220. https://doi.org/10.3390/diagnostics11071220
Chen S-L, Chan K-C. Anaplastic Lymphoma Kinase- and CD30-Positive Anaplastic Large-Cell Lymphoma of the External Auditory Canal. Diagnostics. 2021; 11(7):1220. https://doi.org/10.3390/diagnostics11071220
Chicago/Turabian StyleChen, Shih-Lung, and Kai-Chieh Chan. 2021. "Anaplastic Lymphoma Kinase- and CD30-Positive Anaplastic Large-Cell Lymphoma of the External Auditory Canal" Diagnostics 11, no. 7: 1220. https://doi.org/10.3390/diagnostics11071220
APA StyleChen, S.-L., & Chan, K.-C. (2021). Anaplastic Lymphoma Kinase- and CD30-Positive Anaplastic Large-Cell Lymphoma of the External Auditory Canal. Diagnostics, 11(7), 1220. https://doi.org/10.3390/diagnostics11071220






