Performance of Integrated Near-Infrared Spectroscopy and Intravascular Ultrasound (NIRS-IVUS) System against Quantitative Flow Ratio (QFR)
Abstract
:1. Introduction
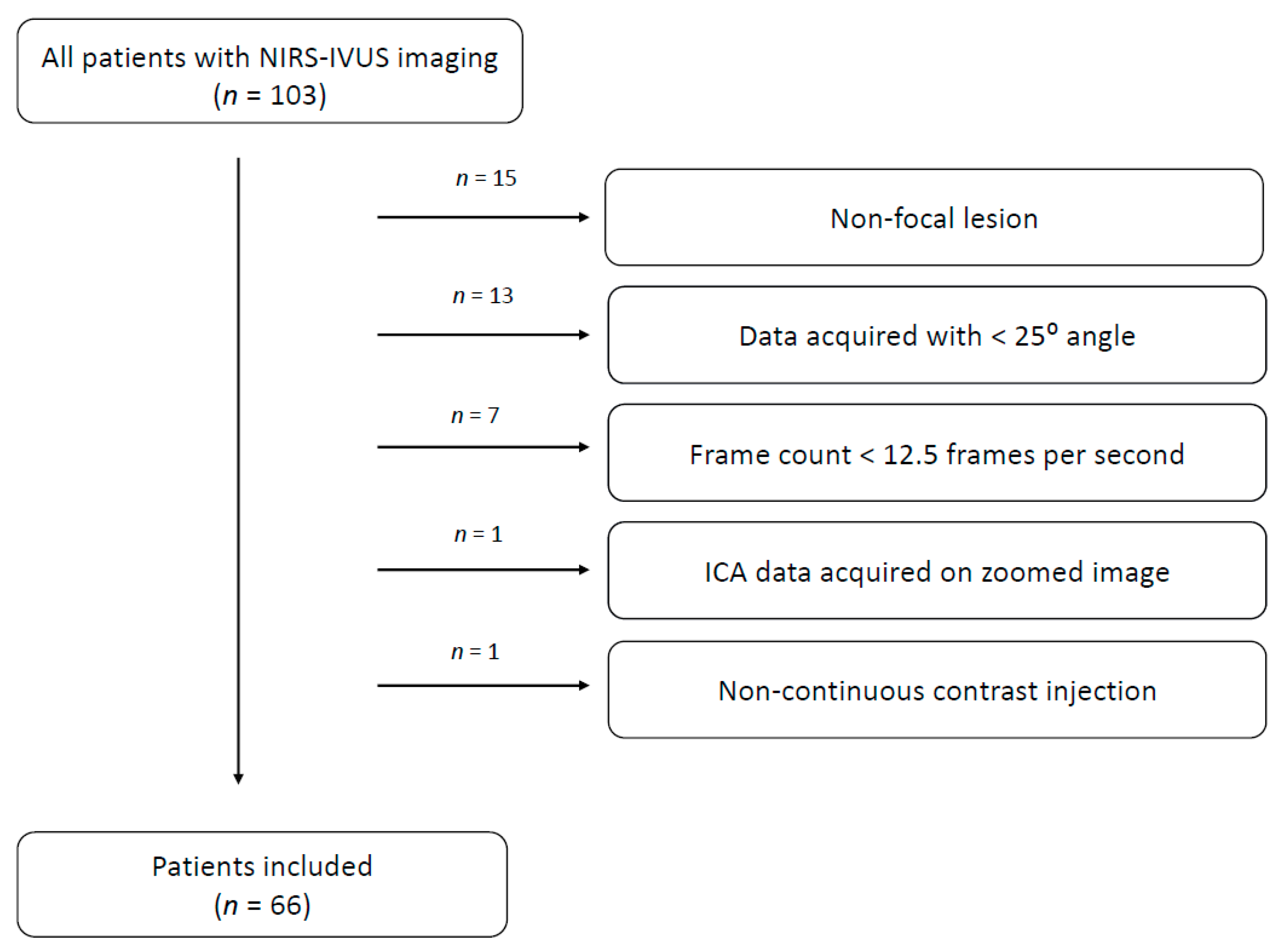
2. Materials and Methods
2.1. Study Population
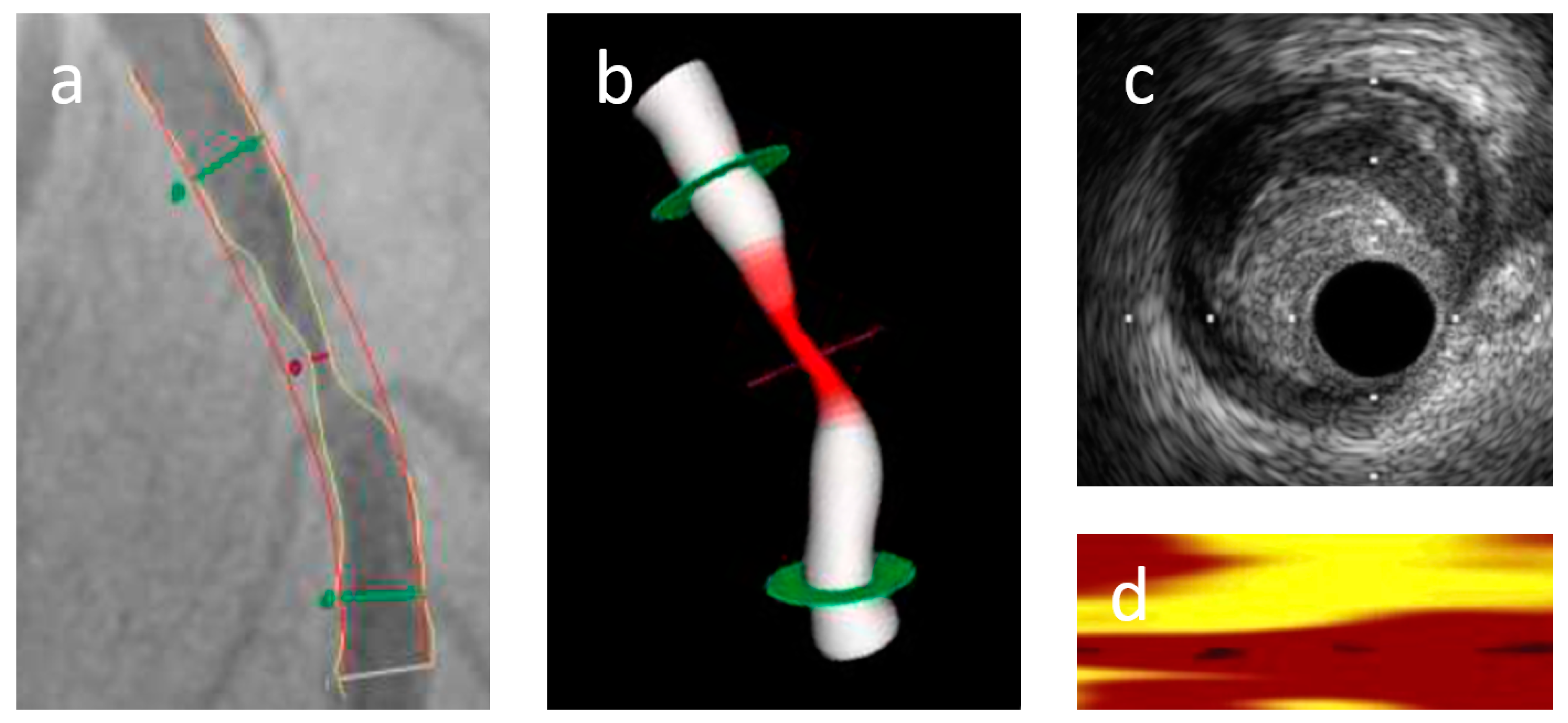
2.2. Invasive Coronary Angiography
2.3. QFR Measurement
2.4. NIRS-IVUS Analysis
2.5. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Lesion Characteristics
3.3. QFR and NIRS-IVUS Lesion Analysis
3.4. QFR and NIRS-IVUS Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Nunen, L.X.; Zimmermann, F.M.; Tonino, P.A.L.; Barbato, E.; Baumbach, A.; Engstrøm, T.; Klauss, V.; Maccarthy, P.A.; Manoharan, G.; Oldroyd, K.G.; et al. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME): 5-year follow-up of a randomised controlled trial. Lancet 2015, 386, 1853–1860. [Google Scholar] [CrossRef]
- Zimmermann, F.M.; Ferrara, A.; Johnson, N.P.; van Nunen, L.X.; Escaned, J.; Albertsson, P.; Erbel, R.; Legrand, V.; Gwon, H.-C.; Remkes, W.S.; et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur. Heart J. 2015, 36, 3182–3188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, S.; Westra, J.; Yang, J.; von Birgelen, C.; Ferrara, A.; Pellicano, M.; Nef, H.; Tebaldi, M.; Murasato, Y.; Lansky, A.; et al. Diagnostic Accuracy of Fast Computational Approaches to Derive Fractional Flow Reserve From Diagnostic Coronary Angiography: The International Multicenter FAVOR Pilot Study. JACC Cardiovasc. Interv. 2016, 9, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Tu, S.; Qiao, S.; Qu, X.; Chen, Y.; Yang, J.; Guo, L.; Sun, Z.; Li, Z.; Tian, F.; et al. Diagnostic Accuracy of Angiography-Based Quantitative Flow Ratio Measurements for Online Assessment of Coronary Stenosis. J. Am. Coll. Cardiol. 2017. [Google Scholar] [CrossRef]
- Smit, J.M.; Koning, G.; van Rosendael, A.R.; El Mahdiui, M.; Mertens, B.J.; Schalij, M.J.; Jukema, J.W.; Delgado, V.; Reiber, J.H.C.; Bax, J.J.; et al. Referral of patients for fractional flow reserve using quantitative flow ratio. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1231–1238. [Google Scholar] [CrossRef]
