Cerebrospinal Fluid Chitinases as Biomarkers for Amyotrophic Lateral Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Material
2.2. ELISA Quantifications
2.3. Statistical Analysis
2.4. UHPLC-MS Analysis of CSF
3. Results
3.1. Chitinases and pNFH Analysis from the CSF
3.2. UHPLC-MS Analysis of CSF
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ryan, M.; Heverin, M.; McLaughlin, R.L.; Hardiman, O. Lifetime Risk and Heritability of Amyotrophic Lateral Sclerosis. JAMA Neurol. 2019, 76, 1367–1374. [Google Scholar] [CrossRef]
- Volk, A.E.; Weishaupt, J.H.; Andersen, P.M.; Ludolph, A.C.; Kubisch, C. Current knowledge and recent insights into the genetic basis of amyotrophic lateral sclerosis. Med. Genet. 2018, 30, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Moll, T.; Shaw, P.J.; Cooper-Knock, J. Disrupted glycosylation of lipids and proteins is a cause of neurodegeneration. Brain 2020, 143, 1332–1340. [Google Scholar] [CrossRef]
- Cooper-Knock, J.; Moll, T.; Ramesh, T.; Castelli, L.; Beer, A.; Robins, H.; Fox, I.; Niedermoser, I.; Van Damme, P.; Moisse, M.; et al. Mutations in the Glycosyltransferase Domain of GLT8D1 Are Associated with Familial Amyotrophic Lateral Sclerosis. Cell Rep. 2019, 26, 2298–2306.e2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, J.; Gomes, C.; de Carvalho, M. Diagnosis, pathogenesis and therapeutic targets in amyotrophic lateral sclerosis. CNS Neurol. Disord. Drug Targets 2010, 9, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.P.; Brown, R.H., Jr.; Cleveland, D.W. Decoding ALS: From genes to mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Clement, A.M.; Nguyen, M.D.; Roberts, E.A.; Garcia, M.L.; Boillee, S.; Rule, M.; McMahon, A.P.; Doucette, W.; Siwek, D.; Ferrante, R.J.; et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science 2003, 302, 113–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahsen, B.F.; Gray, E.; Thompson, A.G.; Ansorge, O.; Anthony, D.C.; Cowley, S.A.; Talbot, K.; Turner, M.R. Non-neuronal cells in amyotrophic lateral sclerosis—From pathogenesis to biomarkers. Nat. Rev. Neurol. 2021. [Google Scholar] [CrossRef]
