Adverse Impact of DNA Methylation Regulatory Gene Mutations on the Prognosis of AML Patients in the 2017 ELN Favorable Risk Group, Particularly Those Defined by NPM1 Mutation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. NGS Study and FLT3-ITD Measurement
2.3. Patient Group
2.4. Statistical Analyses
3. Results
3.1. Patients’ Characteristics
3.2. Incidence of DMRGM
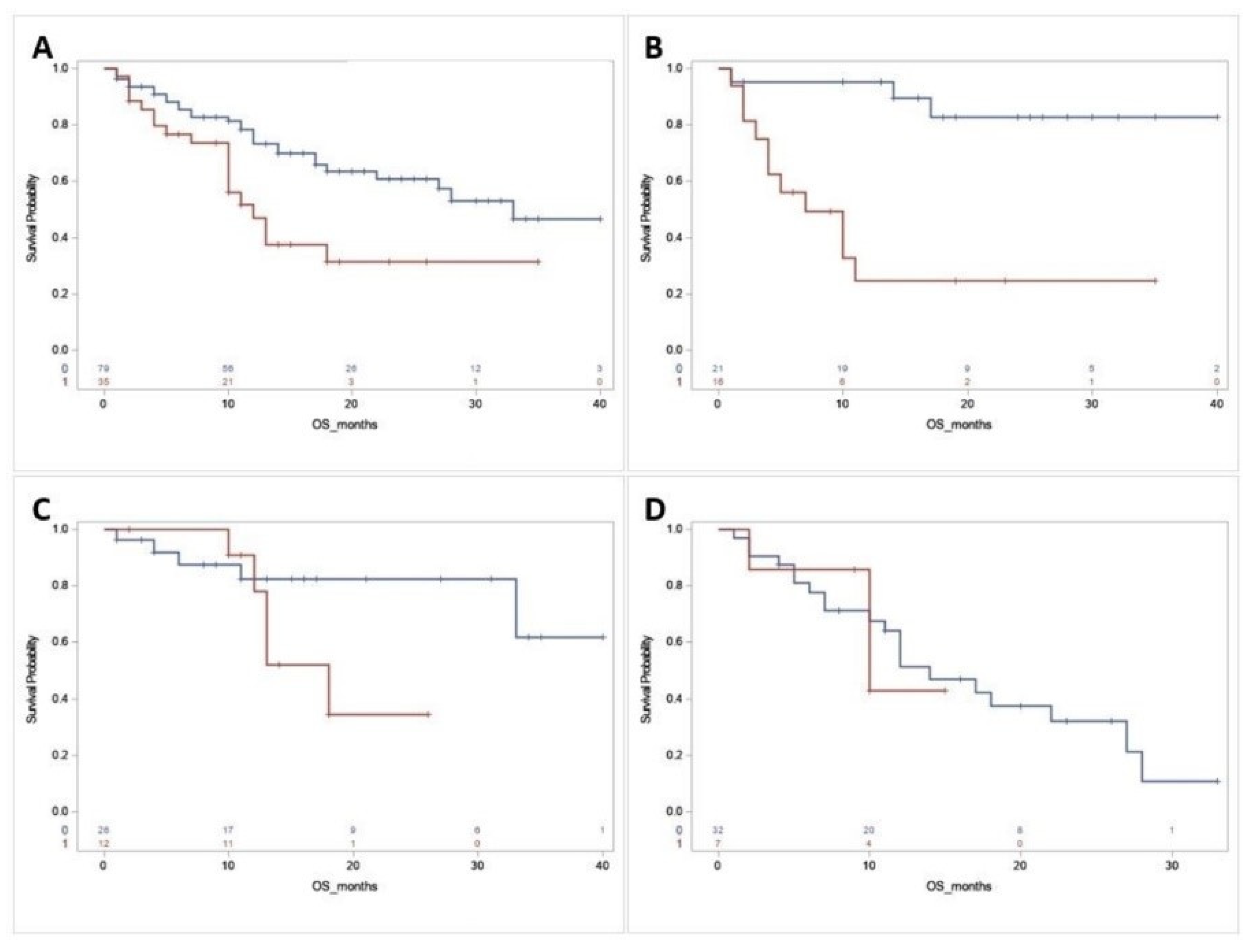
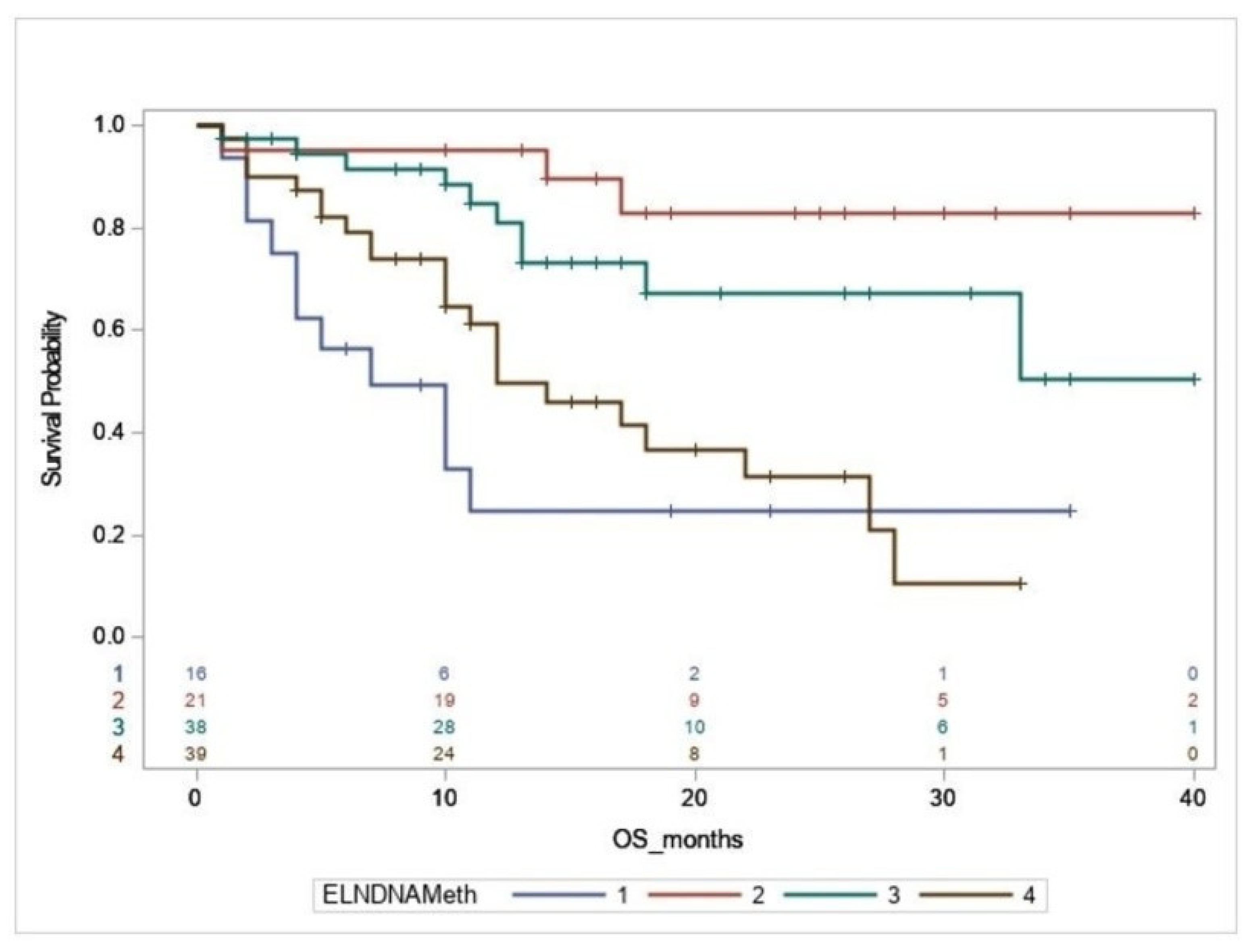
3.3. Survival Analysis
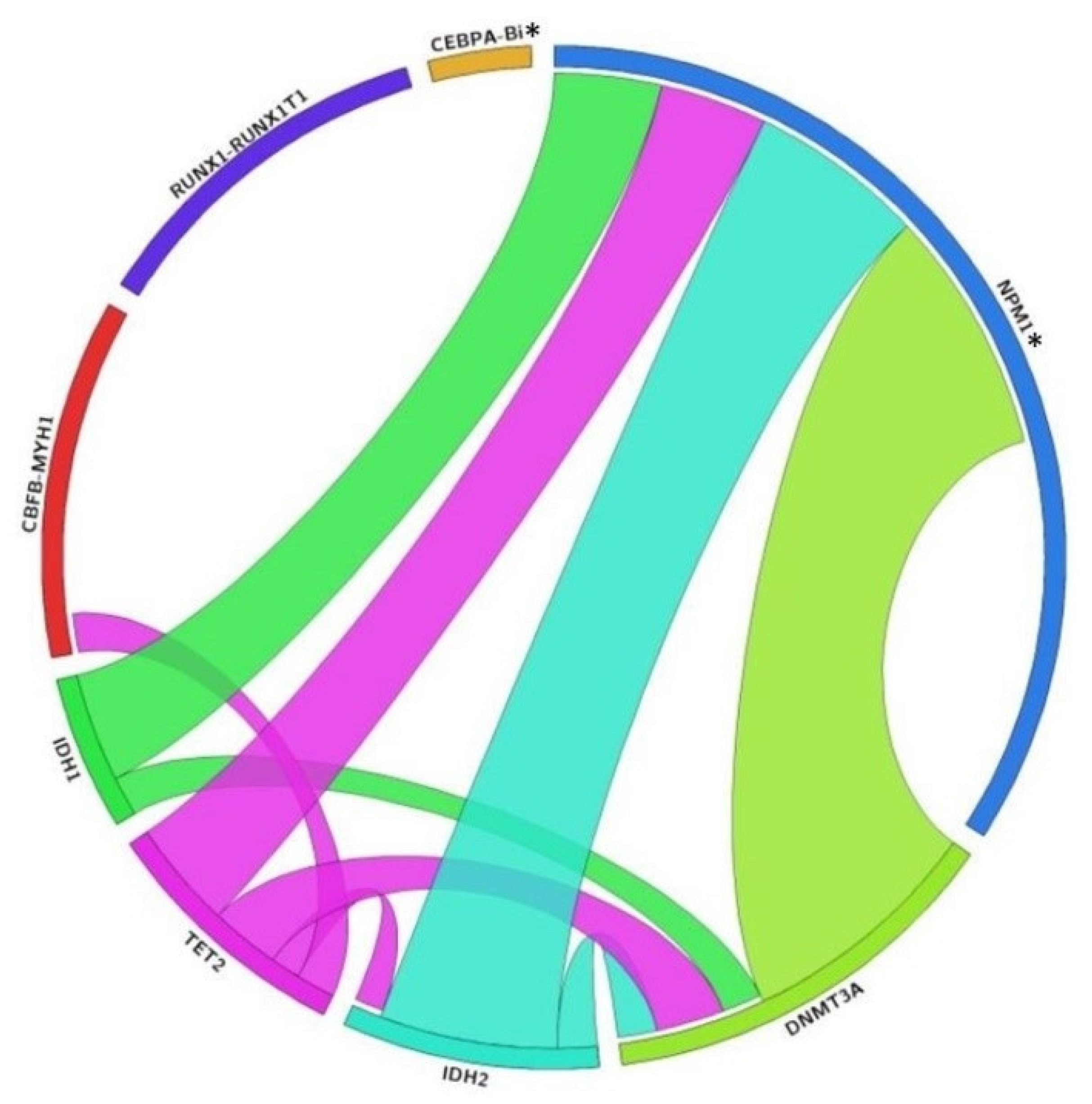
3.4. Genetic Association of DMRGM with Other Mutations in the 2017 ELN Favorable Risk Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Ley, T.J.; Mardis, E.R.; Ding, L.; Fulton, B.; McLellan, M.D.; Chen, K.; Dooling, D.; Dunford-Shore, B.H.; McGrath, S.; Hickenbotham, M.; et al. DNA sequencing of a cytogenetically normal acute myeloid leukemia genome. Nature 2008, 456, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Mardis, E.R.; Ding, L.; Dooling, D.J.; Larson, D.E.; McLellan, M.D.; Chen, K.; Koboldt, D.C.; Fulton, R.S.; Delehaunty, K.D.; McGrath, S.D.; et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med. 2009, 361, 1058–1066. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Ley, T.J.; Miller, C.; Ding, L.; Raphael, B.J.; Mungall, A.J.; Robertson, A.G.; Hoadley, K.; Triche, T.J., Jr.; Laird, P.W.; et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar] [CrossRef] [PubMed]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef]
- Patel, J.P.; Gönen, M.; Figueroa, M.E.; Fernandez, H.; Sun, Z.; Racevskis, J.; Van Vlierberghe, P.; Dolgalev, I.; Thomas, S.; Aminova, O.; et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N. Engl. J. Med. 2012, 366, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, G.; Haferlach, T.; Döhner, H. Molecular genetics of adult acute myeloid leukemia: Prognostic and therapeutic implications. J. Clin. Oncol. 2011, 29, 475–486. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Hou, H.-A.; Tang, J.-L.; Liu, C.-Y.; Lin, C.