Guidelines for Point-of-Care Fluorescence Imaging for Detection of Wound Bacterial Burden Based on Delphi Consensus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Expert Panel
2.2. Delphi Survey
3. Results & Discussion
3.1. Delphi Participants
3.2. Delphi Results
3.3. Recommendations
3.3.1. Competencies Required to Perform Fluorescence Imaging
3.3.2. Fluorescence Imaging Clinical Workflow
3.3.3. Clinical Indications for Fluorescence Imaging to Detect Bacterial Burden in Wounds
3.3.4. Recommended Frequency of Fluorescence Imaging
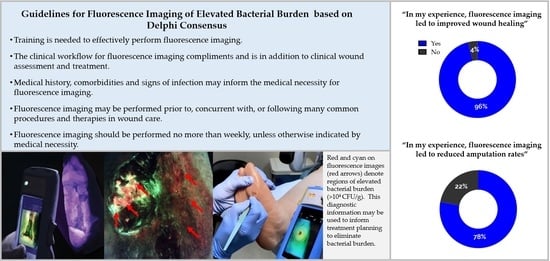
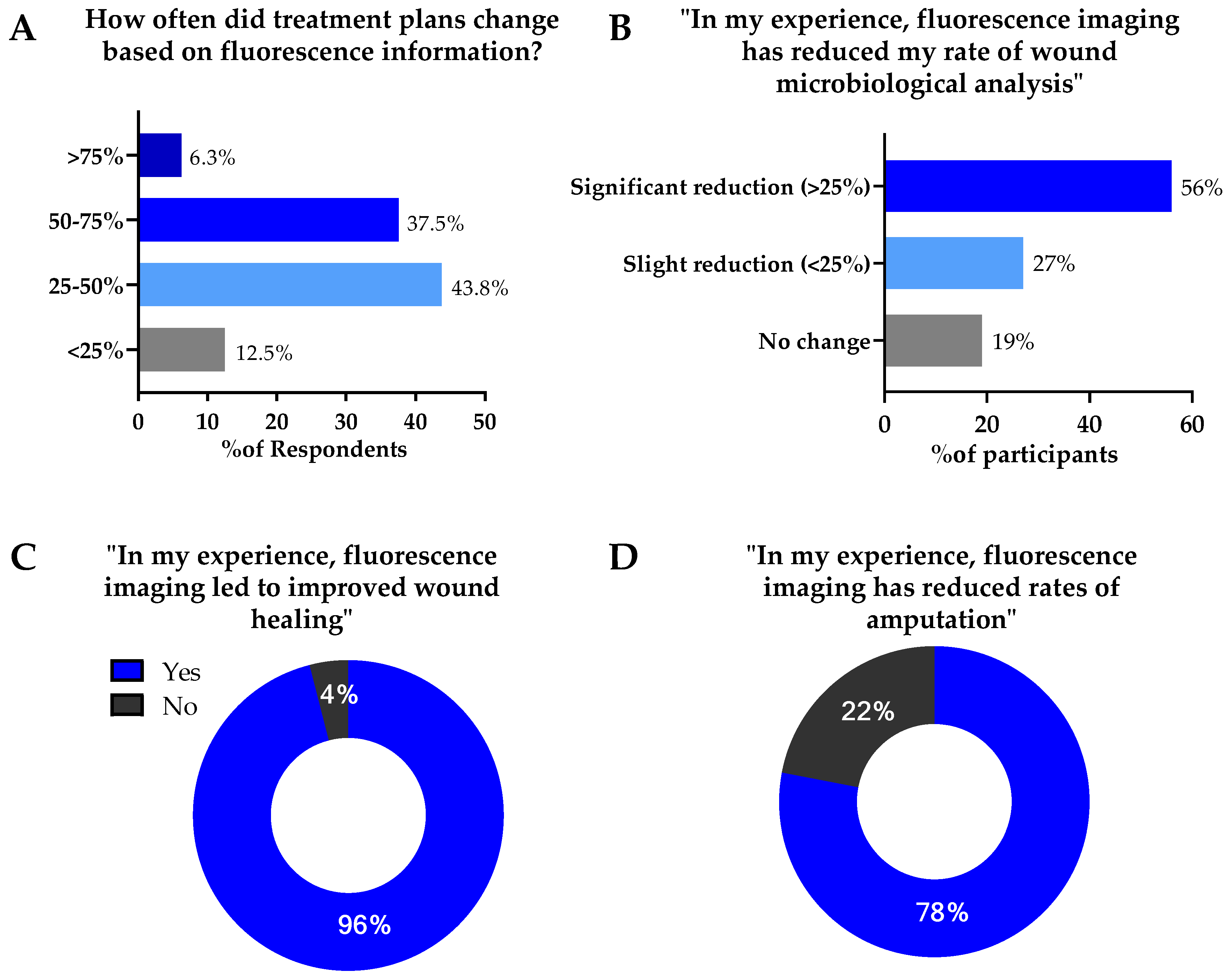
3.4. Reported Impact of Fluorescence Imaging on Treatment Planning & Wound Outcomes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Le, L.; Baer, M.; Briggs, P.; Bullock, N.; Cole, W.; DiMarco, D.; Hamil, R.; Harrell, K.; Kasper, M.; Li, W.; et al. Diagnostic Accuracy of Point-of-Care Fluorescence Imaging for the Detection of Bacterial Burden in Wounds: Results from the 350-Patient Fluorescence Imaging Assessment and Guidance Trial. Adv. Wound Care 2021, 10, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Serena, T.E.; Harrell, K.; Serena, L.; Yaakov, R.A. Real-time bacterial fluorescence imaging accurately identifies wounds with moderate-to-heavy bacterial burden. J. Wound Care 2019, 28, 346–357. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.; Gill, S.S.; Wu, W.; Kalkar, S.R.; Rochon, P.A. Does this patient have an infection of a chronic wound? JAMA 2012, 307, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Lantis, J.C.; Marston, W.A.; Farber, A.; Kirsner, R.S.; Zhang, Y.; Lee, T.D.; Cargill, D.I.; Slade, H.B. The influence of patient and wound variables on healing of venous leg ulcers in a randomized controlled trial of growth-arrested allogeneic keratinocytes and fibroblasts. J. Vasc. Surg. 2013, 58, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Lookingbill, D.P.; Miller, S.H.; Knowles, R.C. Bacteriology of chronic leg ulcers. Arch. Dermatol. 1978, 114, 1765–1768. [Google Scholar] [CrossRef]
- Majewski, W.; Cybulski, Z.; Napierala, M.; Pukacki, F.; Staniszewski, R.; Pietkiewicz, K.; Zapalski, S. The value of quantitative bacteriological investigations in the monitoring of treatment of ischaemic ulcerations of lower legs. Int. Angiol. 1995, 14, 381–384. [Google Scholar] [PubMed]