- Beers, D.R.; Appel, S.H. Immune dysregulation in amyotrophic lateral sclerosis: Mechanisms and emerging therapies. Lancet Neurol. 2019, 18, 211–220. [Google Scholar] [CrossRef]
- Moreno-Garcia, L.; Miana-Mena, F.J.; Moreno-Martinez, L.; de la Torre, M.; Lunetta, C.; Tarlarini, C.; Zaragoza, P.; Calvo, A.C.; Osta, R. Inflammasome in ALS Skeletal Muscle: NLRP3 as a Potential Biomarker. Int. J. Mol. Sci. 2021, 22, 2523. [Google Scholar] [CrossRef] [PubMed]
- McCauley, M.E.; Baloh, R.H. Inflammation in ALS/FTD pathogenesis. Acta Neuropathol. 2019, 137, 715–730. [Google Scholar] [CrossRef] [Green Version]
- Pinteac, R.; Montalban, X.; Comabella, M. Chitinases and chitinase-like proteins as biomarkers in neurologic disorders. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8. [Google Scholar] [CrossRef]
- Steinacker, P.; Verde, F.; Fang, L.; Feneberg, E.; Oeckl, P.; Roeber, S.; Anderl-Straub, S.; Danek, A.; Diehl-Schmid, J.; Fassbender, K.; et al. Chitotriosidase (CHIT1) is increased in microglia and macrophages in spinal cord of amyotrophic lateral sclerosis and cerebrospinal fluid levels correlate with disease severity and progression. J. Neurol. Neurosurg. Psychiatry 2018, 89, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Da Silva, C.A.; Dela Cruz, C.S.; Ahangari, F.; Ma, B.; Kang, M.J.; He, C.H.; Takyar, S.; Elias, J.A. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu. Rev. Physiol. 2011, 73, 479–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, R.; Hamel, K.; Petersen, L.; Cao, Q.J.; Arenas, R.B.; Bigelow, C.; Bentley, B.; Yan, W. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene 2009, 28, 4456–4468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vu, L.; An, J.; Kovalik, T.; Gendron, T.; Petrucelli, L.; Bowser, R. Cross-sectional and longitudinal measures of chitinase proteins in amyotrophic lateral sclerosis and expression of CHI3L1 in activated astrocytes. J. Neurol. Neurosurg. Psychiatry 2020, 91, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yang, Y.; Duan, H.; He, J.; Sun, L.; Hu, W.; Zeng, J. CHI3L2 Is a Novel Prognostic Biomarker and Correlated With Immune Infiltrates in Gliomas. Front. Oncol. 2021, 11, 611038. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, D.; Meneri, M.; Saccomanno, D.; Bresolin, N.; Comi, G.P.; Corti, S. Diagnostic and Prognostic Role of Blood and Cerebrospinal Fluid and Blood Neurofilaments in Amyotrophic Lateral Sclerosis: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 4152. [Google Scholar] [CrossRef] [Green Version]