-C.; Chou, W.-C.; Tseng, M.-H.; Chiang, Y.-C.; Kuo, Y.-Y.; Liu, M.-C.; et al. Genetic alterations and their clinical implications in older patients with acute myeloid leukemia. Leukemia 2016, 30, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Eisfeld, A.-K.; Kohlschmidt, J.; Mims, A.; Nicolet, D.; Walker, C.J.; Blachly, J.S.; Carroll, A.J.; Papaioannou, D.; Kolitz, J.E.; Powell, B.E.; et al. Additional gene mutations may refine the 2017 European LeukemiaNet classification in adult patients with de novo acute myeloid leukemia aged <60 years. Leukemia 2020, 34, 3215–3227. [Google Scholar] [CrossRef]
- Eisfeld, A.-K.; Kohlschmidt, J.; Mrózek, K.; Blachly, J.S.; Walker, C.J.; Nicolet, D.; Orwick, S.; Maharry, S.E.; Carroll, A.J.; Stone, R.M.; et al. Mutation patterns identify adult patients with de novo acute myeloid leukemia aged 60 years or older who respond favorably to standard chemotherapy: An analysis of Alliance studies. Leukemia 2018, 32, 1338–1348. [Google Scholar] [CrossRef]
- Huang, S.-C.; Hou, H.-A.; Tsai, C.-H.; Chou, W.-C.; Tang, J.-L.; Yao, M.; Ko, B.-S.; Tien, F.-M.; Kuo, Y.-Y.; Tseng, M.-H.; et al. Re-examination of 2017 ELN risk classification by a cohort of 739 de novo aml patients in Taiwan: Co-occurring poor-risk mutations may further predict outcome in FLT3-ITD patients. Blood 2018, 132, 3977. [Google Scholar] [CrossRef]
- Metzeler, K.H.; Herold, T.; Rothenberg-Thurley, M.; Amler, S.; Sauerland, M.C.; Görlich, D.; Schneider, S.; Konstandin, N.P.; Dufour, A.; Bräundl, K.; et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood 2016, 128, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Ley, T.J.; Ding, L.; Walter, M.J.; McLellan, M.D.; Lamprecht, T.L.; Larson, D.E.; Kandoth, C.; Payton, J.E.; Baty, J.; Welch, J.J.; et al. DNMT3A mutations in acute myeloid leukemia. N. Engl. J. Med. 2010, 363, 2424–2433. [Google Scholar] [CrossRef]