- Martín, S.; Pérez, A.; Aldecoa, C. Sepsis and Immunosenescence in the Elderly Patient: A Review. Front. Med. 2017, 4, 20. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, M.D. Bacteria and Antibiotics in Wound Healing. Surg. Clin. N. Am. 2020, 100, 757–776. [Google Scholar] [CrossRef] [PubMed]
- Browne, A.C.; Vearncombe, M.; Sibbald, R.G. High bacterial load in asymptomatic diabetic patients with neurotrophic ulcers retards wound healing after application of Dermagraft. Ostomy Wound Manag. 2001, 47, 44–49. [Google Scholar]
- Hogsberg, T.; Bjarnsholt, T.; Thomsen, J.S.; Kirketerp-Moller, K. Success rate of split-thickness skin grafting of chronic venous leg ulcers depends on the presence of Pseudomonas aeruginosa: A retrospective study. PLoS ONE 2011, 6, e20492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ai-Jalodi, O.; Sabo, M.; Patel, K.; Bullock, N.; Serena, L.; Breisinger, K.; Serena, T.E. Efficacy and safety of a porcine peritoneum-derived matrix in diabetic foot ulcer treatment: A pilot study. J. Wound Care 2021, 30, S18–S23. [Google Scholar] [CrossRef]
- Esper, A.M.; Moss, M.; Lewis, C.A.; Nisbet, R.; Mannino, D.M.; Martin, G.S. The role of infection and comorbidity: Factors that influence disparities in sepsis. Crit. Care Med. 2006, 34, 2576–2582. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Swerdlow, M.A.; Armstrong, A.A.; Conte, M.S.; Padula, W.V.; Bus, S.A. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J. Foot Ankle Res. 2020, 13, 16. [Google Scholar] [CrossRef] [Green Version]
- Rennie, M.Y.; Dunham, D.; Lindvere-Teene, L.; Raizman, R.; Hill, R.; Linden, R. Understanding Real-Time Fluorescence Signals from Bacteria and Wound Tissues Observed with the MolecuLight i:X(TM). Diagnostics 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, R.; Woo, K. A Prospective Multisite Observational Study Incorporating Bacterial Fluorescence Information Into the UPPER/LOWER Wound Infection Checklists. Wounds 2020, 32, 299–308. [Google Scholar]