- Cortés, C.; Carrasco-Moraleja, M.; Aparisi, A.; Rodriguez-Gabella, T.; Campo, A.; Gutiérrez, H.; Julca, F.; Gómez, I.; San Román, J.A.; Amat-Santos, I.J. Quantitative flow ratio-Meta-analysis and systematic review. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2021, 97, 807–814. [Google Scholar] [CrossRef]
- Parise, H.; Maehara, A.; Stone, G.W.; Leon, M.B.; Mintz, G.S. Meta-analysis of randomized studies comparing intravascular ultrasound versus angiographic guidance of percutaneous coronary intervention in predrug-eluting stent era. Am. J. Cardiol. 2011, 107, 374–382. [Google Scholar] [CrossRef]
- Buccheri, S.; Franchina, G.; Romano, S.; Puglisi, S.; Venuti, G.; D’Arrigo, P.; Francaviglia, B.; Scalia, M.; Condorelli, A.; Barbanti, M.; et al. Clinical Outcomes Following Intravascular Imaging-Guided Versus Coronary Angiography–Guided Percutaneous Coronary Intervention with Stent Implantation: A Systematic Review and Bayesian Network Meta-Analysis of 31 Studies and 17,882 Patients. JACC Cardiovasc. Interv. 2017, 10, 2488–2498. [Google Scholar] [CrossRef]
- De Korte, C.L.; Pasterkamp, G.; van der Steen, A.F.; Woutman, H.A.; Bom, N. Characterization of plaque components with intravascular ultrasound elastography in human femoral and coronary arteries in vitro. Circulation 2000, 102, 617–623. [Google Scholar] [CrossRef] [Green Version]
- Waksman, R.; Legutk, J.; Singh, J.; Orlando, Q.; Marso, S.; Schloss, T.; Tugaoen, J.; DeVries, J.; Palmer, N.; Haude, M.; et al. Erratum: FIRST: Fractional Flow Reserve and Intravascular Ultrasound Relationship Study (Journal of the American College of Cardiology (2013) 61(917–23)). J. Am. Coll. Cardiol. 2015, 66, 335. [Google Scholar] [CrossRef]
- Park, S.J.; Ahn, J.M.; Kang, S.J.; Yoon, S.H.; Koo, B.K.; Lee, J.Y.; Kim, W.J.; Park, D.W.; Lee, S.W.; Kim, Y.H.; et al. Intravascular ultrasound-derived minimal lumen area criteria for functionally significant left main coronary artery stenosis. JACC Cardiovasc. Interv. 2014, 7, 868–874. [Google Scholar] [CrossRef] [Green Version]