- Ribeiro, A.F.T.; Pratcorona, M.; Erpelinck-Verschueren, C.; Rockova, V.; Sanders, M.; Abbas, S.; Figueroa, M.E.; Zeilemaker, A.; Melnick, A.; Löwenberg, B.; et al. Mutant DNMT3A: A marker of poor prognosis in acute my-eloid leukemia. Blood 2012, 119, 5824–5831. [Google Scholar] [CrossRef]
- Abbas, S.; Lugthart, S.; Kavelaars, F.; Schelen, A.; Koenders, J.; Zeilemaker, A.; Van Putten, W.J.L.; Rijneveld, A.; Löwenberg, B.; Valk, P. Acquired mutations in the genes encoding IDH1 and IDH2 both are recurrent aberrations in acute myeloid leukemia: Prevalence and prognostic value. Blood 2010, 116, 2122–2126. [Google Scholar] [CrossRef] [PubMed]
- Paschka, P.; Schlenk, R.F.; Gaidzik, V.I.; Habdank, M.; Krönke, J.; Bullinger, L.; Späth, D.; Kayser, S.; Zucknick, M.; Götze, K.; et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J. Clin. Oncol. 2010, 28, 3636–3643. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-C.; Chou, S.-C.; Liu, C.-Y.; Chen, C.-Y.; Hou, H.-A.; Kuo, Y.-Y.; Lee, M.-C.; Ko, B.-S.; Tang, J.-L.; Yao, M.; et al. TET2 mutation is an unfavorable prognostic factor in acute myeloid leukemia patients with intermediate-risk cytogenetics. Blood 2011, 118, 3803–3810. [Google Scholar] [CrossRef] [PubMed]
- Gaidzik, V.I.; Schlenk, R.F.; Paschka, P.; Stölzle, A.; Späth, D.; Kuendgen, A.; Von Lilienfeld-Toal, M.; Brugger, W.; Derigs, H.G.; Kremers, S.; et al. Clinical impact of DNMT3A mutations in younger adult patients with acute myeloid leukemia: Results of the AML Study Group (AMLSG). Blood 2013, 121, 4769–4777. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, Y.-M.; Fan, X.; Shi, J.-Y.; Wang, Q.-R.; Yan, X.-J.; Gu, Z.-H.; Wang, Y.-Y.; Chen, B.; Jiang, C.-L.; et al. Gene mutation patterns and their prognostic impact in a cohort of 1185 patients with acute myeloid leukemia. Blood 2011, 118, 5593–5603. [Google Scholar] [CrossRef]
- Cairns, R.A.; Mak, T.W. Oncogenic isocitrate dehydrogenase mutations: Mechanisms, models, and clinical opportunities. Cancer Discov. 2013, 3, 730–741. [Google Scholar] [CrossRef]