- Hurley, C.M.; McClusky, P.; Sugrue, R.M.; Clover, J.A.; Kelly, J.E. Efficacy of a bacterial fluorescence imaging device in an outpatient wound care clinic: A pilot study. J. Wound Care 2019, 28, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.M.; Dunham, D.; Rennie, M.Y.; Kirman, J.; Lopez, A.J.; Keim, K.C.; Little, W.; Gomez, A.; Bourke, J.; Ng, H.; et al. In vitro detection of porphyrin-producing wound bacteria with real-time fluorescence imaging. Future Microbiol. 2020, 15, 319–332. [Google Scholar] [CrossRef] [Green Version]
- Raizman, R.; Little, W.; Smith, A.C. Rapid Diagnosis of Pseudomonas aeruginosa in Wounds with Point-Of-Care Fluorescence Imaing. Diagnostics 2021, 11, 280. [Google Scholar] [CrossRef]
- Rennie, M.Y.; Lindvere-Teene, L.; Tapang, K.; Linden, R. Point-of-care fluorescence imaging predicts the presence of pathogenic bacteria in wounds: A clinical study. J. Wound Care 2017, 26, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.; Rennie, M.Y.; Douglas, J. Using Bacterial Fluorescence Imaging and Antimicrobial Stewardship to Guide Wound Management Practices: A Case Series. Ostomy Wound Manag. 2018, 64, 18–28. [Google Scholar] [CrossRef]
- Price, N. Routine fluorescence imaging to detect wound bacteria reduces antibiotic use and antimicrobial dressing expenditure while improving healing rates: Retrospective analysis of 229 foot ulcers. Diagnostics 2020, 10, 927. [Google Scholar] [CrossRef] [PubMed]
- Cole, W.; Coe, S. Use of a bacterial fluorescence imaging system to target wound debridement and accelerate healing: A pilot study. J. Wound Care 2020, 29, S44–S52. [Google Scholar] [CrossRef]
- Eubank, B.H.; Mohtadi, N.G.; Lafave, M.R.; Wiley, J.P.; Bois, A.J.; Boorman, R.S.; Sheps, D.M. Using the modified Delphi method to establish clinical consensus for the diagnosis and treatment of patients with rotator cuff pathology. BMC Med. Res. Methodol. 2016, 16, 56. [Google Scholar] [CrossRef] [Green Version]
- Kim, P.J.; Attinger, C.E.; Steinberg, J.S.; Evans, K.K.; Lehner, B.; Willy, C.; Lavery, L.; Wolvos, T.; Orgill, D.; Ennis, W.; et al. Negative-pressure wound therapy with instillation: International consensus guidelines. Plast. Reconstr. Surg. 2013, 132, 1569–1579. [Google Scholar] [CrossRef]
- Russell, D.; Atkin, L.; Betts, A.; Dowsett, C.; Fatoye, F.; Gardner, S.; Green, J.; Manu, C.; McKenzie, T.; Meally, H.; et al. Using a modified Delphi methodology to gain consensus on the use of dressings in chronic wounds management. J. Wound Care 2018, 27, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Cutting, K.F.; White, R.J.; Mahoney, P. Developing clinical criteria for wound infection—A delphi approach: 680. J. Wound Ostomy Cont. Nurs. 2005, 32, S26. [Google Scholar] [CrossRef]