- Chio, A.; Mazzini, L.; Mora, G. Disease-modifying therapies in amyotrophic lateral sclerosis. Neuropharmacology 2020, 167, 107986. [Google Scholar] [CrossRef]
- Feneberg, E.; Oeckl, P.; Steinacker, P.; Verde, F.; Barro, C.; Van Damme, P.; Gray, E.; Grosskreutz, J.; Jardel, C.; Kuhle, J.; et al. Multicenter evaluation of neurofilaments in early symptom onset amyotrophic lateral sclerosis. Neurology 2018, 90, e22–e30. [Google Scholar] [CrossRef]
- Goncalves, M.; Tillack, L.; de Carvalho, M.; Pinto, S.; Conradt, H.S.; Costa, J. Phosphoneurofilament heavy chain and N-glycomics from the cerebrospinal fluid in amyotrophic lateral sclerosis. Clin. Chim. Acta 2015, 438, 342–349. [Google Scholar] [CrossRef]
- Goncalves, M.; De Carvalho, M.; Peixoto, C.; Alves, P.; Barreto, C.; Oliva, A.; Pinto, S.; Laborinho-Pronto, A.; Gromicho, M.; Costa, J. Phosphoneurofilament heavy chain and vascular endothelial growth factor as cerebrospinal fluid biomarkers for ALS. Amyotroph. Lateral Scler. Frontotemporal. Degener. 2017, 18, 134–136. [Google Scholar] [CrossRef]
- Lu, C.H.; Macdonald-Wallis, C.; Gray, E.; Pearce, N.; Petzold, A.; Norgren, N.; Giovannoni, G.; Fratta, P.; Sidle, K.; Fish, M.; et al. Neurofilament light chain: A prognostic biomarker in amyotrophic lateral sclerosis. Neurology 2015, 84, 2247–2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oeckl, P.; Jardel, C.; Salachas, F.; Lamari, F.; Andersen, P.M.; Bowser, R.; de Carvalho, M.; Costa, J.; van Damme, P.; Gray, E.; et al. Multicenter validation of CSF neurofilaments as diagnostic biomarkers for ALS. Amyotroph. Lateral Scler. Frontotemporal. Degener. 2016, 17, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Steinacker, P.; Feneberg, E.; Weishaupt, J.; Brettschneider, J.; Tumani, H.; Andersen, P.M.; von Arnim, C.A.; Bohm, S.; Kassubek, J.; Kubisch, C.; et al. Neurofilaments in the diagnosis of motoneuron diseases: A prospective study on 455 patients. J. Neurol. Neurosurg. Psychiatry 2016, 87, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Gaur, N.; Perner, C.; Witte, O.W.; Grosskreutz, J. The Chitinases as Biomarkers for Amyotrophic Lateral Sclerosis: Signals From the CNS and Beyond. Front. Neurol. 