- Figueroa, M.E.; Abdel-Wahab, O.; Lu, C.; Ward, P.; Patel, J.; Shih, A.; Li, Y.; Bhagwat, N.; VasanthaKumar, A.; Fernandez, H.F.; et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010, 18, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Rakheja, D.; Konoplev, S.; Medeiros, L.J.; Chen, W. IDH mutations in acute myeloid leukemia. Hum. Pathol. 2012, 43, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Ryotokuji, T.; Yamaguchi, H.; Ueki, T.; Usuki, K.; Kurosawa, S.; Kobayashi, Y.; Kawata, E.; Tajika, K.; Gomi, S.; Kanda, J.; et al. Clinical characteristics and prognosis of acute myeloid leukemia associated with DNA-methylation regulatory gene mutations. Haematologica 2016, 101, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Betensky, R.A. Measures of follow-up in time-to-event studies: Why provide them and what should they be? Clin. Trials. 2015, 12, 403–408. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Therneau, T.M.; Grambsch, P.M. The cox model. In Modeling Survival Data: Extending the Cox Model. Statistics for Biology and Health; Therneau, T.M., Grambsch, P.M., Eds.; Springer: Cham, Switzerland, 2000; pp. 39–77. [Google Scholar] [CrossRef]
- Shivarov, V.; Gueorguieva, R.; Stoimenov, A.; Tiu, R. DNMT3A mutation is a poor prognosis biomarker in AML: Results of a meta-analysis of 4500 AML patients. Leuk. Res. 2013, 37, 1445–1450. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Y.; Lv, N.; Jing, Y.; Xu, Y.; Li, Y.; Li, W.; Yao, Z.; Chen, X.; Huang, S.; et al. Correlation between isocitrate dehydrogenase gene aberrations and prognosis of patients with acute myeloid leukemia: A systematic review and meta-analysis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 4511–4522. [Google Scholar] [CrossRef]
- Ravandi, F.; Patel, K.; Luthra, R.; Faderl, S.; Konopleva, M.; Kadia, T.; Brandt, M.; Pierce, S.; Kornblau, S.; Andreeff, M.; et al. Prognostic significance of alterations in IDH enzyme isoforms in patients with AML treated with high-dose cytarabine and idarubicin. Cancer 2012, 118, 2665–2673. [Google Scholar] [CrossRef]