- Hohmann, E.; Brand, J.C.; Rossi, M.J.; Lubowitz, J.H. Expert Opinion Is Necessary: Delphi Panel Methodology Facilitates a Scientific Approach to Consensus. Arthroscopy 2018, 34, 349–351. [Google Scholar] [CrossRef] [Green Version]
- Hasson, F.; Keeney, S.; McKenna, H. Research guidelines for the Delphi survey technique. J. Adv. Nurs. 2000, 32, 1008–1015. [Google Scholar]
- Keeney, S.; Hasson, F.; McKenna, H. Consulting the oracle: Ten lessons from using the Delphi technique in nursing research. J. Adv. Nurs. 2006, 53, 205–212. [Google Scholar] [CrossRef]
- MacLeod, B.G.; Klarich, C.S.; Wessman, L.L.; Gaddis, K.P.; Konstantinov, N.K.; Wubben, A.; Melin, M.M. Novel Diagnostic Fluorescence Imaging and Therapeutic Considerations for the Treatment of Pyoderma Gangrenosum. Adv. Ski. Wound Care 2021, in press. [Google Scholar]
- Raizman, R. Bacterial fluorescence imaging guides wound cleaning and facilitates patient education in pilonidal sinus wound care patients. In Proceedings of the European Wound Management Association, Amsterdam, The Netherlands, 3–6 May 2017. [Google Scholar]
- Wu, Y.C.; Smith, M.; Chu, A.; Lindvere-Teene, L.; Starr, D.; Tapang, K.; Shekhman, R.; Wong, O.; Linden, R.; DaCosta, R.S. Handheld fluorescence imaging device detects subclinical wound infection in an asymptomatic patient with chronic diabetic foot ulcer: A case report. Int. Wound J. 2016, 13, 449–453. [Google Scholar] [CrossRef]
- Xu, L.; McLennan, S.V.; Lo, L.; Natfaji, A.; Bolton, T.; Liu, Y.; Twigg, S.M.; Yue, D.K. Bacterial load predicts healing rate in neuropathic diabetic foot ulcers. Diabetes Care 2007, 30, 378–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, S.E.; Hillis, S.L.; Frantz, R.A. Clinical signs of infection in diabetic foot ulcers with high microbial load. Biol. Res. Nurs. 2009, 11, 119–128. [Google Scholar] [CrossRef]
- Zekri, A.; King, W. Success of skin grafting on a contaminated recipient surface. Eur. J. Plast. Surg. 1995, 18, 40–42. [Google Scholar] [CrossRef]
- Aung, B. Can Fluorescence Imaging Predict the Success of CTPs for Wound Closure and Save Costs? Today’s Wound Clin. 2019, 13, 22–25. [Google Scholar]