- Usui, E.; Yonetsu, T.; Kanaji, Y.; Hoshino, M.; Yamaguchi, M.; Hada, M.; Hamaya, R.; Kanno, Y.; Murai, T.; Lee, T.; et al. Efficacy of optical coherence tomography-derived morphometric assessment in predicting the physiological significance of coronary stenosis: Head-to-head comparison with intravascular ultrasound. EuroIntervention 2018, 13, e2210–e2218. [Google Scholar] [CrossRef]
- Erlinge, D.; Maehara, A.; Ben-Yehuda, O.; Bøtker, H.E.; Maeng, M.; Kjøller-Hansen, L.; Engstrøm, T.; Matsumura, M.; Crowley, A.; Dressler, O.; et al. Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): A prospective natural history study. Lancet 2021, 397, 985–995. [Google Scholar] [CrossRef]
- Roleder, T.; Kovacic, J.C.; Ali, Z.; Sharma, R.; Cristea, E.; Moreno, P.; Sharma, S.K.; Narula, J.; Kini, A.S. Combined NIRS and IVUS imaging detects vulnerable plaque using a single catheter system: A head-to-head comparison with OCT. EuroIntervention 2014, 10, 303–311. [Google Scholar] [CrossRef]
- Goldstein, J.A.; Maini, B.; Dixon, S.R.; Brilakis, E.S.; Grines, C.L.; Rizik, D.G.; Powers, E.R.; Steinberg, D.H.; Shunk, K.A.; Weisz, G.; et al. Detection of lipid-core plaques by intracoronary near-infrared spectroscopy identifies high risk of periprocedural myocardial infarction. Circ. Cardiovasc. Interv. 2011, 4, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Sakhuja, R.; Suh, W.M.; Jaffer, F.A.; Jang, I.K. Residual thrombogenic substrate after rupture of a lipid-rich plaque: Possible mechanism of acute stent thrombosis?: A near-infrared spectroscopy study. Circulation 2010, 122, 2349–2350. [Google Scholar] [CrossRef] [Green Version]
- Tu, S.; Reiber, J.H.C.; Li, Y. Method and Device for Determining Deviation in Pressure in a Blood Vessel. U.S. Patent US9891044B2, 13 February 2018. [Google Scholar]
- Choi, H.; Choe, S. Therapeutic Effect Enhancement by Dual-Bias High-Voltage Circuit of Transmit Amplifier for Immersion Ultrasound Transducer Applications. Sensors 2018, 18, 4210. [Google Scholar] [CrossRef] [Green Version]
- Mirmiran, P.; Esmaillzadeh, A.; Azizi, F. Detection of cardiovascular risk factors by anthropometric measures in Tehranian adults: Receiver operating characteristic (ROC) curve analysis. Eur. J. Clin. Nutr. 2004, 58, 1110–1118. [Google Scholar] [CrossRef] [Green Version]