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Swash, M. Chitinases, neuroinflammation and biomarkers in ALS. J. Neurol. Neurosurg. Psychiatry 2020, 91, 338. [Google Scholar] [CrossRef]
- Steinacker, P.; Feneberg, E.; Halbgebauer, S.; Witzel, S.; Verde, F.; Oeckl, P.; Van Damme, P.; Gaur, N.; Gray, E.; Grosskreutz, J.; et al. Chitotriosidase as biomarker for early stage amyotrophic lateral sclerosis: A multicenter study. Amyotroph. Lateral Scler. Frontotemporal. Degener. 2021, 22, 276–286. [Google Scholar] [CrossRef]
- Thompson, A.G.; Gray, E.; Thezenas, M.L.; Charles, P.D.; Evetts, S.; Hu, M.T.; Talbot, K.; Fischer, R.; Kessler, B.M.; Turner, M.R. Cerebrospinal fluid macrophage biomarkers in amyotrophic lateral sclerosis. Ann. Neurol. 2018, 83, 258–268. [Google Scholar] [CrossRef]
- Thompson, A.G.; Gray, E.; Bampton, A.; Raciborska, D.; Talbot, K.; Turner, M.R. CSF chitinase proteins in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1215–1220. [Google Scholar] [CrossRef]
- Varghese, A.M.; Sharma, A.; Mishra, P.; Vijayalakshmi, K.; Harsha, H.C.; Sathyaprabha, T.N.; Bharath, S.M.; Nalini, A.; Alladi, P.A.; Raju, T.R. Chitotriosidase—A putative biomarker for sporadic amyotrophic lateral sclerosis. Clin. Frontotemporal. 2013, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Barschke, P.; Oeckl, P.; Steinacker, P.; Al Shweiki, M.R.; Weishaupt, J.H.; Landwehrmeyer, G.B.; Anderl-Straub, S.; Weydt, P.; Diehl-Schmid, J.; Danek, A.; et al. Different CSF protein profiles in amyotrophic lateral sclerosis and frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. J. Neurol. Neurosurg. Psychiatry 2020, 91, 503–511. [Google Scholar] [CrossRef]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L.; World Federation of Neurology Research Group on Motor Neuron Disease. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000, 1, 293–299. [Google Scholar] [CrossRef]