- Raizman, R. Fluorescence imaging guided dressing change frequency during negative pressure wound therapy: A case series. J. Wound Care 2019, 28, S28–S37. [Google Scholar] [CrossRef]
- Serena, T. A Paradigm Shift in Chronic Wound Assessment: Incorporating Bacterial Fluorescence Imaging into Standard of Care. In Photonic Diagnosis, Monitoring, Prevention, and Treatment of Infections and Inflammatory Diseases; SPIE: San Fransico, CA, USA, 2020; Volume 112230F. [Google Scholar] [CrossRef]
- Girard, T.D.; Ely, E.W. Bacteremia and sepsis in older adults. Clin. Geriatr. Med. 2007, 23, 633–647. [Google Scholar] [CrossRef]
- Nasa, P.; Juneja, D.; Singh, O. Severe sepsis and septic shock in the elderly: An overview. World J. Crit. Care Med. 2012, 1, 23–30. [Google Scholar] [CrossRef]
- Raizman, R.; Dunham, D.; Lindvere-Teene, L.; Jones, L.M.; Tapang, K.; Linden, R.; Rennie, M.Y. Use of a bacterial fluorescence imaging device: Wound measurement, bacterial detection and targeted debridement. J. Wound Care 2019, 28, 824–834. [Google Scholar] [CrossRef] [Green Version]
- Bonham, P.A. Swab cultures for diagnosing wound infections: A literature review and clinical guideline. J. Wound Ostomy Cont. Nurs. 2009, 36, 389–395. [Google Scholar] [CrossRef]
- Rondas, A.A.; Schols, J.M.; Halfens, R.J.; Stobberingh, E.E. Swab versus biopsy for the diagnosis of chronic infected wounds. Adv. Ski. Wound Care 2013, 26, 211–219. [Google Scholar] [CrossRef]
- Rahma, S.; Woods, J.; Nixon, J.; Brown, S.; Russel, D. The use of Point-of-Care Bacterial Autofluorescence Imaging in the Management of Diabetic Foot Ulcers; A Pilot Randomised Controlled Trial. In Proceedings of the Symposium on Advanced Wound Care Virtual, Online, 4–6 November 2020. [Google Scholar]
- Oropallo, A.; Gawlik, S.; Vayser, D. 12-week RCT Evaluating Impact of Routine Fluorescence Imaging of Bacteria on DFU Healing Rates. In Proceedings of the Symposium on Advanced Wound Care Virtual, Online, 10–14 May 2021. [Google Scholar]
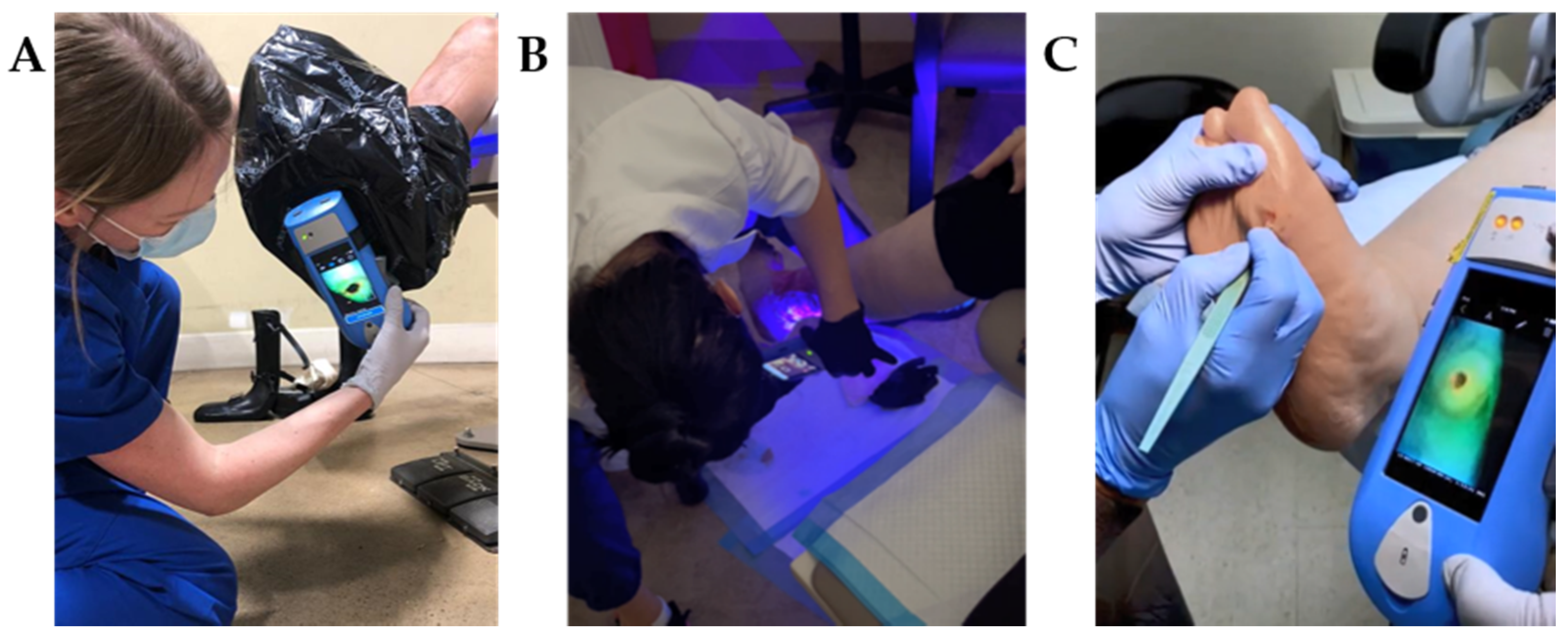

| Country | Licensed Profession | ||
|---|---|---|---|
| USA | 84.0% | Medical doctor | 56.3% |
| Canada | 6.3% | Podiatrist | 21.9% |
| United Kingdom | 9.4% | Nurse Practitioner | 9.4% |
| Physical Therapist | 3.1% | ||
| Sites of Service | Nurse | 9.4% | |