- Echavarría-Pinto, M.; Gonzalo, N.; Ibañez, B.; Petraco, R.; Jimenez-Quevedo, P.; Sen, S.; Nijjer, S.; Tarkin, J.; Alfonso, F.; Núñez-Gil, I.J.; et al. Low coronary microcirculatory resistance associated with profound hypotension during intravenous adenosine infusion implications for the functional assessment of coronary stenoses. Circ. Cardiovasc. Interv. 2014. [Google Scholar] [CrossRef] [Green Version]
- Mejía-Rentería, H.; Lauri, F.M.; Lee, J.M.; McInerney, A.; van der Hoeven, N.W.; de Waard, G.A.; Fernández-Ortiz, A.; Macaya, C.; Knaapen, P.; van Royen, N.; et al. Interindividual Variations in the Adenosine-Induced Hemodynamics During Fractional Flow Reserve Evaluation: Implications for the Use of Quantitative Flow Ratio in Assessing Intermediate Coronary Stenoses. J. Am. Heart Assoc. 2019. [Google Scholar] [CrossRef]
- Wilson, R.F.; Wyche, K.; Christensen, B.V.; Zimmer, S.; Laxson, D.D. Effects of adenosine on human coronary arterial circulation. Circulation 1990. [Google Scholar] [CrossRef] [Green Version]
- Westra, J.; Andersen, B.K.; Campo, G.; Matsuo, H.; Koltowski, L.; Eftekhari, A.; Liu, T.; Di Serafino, L.; Di Girolamo, D.; Escaned, J.; et al. Diagnostic performance of in-procedure angiography-derived quantitative flow reserve compared to pressure-derived fractional flow reserve: The FAVOR II Europe-Japan study. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Hwang, D.; Choi, K.H.; Lee, J.M.; Mejía-Rentería, H.; Kim, J.; Park, J.; Rhee, T.M.; Jeon, K.H.; Lee, H.J.; Kim, H.K.; et al. Diagnostic Agreement of Quantitative Flow Ratio with Fractional Flow Reserve and Instantaneous Wave-Free Ratio. J. Am. Heart Assoc. 2019. [Google Scholar] [CrossRef] [Green Version]
- Westra, J.; Tu, S.; Winther, S.; Nissen, L.; Vestergaard, M.B.; Andersen, B.K.; Holck, E.N.; Maule, C.F.; Johansen, J.K.; Andreasen, L.N.; et al. Evaluation of Coronary Artery Stenosis by Quantitative Flow Ratio during Invasive Coronary Angiography: The WIFI II Study (Wire-Free Functional Imaging II). Circ. Cardiovasc. Imaging 2018. [Google Scholar] [CrossRef]