- Liu, W.-J.; Tan, X.-H.; Luo, X.-P.; Guo, B.-P.; Wei, Z.-J.; Ke, Q.; He, S.; Cen, H. Prognostic significance of Tet methylcytosine dioxygenase 2 (TET2) gene mutations in adult patients with acute myeloid leukemia: A meta-analysis. Leuk. Lymphoma. 2014, 55, 2691–2698. [Google Scholar] [CrossRef]
- Tian, X.; Xu, Y.; Yin, J.; Tian, H.; Chen, S.; Wu, D.; Sun, A. TET2 gene mutation is unfavorable prognostic factor in cytogenetically normal acute myeloid leukemia patients with NPM1+ and FLT3-ITD− mutations. Int. J. Hematol. 2014, 100, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Mason, E.F.; Kuo, F.C.; Hasserjian, R.P.; Seegmiller, A.C.; Pozdnyakova, O. A distinct immunophenotype identifies a subset of NPM1-mutated AML with TET2 or IDH1/2 mutations and improved outcome. Am. J. Hematol. 2018, 93, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Gaidzik, V.I.; Paschka, P.; Späth, D.; Habdank, M.; Köhne, C.-H.; Germing, U.; Von Lilienfeld-Toal, M.; Held, G.; Horst, H.-A.; Haase, D.; et al. TET2 mutations in acute myeloid leukemia (AML): Results from a comprehensive genetic and clinical analysis of the AML study group. J. Clin. Oncol Off. J. Am. Soc. Clin. Oncol. 2012, 30, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.; Patel, J.; Wise, D.R.; Abdel-Wahab, O.; Bennett, B.D.; Coller, H.A.; Cross, J.R.; Fantin, V.R.; Hedvat, C.V.; Perl, A.E.; et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010, 17, 225–234. [Google Scholar] [CrossRef]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009, 462, 739–744. [Google Scholar] [CrossRef]
- Im, A.P.; Sehgal, A.R.; Carroll, M.P.; Smith, B.D.; Tefferi, A.; E Johnson, D.; Boyiadzis, M. DNMT3A and IDH mutations in acute myeloid leukemia and other myeloid ma-lignancies: Associations with prognosis and potential treatment strategies. Leukemia 2014, 28, 1774–1783. [Google Scholar] [CrossRef]
- Hájková, H.; Marková, J.; Haškovec, C.; Šárová, I.; Fuchs, O.; Kostečka, A.; Cetkovský, P.; Michalová, K.; Schwarz, J. Decreased DNA methylation in acute myeloid leukemia patients with DNMT3A mutations and prognostic implications of DNA methylation. Leuk. Res. 2012, 36, 1128–1133. [Google Scholar] [CrossRef]