| Hospital Outpatient | 75.0% | ||
| Hospital Inpatient | 62.5% | Years of Experience in wound care | |
| Private Office | 53.1% | >20 | 40.6% |
| Telehealth | 34.4% | 15 to 20 | 21.9% |
| Long Term Care Facility | 18.8% | 10 to 15 | 25.0% |
| Long Term Acute Care Hospital | 15.6% | 5 to 10 | 6.3% |
| Home Health | 9.4% | 0 to 5 | 6.3% |
| Other (i.e., Mobile Unit, Urgent Care) | 37.5% | ||
| Fundamental Competencies | Advanced Competencies | |
|---|---|---|
|
|
|
| Fluorescence Imaging May Be Performed on (but Not Limited to) Any of the Following Wound Types: |
|---|
| Diabetic foot ulcer Venous leg Ulcer Pressure Ulcer Surgical Site infection Post-operative wound Traumatic wound |
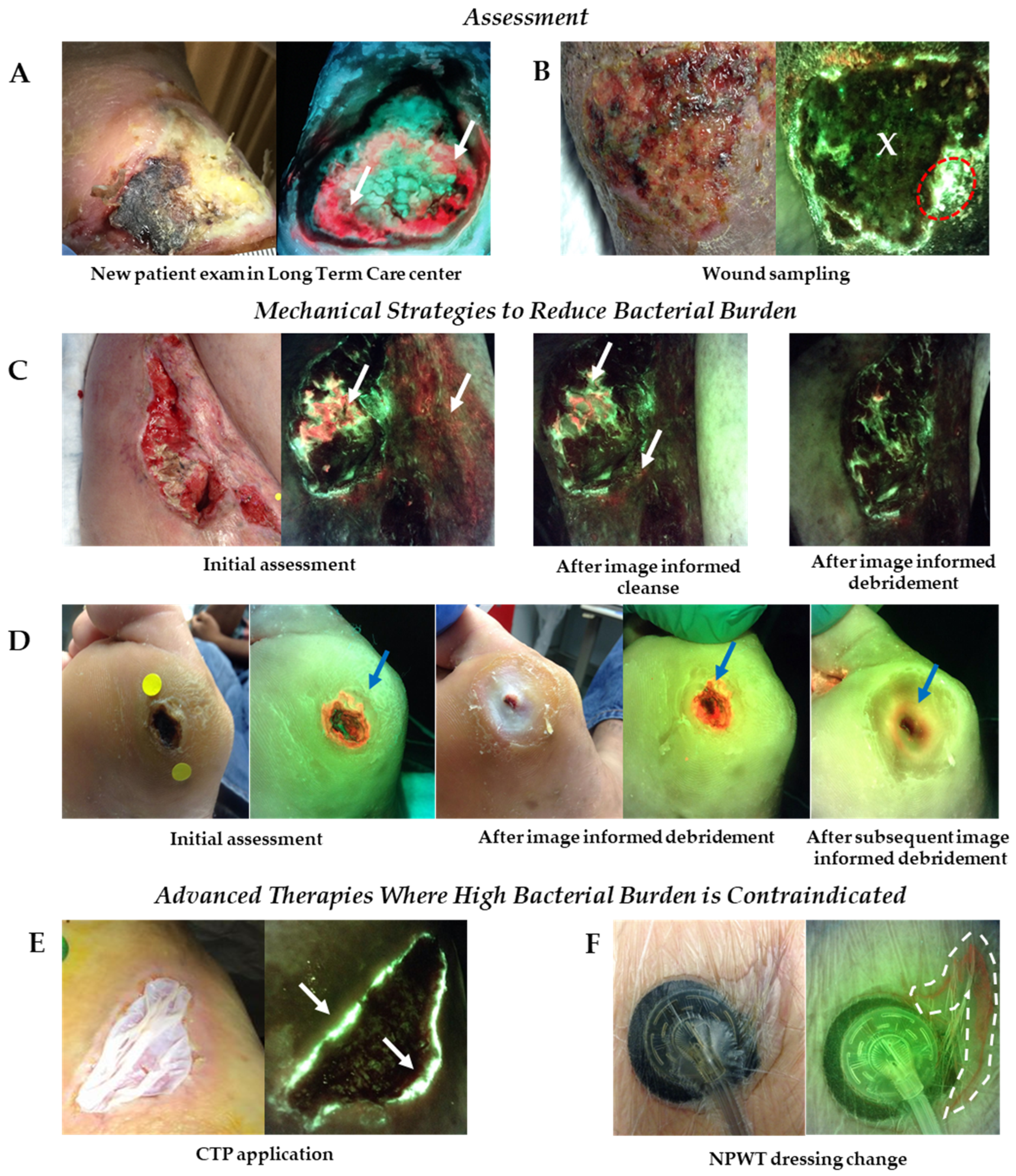
| New Patient Exam |
|---|
| Prior history or comorbidities |
| History of delayed wound healing (>4 weeks) History of wound infection Failure of prior treatment Positive for clinical signs and symptoms present at visit Co-morbid medical conditions that mask signs and symptoms (i.e., diabetes, autoimmune disorders, elderly) Multiple co-morbid medical conditions that elevate risk of life/limb loss |
| At least one of the following signs or symptoms: |
| Delayed wound healing Wound breakdown and enlargement Increased malodor Local warmth New or increase pain Erythema Purulent discharge Extending induration Lymphangitis Crepitus Bleeding, friable granulation |
| Procedures or treatment |
| Follow up on positive fluorescence image Prior to, concurrent with or after debridement Suspected or confirmed biofilm Prescription of antimicrobials (including antibiotics) Follow up on application of antimicrobial Prior to or follow up on CTP/graft application Prior to, concurrent with or after NPWT Baseline assessment of new wound/patient in LTC/nursing facility |
| Wound sampling |
| Follow up on positive microbiology Inform need for and location of microbiological sample Inform referral for microbiological analysis |
| Recommended Frequency of Fluorescence Imaging. |
|---|
| Fluorescence imaging may be performed on wounds at baseline, during the first 4 weeks and/or if an increase in wound size is observed |
| Fluorescence imaging may be performed weekly on patients that meet criteria for imaging (e.g., clinical signs and symptoms) |