- Tanigaki, T.; Emori, H.; Kawase, Y.; Kubo, T.; Omori, H.; Shiono, Y.; Sobue, Y.; Shimamura, K.; Hirata, T.; Matsuo, Y.; et al. QFR Versus FFR Derived from Computed Tomography for Functional Assessment of Coronary Artery Stenosis. JACC Cardiovasc. Interv. 2019. [Google Scholar] [CrossRef]
- Zuo, W.; Yang, M.; Chen, Y.; Xie, A.; Chen, L.; Ma, G. Meta-Analysis of Diagnostic Performance of Instantaneous Wave-Free Ratio versus Quantitative Flow Ratio for Detecting the Functional Significance of Coronary Stenosis. Biomed Res. Int. 2019, 2019, 5828931. [Google Scholar] [CrossRef]
- Kleczyński, P.; Dziewierz, A.; Rzeszutko, Ł.; Dudek, D.; Legutko, J. Borderline coronary lesion assessment with quantitative flow ratio and its relation to the instantaneous wave-free ratio. Adv. Med. Sci. 2021, 66, 1–5. [Google Scholar] [CrossRef]
- Masdjedi, K.; van Zandvoort, L.J.C.; Balbi, M.M.; Gijsen, F.J.H.; Ligthart, J.M.R.; Rutten, M.C.M.; Lemmert, M.E.; Wilschut, J.; Diletti, R.; De Jaegere, P.; et al. Validation of 3-Dimensional Quantitative Coronary Angiography based software to calculate Fractional Flow Reserve: Fast Assessment of STenosis severity (FAST)-study. EuroIntervention 2019. [Google Scholar] [CrossRef]
- Abizaid, A.S.; Mintz, G.S.; Mehran, R.; Abizaid, A.; Lansky, A.J.; Pichard, A.D.; Satler, L.F.; Wu, H.; Pappas, C.; Kent, K.M.; et al. Long-term follow-up after percutaneous transluminal coronary angioplasty was not performed based on intravascular ultrasound findings: Importance of lumen dimensions. Circulation 1999. [Google Scholar] [CrossRef] [Green Version]
- Okabe, T.; Mintz, G.S.; Buch, A.N.; Roy, P.; Hong, Y.J.; Smith, K.A.; Torguson, R.; Gevorkian, N.; Xue, Z.; Satler, L.F.; et al. Intravascular Ultrasound Parameters Associated with Stent Thrombosis After Drug-Eluting Stent Deployment. Am. J. Cardiol. 2007. [Google Scholar] [CrossRef]
- Voros, S.; Rinehart, S.; Vazquez-Figueroa, J.G.; Kalynych, A.; Karmpaliotis, D.; Qian, Z.; Joshi, P.H.; Anderson, H.; Murrieta, L.; Wilmer, C.; et al. Prospective, head-to-head comparison of quantitative coronary angiography, quantitative computed tomography angiography, and intravascular ultrasound for the prediction of hemodynamic significance in intermediate and severe lesions, using fractional flow. Am. J. Cardiol. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.J.; Lee, J.Y.; Ahn, J.M.; Mintz, G.S.; Kim, W.J.; Park, D.W.; Yun, S.C.; Lee, S.W.; Kim, Y.H.; Lee, C.W.; et al. Validation of intravascular ultrasound-derived parameters with fractional flow reserve for assessment of coronary stenosis severity. Circ. Cardiovasc. Interv. 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ascenzo, F.; Barbero, U.; Cerrato, E.; Lipinski, M.J.; Omedè, P.; Montefusco, A.; Taha, S.; Naganuma, T.; Reith, S.; Voros, S.; et al. Accuracy of intravascular ultrasound and optical coherence tomography in identifying functionally significant coronary stenosis according to vessel diameter: A meta-analysis of 2581 patients and 2807 lesions. Am. Heart J. 2015. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, N.; Escaned, J.; Alfonso, F.; Nolte, C.; Rodriguez, V.; Jimenez-Quevedo, P.; Bañuelos, C.; Fernández-Ortiz, A.; Garcia, E.; Hernandez-Antolin, R.; et al. Morphometric Assessment of Coronary Stenosis Relevance With Optical Coherence Tomography. J. Am. Coll. Cardiol. 2012. [Google Scholar] [CrossRef] [Green Version]
- Waxman, S.; Dixon, S.R.; L’Allier, P.; Moses, J.W.; Petersen, J.L.; Cutlip, D.; Tardif, J.C.; Nesto, R.W.; Muller, J.E.; Hendricks, M.J.; et al. In Vivo Validation of a Catheter-Based Near-Infrared Spectroscopy System for Detection of Lipid Core Coronary Plaques. Initial Results of the SPECTACL Study. JACC Cardiovasc. Imaging 2009, 2, 858–868. [Google Scholar] [CrossRef] [Green Version]
- Gardner, C.M.; Tan, H.; Hull, E.L.; Lisauskas, J.B.; Sum, S.T.; Meese, T.M.; Jiang, C.; Madden, S.P.; Caplan, J.D.; Burke, A.P.; et al. Detection of Lipid Core Coronary Plaques in Autopsy Specimens with a Novel Catheter-Based Near-Infrared Spectroscopy System. JACC Cardiovasc. Imaging 2008, 1, 638–648. [Google Scholar] [CrossRef] [Green Version]
- Farb, A.; Burke, A.P.; Kolodgie, F.D.; Virmani, R. Pathological mechanisms of fatal late coronary stent thrombosis in humans. Circulation 2003, 108, 1701–1706. [Google Scholar] [CrossRef] [Green Version]
