Using Anti-Malondialdehyde Modified Peptide Autoantibodies to Import Machine Learning for Predicting Coronary Artery Stenosis in Taiwanese Patients with Coronary Artery Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples
2.2. Detection of Plasma MDA and MDA Protein Adducts
2.3. Detection of Plasma Autoantibodies against Unmodified and Modified Peptides
2.4. Statistical Analysis
3. Results
3.1. Determination of Autoantibodies against MDA-Modified BSA in RA with CAD Patients
3.2. Detection of MDA and MDA Protein Adducts
3.3. Measuring of Autoantibodies against Unmodified and MDA-Modified Peptides
3.4. Associations of Plasma Autoantibodies against Unmodified and MDA-Modified Peptides
3.5. Using Plasma Anti-Unmodified and Anti-MDA-Modified Peptide Autoantibodies to Identify CAD Patients from HCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaziano, T.A.; Bitton, A.; Anand, S.; Abrahams-Gessel, S.; Murphy, A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr. Probl. Cardiol. 2010, 35, 72–115. [Google Scholar] [CrossRef]
- Lee, C.-H.; Fang, C.-C.; Tsai, L.-M.; Gan, S.-T.; Lin, S.-H.; Li, Y.-H. Patterns of acute myocardial infarction in Taiwan from 2009 to 2015. Am. J. Cardiol. 2018, 122, 1996–2004. [Google Scholar] [CrossRef]
- Kannel, W.B.; McGee, D.; Gordon, T. A general cardiovascular risk profile: The Framingham study. Am. J. Cardiol. 1976, 38, 46–51. [Google Scholar] [CrossRef]
- Ungvari, Z.; Kaley, G.; de Cabo, R.; Sonntag, W.E.; Csiszar, A. Mechanisms of vascular aging: New perspectives. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2010, 65, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Kianoush, S.; Yakoob, M.Y.; Al-Rifai, M.; DeFilippis, A.P.; Bittencourt, M.S.; Duncan, B.B.; Bensenor, I.M.; Bhatnagar, A.; Lotufo, P.A.; Blaha, M.J. Associations of cigarette smoking with subclinical inflammation and atherosclerosis: ELSA-Brasil (The Brazilian longitudinal study of adult health). J. Am. Heart. Assoc. 2017, 6, e005088. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Li, Y.; Ren, X.; Zhang, X.; Hu, D.; Gao, Y.; Xing, Y.; Shang, H. Oxidative stress-mediated atherosclerosis: Mechanisms and therapies. Front. Physiol. 2017, 8, 600. [Google Scholar] [CrossRef]
- Cipollone, F.; Fazia, M.L.; Mezzetti, A. Oxidative stress, inflammation and atherosclerotic plaque development. Int. Congr. Ser. 2007, 1303, 35–40. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative stress in atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, S.; Raghavamenon, A.; Garelnabi, M.O.; Santanam, N. Oxidized low-density lipoprotein. Methods Mol. Biol. 2010, 610, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Z.; Fang, A.; Jin, Q.; Fang, D.; Liu, Y.; Wu, J.; Tan, X.; Wei, Y.; Jiang, C.; et al. Macrophage foam cell-targeting immunization attenuates atherosclerosis. Front. Immunol. 2018, 9, 3127. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Witztum, J.L. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2311–2316. [Google Scholar] [CrossRef]
- Boaz, M.; Matas, Z.; Biro, A.; Katzir, Z.; Green, M.; Fainaru, M.; Smetana, S. Serum malondialdehyde and prevalent cardiovascular disease in hemodialysis. Kidney Int. 1999, 56, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Leonarduzzi, G.; Chiarpotto, E.; Biasi, F.; Poli, G. 4-Hydroxynonenal and cholesterol oxidation products in atherosclerosis. Mol. Nutr. Food Res. 2005, 49, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- DeJarnett, N.; Conklin, D.J.; Riggs, D.W.; Myers, J.A.; O’Toole, T.E.; Hamzeh, I.; Wagner, S.; Chugh, A.; Ramos, K.S.; Srivastava, S.; et al. Acrolein exposure is associated with increased cardiovascular disease risk. J. Am. Heart Assoc. 2014, 3, e000934. [Google Scholar] [CrossRef]