- Costa, J.; Gatermann, M.; Nimtz, M.; Kandzia, S.; Glatzel, M.; Conradt, H.S. N-Glycosylation of Extracellular Vesicles from HEK-293 and Glioma Cell Lines. Anal Chem 2018, 90, 7871–7879. [Google Scholar] [CrossRef]
- Larsen, T.; Yoshimura, Y.; Voldborg, B.G.; Cazzamali, G.; Bovin, N.V.; Westerlind, U.; Palcic, M.M.; Leisner, J.J. Human chitotriosidase CHIT1 cross reacts with mammalian-like substrates. FEBS Lett 2014, 588, 746–751. [Google Scholar] [CrossRef] [Green Version]
- Renkema, G.H.; Boot, R.G.; Muijsers, A.O.; Donker-Koopman, W.E.; Aerts, J.M. Purification and characterization of human chitotriosidase, a novel member of the chitinase family of proteins. J. Biol. Chem. 1995, 270, 2198–2202. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhang, F.; Colon, W.; Linhardt, R.J.; Xia, K. Glycosaminoglycans in human cerebrospinal fluid determined by LC-MS/MS MRM. Anal. Biochem. 2019, 567, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Gille, B.; De Schaepdryver, M.; Dedeene, L.; Goossens, J.; Claeys, K.G.; Van Den Bosch, L.; Tournoy, J.; Van Damme, P.; Poesen, K. Inflammatory markers in cerebrospinal fluid: Independent prognostic biomarkers in amyotrophic lateral sclerosis? J. Neurol. Neurosurg. Psychiatry 2019, 90, 1338–1346. [Google Scholar] [CrossRef]
- Czaplinski, A.; Yen, A.A.; Appel, S.H. Forced vital capacity (FVC) as an indicator of survival and disease progression in an ALS clinic population. J. Neurol. Neurosurg. Psychiatry 2006, 77, 390–392. [Google Scholar] [CrossRef] [Green Version]
- Oldoni, E.; Smets, I.; Mallants, K.; Vandebergh, M.; Van Horebeek, L.; Poesen, K.; Dupont, P.; Dubois, B.; Goris, A. CHIT1 at Diagnosis Reflects Long-Term Multiple Sclerosis Disease Activity. Ann. Neurol. 2020, 87, 633–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef]
- Choi, J.Y.; Yeo, I.J.; Kim, K.C.; Choi, W.R.; Jung, J.K.; Han, S.B.; Hong, J.T. K284-6111 prevents the amyloid beta-induced neuroinflammation and impairment of recognition memory through inhibition of NF-kappaB-mediated CHI3L1 expression. J Neuroinflamm. 2018, 15, 224. [Google Scholar] [CrossRef] [Green Version]