- Schuurman, A.S.; Vroegindewey, M.; Kardys, I.; Oemrawsingh, R.M.; Cheng, J.M.; De Boer, S.; Garcia-Garcia, H.M.; Van Geuns, R.J.; Regar, E.S.; Daemen, J.; et al. Near-infrared spectroscopy-derived lipid core burden index predicts adverse cardiovascular outcome in patients with coronary artery disease during long-term follow-up. Eur. Heart J. 2018, 39, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Oemrawsingh, R.M.; Cheng, J.M.; García-García, H.M.; Van Geuns, R.J.; De Boer, S.P.M.; Simsek, C.; Kardys, I.; Lenzen, M.J.; Van Domburg, R.T.; Regar, E.; et al. Near-infrared spectroscopy predicts cardiovascular outcome in patients with coronary artery disease. J. Am. Coll. Cardiol. 2014, 64, 2510–2518. [Google Scholar] [CrossRef]
| QFR-Positive n = 37 | QFR-Negative n = 29 | p | |
|---|---|---|---|
| Clinical Demographics | |||
| Age (years) | 63.3 ± 10.34 | 62.8 ± 10.9 | 0.767 |
| Body mass index kg/m2 | 21.89 ± 12.87 | 18.7 ± 12.3 | 0.538 |
| Prior MI n% | 4 (10.8) | 7 (24.1) | 0.833 |
| Prior PCI n% | 4 (10.8) | 10 (34.5) | 0.016 |
| Prior CABG n% | 0 (0) | 0 (0.0) | |
| Dyslipidemia n% | 13 (35.1) | 18 (62.0) | 0.114 |
| Hypertension n% | 16 (43.2) | 23 (79.3) | 0.246 |
| Diabetes mellitus n% | 8 (21.6) | 6 (20.7) | 0.925 |
| TCH | 154.3 ± 36.9 | 128.0 ± 45.0 | 0.093 |
| LDL | 71.8 ± 38.4 | 80.0 ± 28.7 | 0.511 |
| HDL | 41.46 ± 13.52 | 31.4 ± 19.9 | 0.120 |
| TG | 124.5 (77.75, 145.25) | 76 (48.0, 136.0) | 0.656 |
| GFR | 61.6 ± 23.15 | 57.8 ± 27.47 | 0.681 |
| QFR-Positive | QFR-Negative | p | |
|---|---|---|---|
| Indication for Coronary Angiography | |||
| ACS | 10 (27.0) | 6 (20.7) | 0.771 |
| Lesion Location | |||
| LAD | 19 (51.3) | 12 (41.4) | |
| Cx | 11 (29.7) | 7 (24.1) | |
| RCA | 7 (19.0) | 10 (34.5) | |
| QFR Analysis | |||
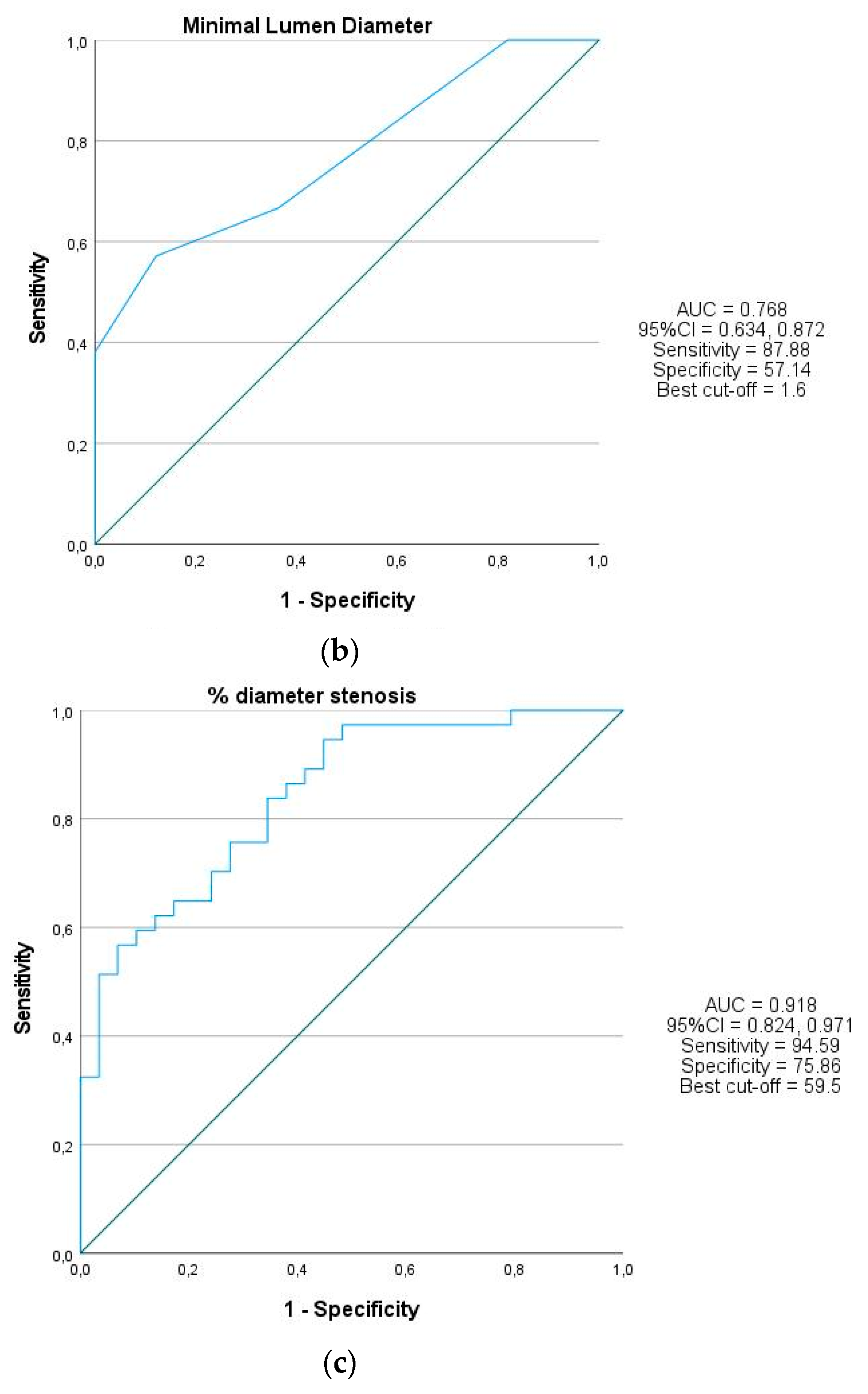
| Diameter stenosis | 71.98 ± 8.67 | 49.42 ± 12.13 | 0.000 |
| Vessel QFR contrast | 0.67 ± 0.14 | 0.9 ± 0.06 | 0.000 |
| IVUS Analysis | |||
| Stenosis length | 27.7 ± 10.74 | 22.91 ± 11.02 | 0.480 |
| Lumen volume | 126.97 ± 68.51 | 133.93 ± 81.56 | 0.737 |
| EEM volume | 353.74 ± 207.49 | 327.2 ± 222.5 | 0.658 |
| EEM area at MLA | 10.59 ± 3.4 | 13.04 ± 5.5 | 0.048 |
| Plaque volume | 226.16 ± 155.57 | 193.27 ± 149.16 | 0.252 |
| Plaque burden | 77.2 ± 7.82 | 75.01 ± 7.43 | 0.286 |