- Yan, X.-J.; Xu, J.; Gu, Z.-H.; Pan, C.-M.; Lu, G.; Shen, Y.; Shi, J.-Y.; Zhu, Y.-M.; Tang, L.; Zhang, X.-W.; et al. Exome sequencing identifies somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nat. Genet. 2011, 43, 309–315. [Google Scholar] [CrossRef]
- Makishima, H.; Yoshida, K.; Nguyen, N.; Przychodzen, B.; Sanada, M.; Okuno, Y.; Ng, K.P.; Gudmundsson, K.O.; Vishwakarma, B.A.; Jerez, A.; et al. Somatic SETBP1 mutations in myeloid malignancies. Nat. Genet. 2013, 45, 942–946. [Google Scholar] [CrossRef]
- Coccaro, N.; Tota, G.; Zagaria, A.; Anelli, L.; Specchia, G.; Albano, F. SETBP1 dysregulation in congenital disorders and myeloid neoplasms. Oncotarget 2017, 8, 51920–51935. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.L.; Schumacher, J.A.; Frizzell, K.; Sorrells, S.; Shen, W.; Clayton, A.; Jattani, R.; Kelley, T.W. Coexisting and cooperating mutations in NPM1-mutated acute myeloid leukemia. Leuk. Res. 2017, 56, 7–12. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | All Patients (n = 114) | DMRGM * Positive (n = 35) | DMRGM Negative (n = 79) | p-Value |
|---|---|---|---|---|
| Age, years | 0.0748 | |||
| Median | 61.5 | 63.16 | 58.92 | |
| IQR * | (48.44, 67.11) | (56.29, 69.31) | (46.52, 66.85) | |
| Sex, No. (%) | 0.1859 | |||
| Male | 69 (60.53) | 18 (51.43) | 51 (64.56) | |
| Female | 45 (39.47) | 17 (48.57) | 28 (35.44) | |
| WBC, 103/uL | 0.3363 | |||
| Median | 7.5 | 11.57 | 7.05 | |
| IQR | (2, 37.57) | (2.1, 55.8) | (1.93, 30) | |
| Missing Values | 0 | 0 | 0 | |
| BM blasts (%) | 0.8825 | |||
| Median | 51 | 58 | 47.6 | |
| IQR | (25, 72) | (25, 73.2) | (25, 69.2) | |
| Missing values | 0 | 0 | 0 | |
| PB blasts (%) | 0.2977 | |||
| Median | 18 | 18 | 18 | |
| IQR | (1, 60) | (0, 60) | (2, 60) | |
| Missing values | 8 | 2 | 6 | |
| Platelet counts, 103/uL | 0.303 | |||
| Median | 52 | 53.5 | 52 | |
| IQR | (24, 89) | (29, 100) | (23, 89) | |