- Matute-Blanch, C.; Calvo-Barreiro, L.; Carballo-Carbajal, I.; Gonzalo, R.; Sanchez, A.; Vila, M.; Montalban, X.; Comabella, M. Chitinase 3-like 1 is neurotoxic in primary cultured neurons. Sci. Rep. 2020, 10, 7118. [Google Scholar] [CrossRef] [PubMed]
- Starossom, S.C.; Campo Garcia, J.; Woelfle, T.; Romero-Suarez, S.; Olah, M.; Watanabe, F.; Cao, L.; Yeste, A.; Tukker, J.J.; Quintana, F.J.; et al. Chi3l3 induces oligodendrogenesis in an experimental model of autoimmune neuroinflammation. Nat. Commun. 2019, 10, 217. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Q.; Yu, W.; Tian, Q.; Fu, X.; Wang, X.; Gu, M.; Lu, Y. Chitinase1 contributed to a potential protection via microglia polarization and Abeta oligomer reduction in D-galactose and aluminum-induced rat model with cognitive impairments. Neuroscience 2017, 355, 61–70. [Google Scholar] [CrossRef]
- Alonso, R.; Pisa, D.; Fernandez-Fernandez, A.M.; Rabano, A.; Carrasco, L. Fungal infection in neural tissue of patients with amyotrophic lateral sclerosis. Neurobiol. Dis. 2017, 108, 249–260. [Google Scholar] [CrossRef]
- Castellani, R.J.; Perry, G.; Smith, M.A. The role of novel chitin-like polysaccharides in Alzheimer disease. Neurotox. Res. 2007, 12, 269–274. [Google Scholar] [CrossRef]
- Danielson, B.; Chen, C.H.; Kaber, G.; Mochly-Rosen, D.; Grimes, K.; Stern, R.; Bollyky, P.L. Human Chitotriosidase Does Not Catabolize Hyaluronan. Int. J. Biol. Macromol. 2018, 109, 629–633. [Google Scholar] [CrossRef]

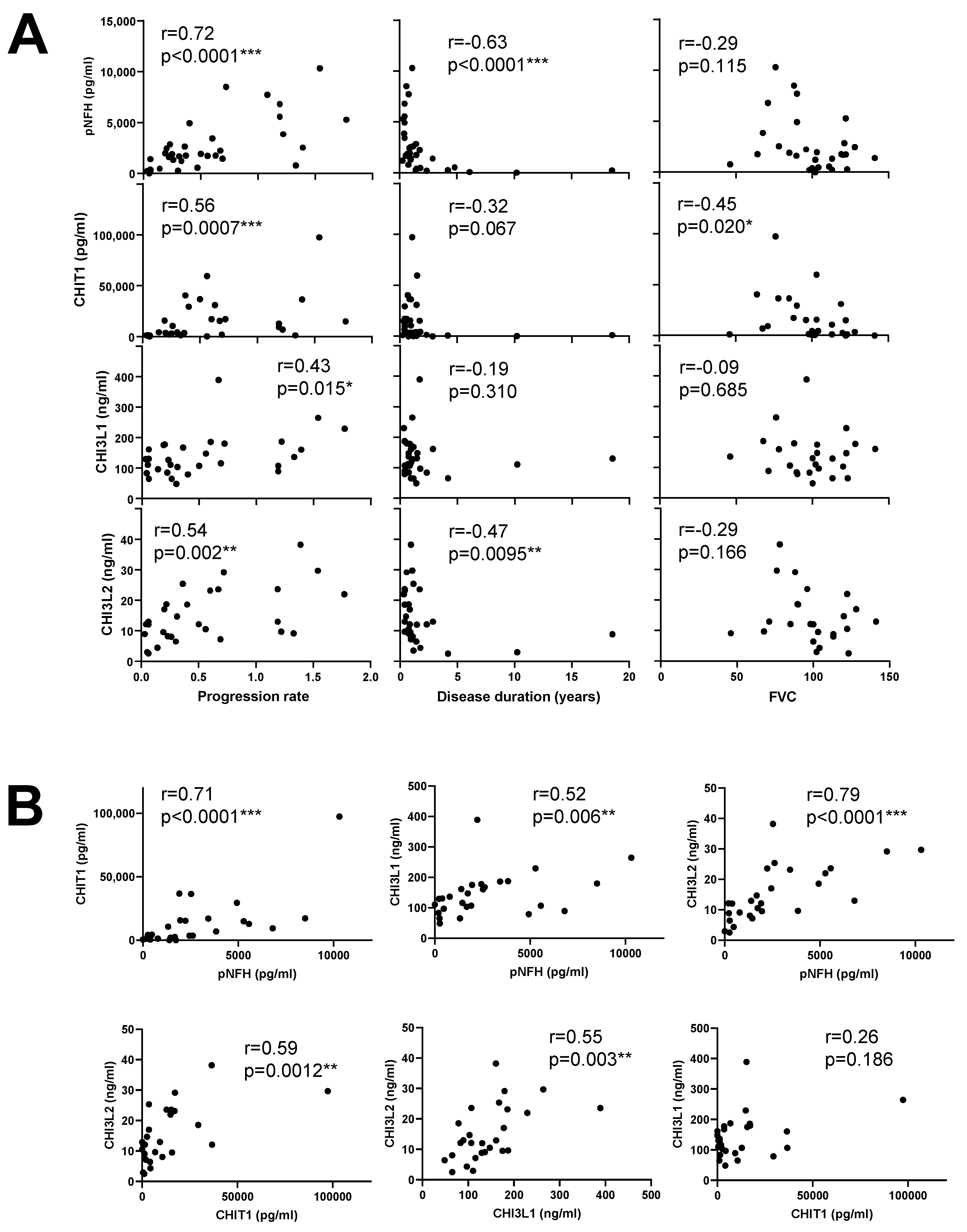
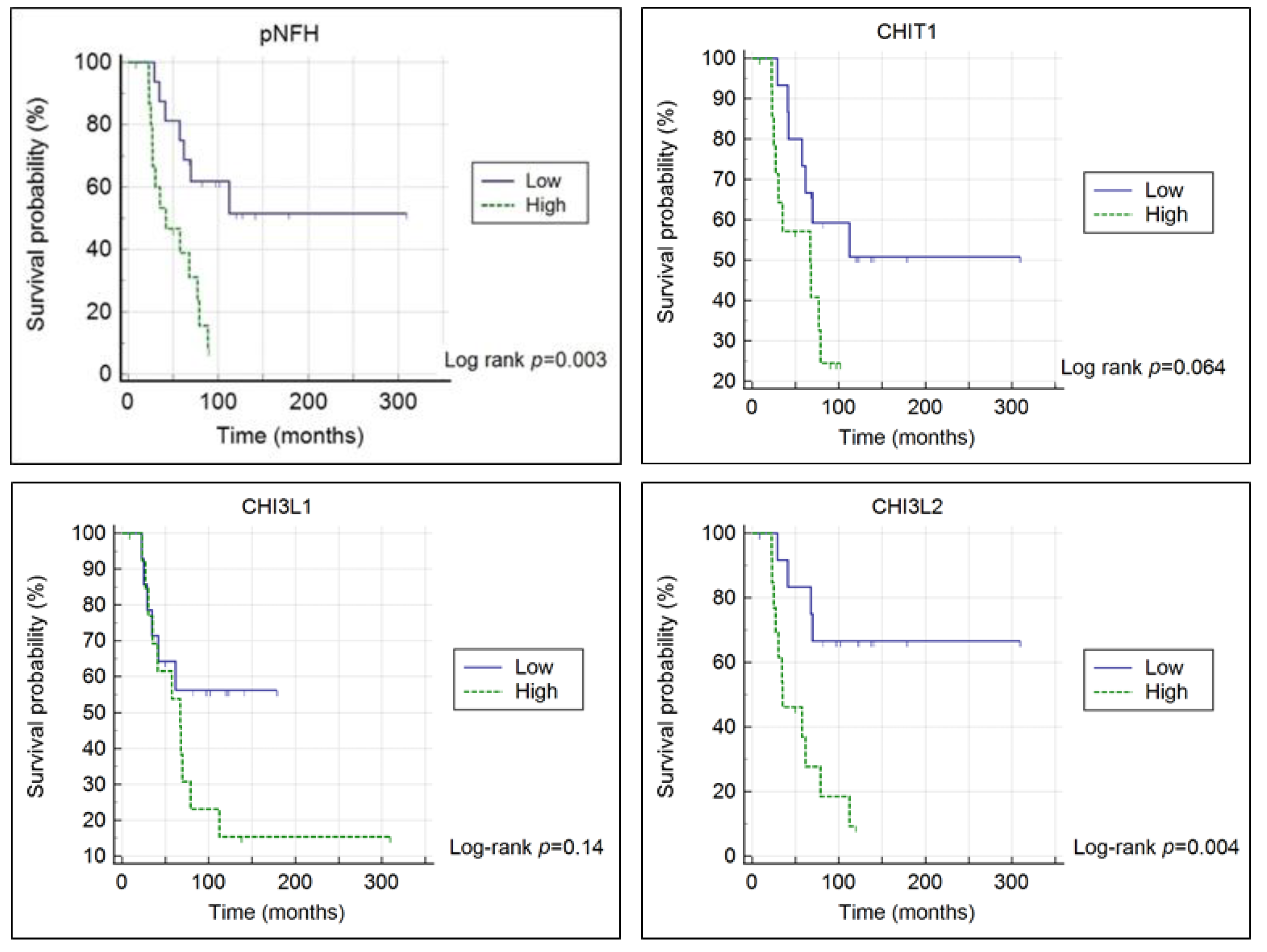
| pNFH | CHIT1 | CHI3L1 | CHI3L2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M/F | Age | CC (pg/mL) | M/F | Age | CC (pg/mL) | M/F | Age | CC (ng/mL) | M/F | Age | CC (ng/mL) | |
| C | 11/8 | 62.3 (52.3–67.0) | 338.7 (114.9–605.7) | 12/12 | 55.9 (43.1–65.5) | 638.9 (273.7–1678) | 9/9 | 56.0 (45.8–67.0) | 111.7 (84.3–147.7) | 8/9 | 57.2 (47.7–67.0) | 8.66 (6.47–12.13) |
| ALS | 26/10 | 56.0 (48.0–66.4) | 1751 (604.1–3285) | 25/9 | 56.7 (47.4–66.3) | 4254 (1293–17074) | 24/8 | 58.0 (49.7–66.4) | 128 (91.4–173.3) | 22/8 | 59.4 (49.8–66.6) | 12.10 (8.17–22.27) |
| p | - | 0.420 | <0.0001 *** | - | 0.826 | <0.0001 *** | - | 0.909 | 0.515 | - | 0.991 | 0.099 |
| Covariate | b | SE | Wald | p | HR | 95% CI of HR |