| If a wound is positive for fluorescence, fluorescence imaging is performed no more than on a weekly basis; unless otherwise indicated by development or change in symptoms |
| Summary of Guidelines for Fluorescence Imaging |
|---|
| Training is needed to effectively perform fluorescence imaging. E.g., how to position the patient and device, how to interpret images The clinical workflow for fluorescence imaging compliments and is in addition to clinical wound assessment and treatment. Clinical judgement should be used to apply fluorescence information to treatment decision making. Medical history, comorbidities and signs of infection may inform the medical necessity for fluorescence imaging. E.g., history of delayed wound healing, presence of diabetes, detection of pain or malodor Fluorescence imaging may be performed prior to, concurrent with, or following many common procedures and therapies in wound care. E.g., Debridement, CTP application, prescription of antimicrobials, wound sampling Fluorescence imaging should be performed no more than weekly, unless otherwise indicated by medical necessity. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oropallo, A.R.; Andersen, C.; Abdo, R.; Hurlow, J.; Kelso, M.; Melin, M.; Serena, T.E. Guidelines for Point-of-Care Fluorescence Imaging for Detection of Wound Bacterial Burden Based on Delphi Consensus. Diagnostics 2021, 11, 1219. https://doi.org/10.3390/diagnostics11071219
Oropallo AR, Andersen C, Abdo R, Hurlow J, Kelso M, Melin M, Serena TE. Guidelines for Point-of-Care Fluorescence Imaging for Detection of Wound Bacterial Burden Based on Delphi Consensus. Diagnostics. 2021; 11(7):1219. https://doi.org/10.3390/diagnostics11071219
Chicago/Turabian StyleOropallo, Alisha R., Charles Andersen, Raymond Abdo, Jenny Hurlow, Martha Kelso, Mark Melin, and Thomas E. Serena. 2021. "Guidelines for Point-of-Care Fluorescence Imaging for Detection of Wound Bacterial Burden Based on Delphi Consensus" Diagnostics 11, no. 7: 1219. https://doi.org/10.3390/diagnostics11071219
APA StyleOropallo, A. R., Andersen, C., Abdo, R., Hurlow, J., Kelso, M., Melin, M., & Serena, T. E. (2021). Guidelines for Point-of-Care Fluorescence Imaging for Detection of Wound Bacterial Burden Based on Delphi Consensus. Diagnostics, 11(7), 1219. https://doi.org/10.3390/diagnostics11071219