| Missing values | 1 | 1 | 0 | |
| Hemoglobin, g/dL | 0.3195 | |||
| Median | 8 | 8.2 | 7.9 | |
| QR | (7, 9.5) | (7.2, 9.5) | (6.5, 9.5) | |
| Missing values | 0 | 0 | 0 | |
| 2017 ELN risk, No. (%) | 0.057 | |||
| Favorable | 37 (32.46) | 16 (45.71) | 21 (26.58) | |
| Intermediate | 38 (33.33) | 12 (34.29) | 26 (32.92) | |
| Adverse | 39 (34.21) | 7 (20.00) | 32 (40.50) | |
| HSCT *, No. (%) | 0.4019 | |||
| Not Received | 65 (57.02) | 22 (62.86) | 43 (54.43) | |
| Received | 49 (42.98) | 13 (37.14) | 36 (45.57) | |
| Classification of AML, No. (%) | ||||
| AML with t(8;21)(q22;q22.1);RUNX1-RUNX1T1 | 7 (6.14%) | 0 (0.00%) | 7 (8.86%) | |
| AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22);CBFB-MYH11 | 7 (6.14%) | 1 (2.86%) | 6 (7.59%) | |
| AML with t(9;11)(p21.3;q23.3);MLLT3-KMT2A | 2 (1.75%) | 2 (5.71%) | 0 (0.00%) | |
| AML with mutated NPM1 | 25 (21.93%) | 16 (45.71%) | 9 (11.39%) | |
| AML with biallelic mutations of CEBPA | 2 (1.75%) | 0 (0.00%) | 2 (2.53%) | |
| AML with myelodysplasia-related changes | 31 (27.19%) | 9 (25.71%) | 22 (27.85%) | |
| AML, NOS | 40 (35.09%) | 7 (20.00%) | 33 (41.77%) |
| DNMT3A | IDH1 | IDH2 | TET2 | SETBP1 | |
|---|---|---|---|---|---|
| Whole AML cohort (n = 114) | 14.0% (16/114) | 5.3% (6/114) | 8.8% (10/114) | 9.6% (11/114) | 1.8% (2/114) |
| Favorable risk (n = 37) | 21.6% (8/37) | 8.1% (3/37) | 13.5% (5/37) | 13.5% (5/37) | 0.0% (0/37) |
| Intermediate risk (n = 38) | 13.2% (5/38) | 7.9% (3/38) | 7.9% (3/38) | 13.2% (5/38) | 0.0% (0/38) |
| Adverse risk (n = 39) | 7.7% (3/39) | 0.0% (0/39) | 5.1% (2/39) | 2.6% (1/39) | 5.1% (2/39) |
| (a) Cox hazard proportional models in all AML patients (n = 114) | ||||
| Variables | HR * | 95% CI * | p-Value | |
| DMRGM * | Yes | 2.704 | (1.451, 5.041) | 0.0017 |
| (Reference, No) | ||||
| 2017 ELN risk | Intermediate | 0.93 | (0.392, 2.208) | 0.8693 |
| High | 2.911 | (1.379, 6.186) | 0.0051 | |
| (Reference, Favor) | ||||
| Age | 65 and over | 1.124 | (0.592, 2.135) | 0.7205 |