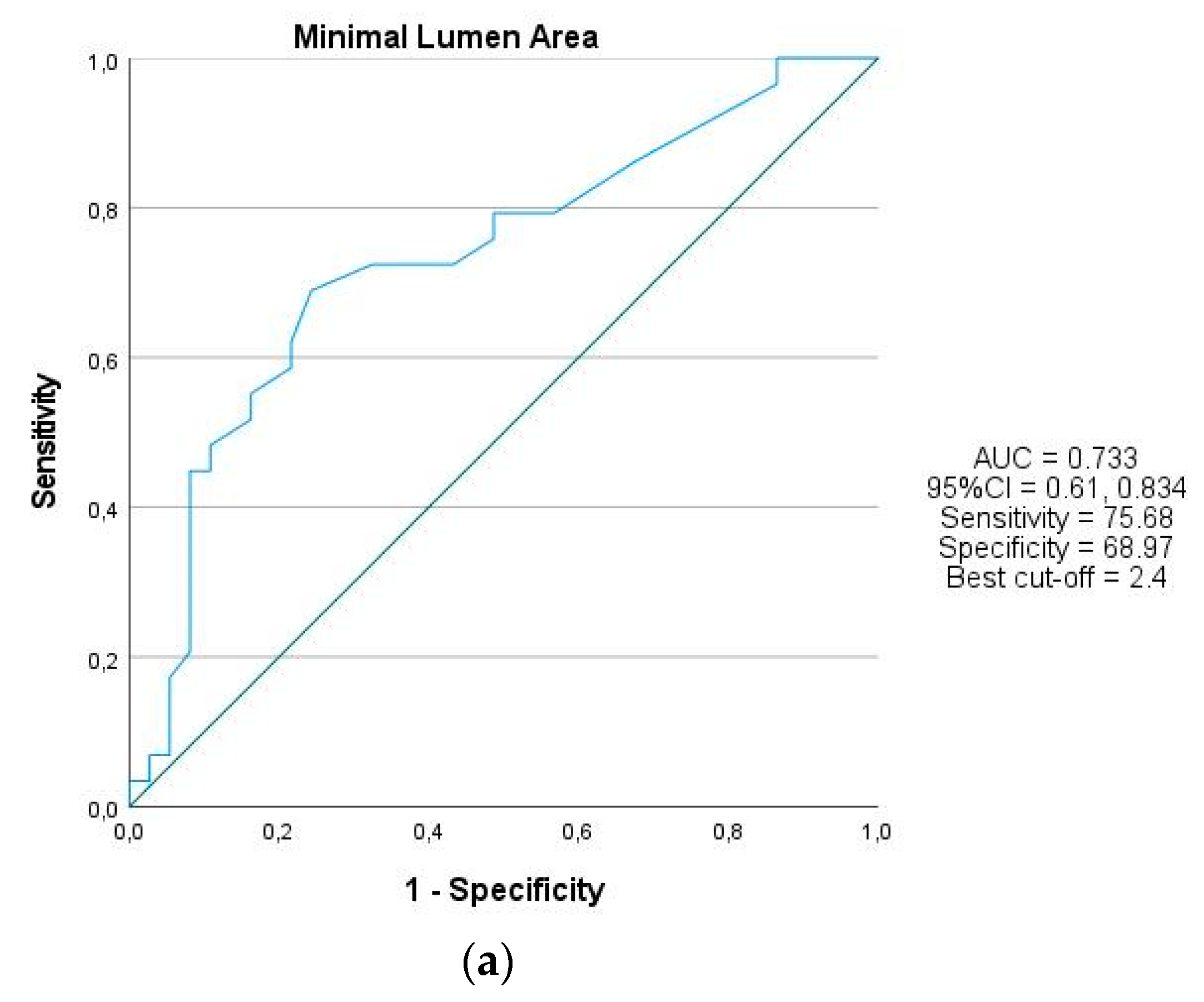
| Minimal lumen area | 2.2 ± 0.42 | 3.12 ± 1.44 | 0.007 |
| Minimal lumen diameter | 1.5 (1.5, 1.6) | 1.7 (1.5, 1.9) | 0.001 |
| Total plaque area | 8.3 (5.45, 10.55) | 8.4 (7.5, 10.85) | 0.347 |
| RI | 1.02 (0.8, 1.27) | 1.00 (0.84, 1.44) | 0.690 |
| MLA < 2.4 mm2 | 28 (75.67) | 9 (31.0) | 0.000 |
| NIRS Analysis | |||
| maxLCBI4mm | 450.12 ± 251.0 | 329.47 ± 191.14 | 0.046 |
| TCFA lesions | 22 (59.45) | 21 (72.41) | 0.276 |
| QFR-Positive | QFR-Negative | |||
|---|---|---|---|---|
| Vessel QFR (r) | p | Vessel QFR (r) | p | |
| MLA | 0.151 | 0.721 | 0.151 | 0.435 |
| PB | −0.227 | 0.176 | −0.177 | 0.358 |
| maxLCBI4mm | −0.198 | 0.239 | −0.033 | 0.866 |
| PV | −0.229 | 0.173 | −0.212 | 0.271 |
| MLD | 0.064 | 0.722 | 0.388 | 0.082 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrolińska, M.M.; Gąsior, P.M.; Pociask, E.; Smolka, G.; Ochala, A.; Wojakowski, W.; Roleder, T. Performance of Integrated Near-Infrared Spectroscopy and Intravascular Ultrasound (NIRS-IVUS) System against Quantitative Flow Ratio (QFR). Diagnostics 2021, 11, 1148. https://doi.org/10.3390/diagnostics11071148
Dobrolińska MM, Gąsior PM, Pociask E, Smolka G, Ochala A, Wojakowski W, Roleder T. Performance of Integrated Near-Infrared Spectroscopy and Intravascular Ultrasound (NIRS-IVUS) System against Quantitative Flow Ratio (QFR). Diagnostics. 2021; 11(7):1148. https://doi.org/10.3390/diagnostics11071148
Chicago/Turabian StyleDobrolińska, Magdalena M., Paweł M. Gąsior, Elżbieta Pociask, Grzegorz Smolka, Andrzej Ochala, Wojciech Wojakowski, and Tomasz Roleder. 2021. "Performance of Integrated Near-Infrared Spectroscopy and Intravascular Ultrasound (NIRS-IVUS) System against Quantitative Flow Ratio (QFR)" Diagnostics 11, no. 7: 1148. https://doi.org/10.3390/diagnostics11071148
APA StyleDobrolińska, M. M., Gąsior, P. M., Pociask, E., Smolka, G., Ochala, A., Wojakowski, W., & Roleder, T. (2021). Performance of Integrated Near-Infrared Spectroscopy and Intravascular Ultrasound (NIRS-IVUS) System against Quantitative Flow Ratio (QFR). Diagnostics, 11(7), 1148. https://doi.org/10.3390/diagnostics11071148