|---|---|---|---|---|---|---|
| Age at onset | 0.076 | 0.033 | 5.206 | 0.023 | 1.079 | 1.011 to 1.153 |
| Disease duration at sampling | −0.099 | 0.043 | 5.428 | 0.020 | 0.906 | 0.833 to 0.984 |
| CHIT1 | −0.777 | 0.541 | 2.060 | 0.151 | 0.460 | 0.159 to 1.328 |
| Age at onset | 0.049 | 0.032 | 2.291 | 0.130 | 1.050 | 0.986 to 1.119 |
| Disease duration at sampling | −0.096 | 0.042 | 5.301 | 0.021 | 0.908 | 0.837 to 0.986 |
| CHI3L1 | −0.398 | 0.531 | 0.562 | 0.453 | 0.672 | 0.237 to 1.902 |
| Age at onset | 0.115 | 0.055 | 4.454 | 0.035 | 1.122 | 1.008 to 1.249 |
| Disease duration at sampling | −0.116 | 0.045 | 6.666 | 0.010 | 0.890 | 0.815 to 0.972 |
| CHI3L2 | −1.604 | 0.685 | 5.478 | 0.019 | 0.201 | 0.053 to 0.771 |
| Age at onset | 0.065 | 0.032 | 4.078 | 0.043 | 1.068 | 1.002 to 1.137 |
| Disease duration at sampling | −0.059 | 0.033 | 3.264 | 0.071 | 0.942 | 0.884 to 1.005 |
| pNFH | −0.890 | 0.537 | 2.741 | 0.098 | 0.411 | 0.143 to 1.178 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.; Gromicho, M.; Pronto-Laborinho, A.; Almeida, C.; Gomes, R.A.; Guerreiro, A.C.L.; Oliva, A.; Pinto, S.; de Carvalho, M. Cerebrospinal Fluid Chitinases as Biomarkers for Amyotrophic Lateral Sclerosis. Diagnostics 2021, 11, 1210. https://doi.org/10.3390/diagnostics11071210
Costa J, Gromicho M, Pronto-Laborinho A, Almeida C, Gomes RA, Guerreiro ACL, Oliva A, Pinto S, de Carvalho M. Cerebrospinal Fluid Chitinases as Biomarkers for Amyotrophic Lateral Sclerosis. Diagnostics. 2021; 11(7):1210. https://doi.org/10.3390/diagnostics11071210
Chicago/Turabian StyleCosta, Júlia, Marta Gromicho, Ana Pronto-Laborinho, Conceição Almeida, Ricardo A. Gomes, Ana C. L. Guerreiro, Abel Oliva, Susana Pinto, and Mamede de Carvalho. 2021. "Cerebrospinal Fluid Chitinases as Biomarkers for Amyotrophic Lateral Sclerosis" Diagnostics 11, no. 7: 1210. https://doi.org/10.3390/diagnostics11071210
APA StyleCosta, J., Gromicho, M., Pronto-Laborinho, A., Almeida, C., Gomes, R. A., Guerreiro, A. C. L., Oliva, A., Pinto, S., & de Carvalho, M. (2021). Cerebrospinal Fluid Chitinases as Biomarkers for Amyotrophic Lateral Sclerosis. Diagnostics, 11(7), 1210. https://doi.org/10.3390/diagnostics11071210