| (Reference, under 65) | ||||
| HSCT * | Yes | 0.37 | (0.19, 0.719) | 0.0034 |
| (Reference, No) | ||||
| (b) Cox hazard proportional models for 2017 ELN favor risk group (n = 37) | ||||
| Variables | HR | 95% CI | p-Value | |
| DMRGM | Yes | 6.882 | (1.24, 38.184) | 0.0274 |
| (Reference, No) | ||||
| Age | 65 and over | 1.235 | (0.314, 4.857) | 0.763 |
| (Reference, under 65) | ||||
| HSCT | Yes | 0.534 | (0.161, 1.77) | 0.3049 |
| (Reference, No) | ||||
| NPM1 Mutation | Yes | 1.629 | (0.251, 10.589) | 0.6092 |
| (Reference No) | ||||
| FLT3-ITDLow | Yes | 1.188 | (0.363, 3.891) | 0.7761 |
| Mutation | (Reference No) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Sun, J.; Du, Y.; Patel, R.; Varela, J.C.; Mori, S.; Chang, C.-C. Adverse Impact of DNA Methylation Regulatory Gene Mutations on the Prognosis of AML Patients in the 2017 ELN Favorable Risk Group, Particularly Those Defined by NPM1 Mutation. Diagnostics 2021, 11, 986. https://doi.org/10.3390/diagnostics11060986
Yu J, Sun J, Du Y, Patel R, Varela JC, Mori S, Chang C-C. Adverse Impact of DNA Methylation Regulatory Gene Mutations on the Prognosis of AML Patients in the 2017 ELN Favorable Risk Group, Particularly Those Defined by NPM1 Mutation. Diagnostics. 2021; 11(6):986. https://doi.org/10.3390/diagnostics11060986
Chicago/Turabian StyleYu, James, Jingxin Sun, Yuan Du, Rushang Patel, Juan Carlos Varela, Shahram Mori, and Chung-Che Chang. 2021. "Adverse Impact of DNA Methylation Regulatory Gene Mutations on the Prognosis of AML Patients in the 2017 ELN Favorable Risk Group, Particularly Those Defined by NPM1 Mutation" Diagnostics 11, no. 6: 986. https://doi.org/10.3390/diagnostics11060986
APA StyleYu, J., Sun, J., Du, Y., Patel, R., Varela, J. C., Mori, S., & Chang, C.-C. (2021). Adverse Impact of DNA Methylation Regulatory Gene Mutations on the Prognosis of AML Patients in the 2017 ELN Favorable Risk Group, Particularly Those Defined by NPM1 Mutation. Diagnostics, 11(6), 986. https://doi.org/10.3390/diagnostics11060986