- Borza, C.; Muntean, D.; Dehelean, C.; Savoiu, G.; Serban, M.-C.; Simu, G.; Mihaiela, A.; Butur, M.; Drag, S. Oxidative stress and lipid peroxidation—A lipid metabolism dysfunction. In Lipid Metabolism; Intech Open: London, UK, 2013. [Google Scholar]
- Papac-Milicevic, N.; Busch, C.J.; Binder, C.J. Malondialdehyde epitopes as targets of immunity and the implications for atherosclerosis. Adv. Immunol. 2016, 131, 1–59. [Google Scholar] [CrossRef]
- Leibundgut, G.; Witztum, J.L.; Tsimikas, S. Oxidation-Specific epitopes and immunological responses: Translational biotheranostic implications for atherosclerosis. Curr. Opin. Pharm. 2013, 13, 168–179. [Google Scholar] [CrossRef]
- Binder, C.J.; Papac-Milicevic, N.; Witztum, J.L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. 2016, 16, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Alizade, E.; Avcı, A.; Açar, G.; Fidan, S.; Öcal, L.; Bulut, M.; Tellice, M.; Akçakoyun, M.; Pala, S.; Esen, A.M. The relationship between rheumatoid factor levels and coronary artery lesion complexity and severity in patients with stable coronary artery disease. Postep. Kardiol. Interwencyjnej Adv. Interv. Cardiol. 2015, 11, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Bartolini Gritti, B.; Binder, C.J. Oxidation-Specific epitopes are major targets of innate immunity in atherothrombosis. Hamostaseologie 2016, 36, 89–96. [Google Scholar] [CrossRef]
- Batuca, J.R.; Amaral, M.C.; Favas, C.; Justino, G.C.; Papoila, A.L.; Ames, P.R.J.; Alves, J.D. Antibodies against HDL Components in ischaemic stroke and coronary artery disease. Thromb. Haemost. 2018, 118, 1088–1100. [Google Scholar] [CrossRef]
- Matsumura, T.; Terada, J.; Kinoshita, T.; Sakurai, Y.; Yahaba, M.; Tsushima, K.; Sakao, S.; Nagashima, K.; Ozaki, T.; Kobayashi, Y.; et al. Circulating autoantibodies against neuroblastoma suppressor of tumorigenicity 1 (NBL1): A potential biomarker for coronary artery disease in patients with obstructive sleep apnea. PLoS ONE 2018, 13, e0195015. [Google Scholar] [CrossRef]
- Kuo, C.F.; Luo, S.F.; See, L.C.; Chou, I.J.; Chang, H.C.; Yu, K.H. Rheumatoid arthritis prevalence, incidence, and mortality rates: A nationwide population study in Taiwan. Rheumatol. Int. 2013, 33, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Nahrendorf, M. Cardioimmunology: The immune system in cardiac homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Chang, Y.S.; Cheng, C.W.; Chi, W.M.; Tsai, K.L.; Chen, W.J.; Kung, T.S.; Tai, C.C.; Lin, Y.F.; Lin, H.T.; et al. Isotypes of autoantibodies against differentially expressed novel malondialdehyde-modified peptide adducts in serum of Taiwanese women with rheumatoid arthritis. J. Proteom. 2018, 170, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.M.D.; Santos, R.C.C.D.; Lima, E.S. A simple automated procedure for thiol measurement in human serum samples. J. Bras. Patol. Med. Lab. 2006, 42, 345–350. [Google Scholar] [CrossRef]
- Schütt, F.; Aretz, S.; Auffarth, G.U.; Kopitz, J. Moderately reduced ATP levels promote oxidative stress and debilitate autophagic and phagocytic capacities in human RPE cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5354–5361. [Google Scholar] [CrossRef]
- Wallberg-Jonsson, S.; Ohman, M.L.; Dahlqvist, S.R. Cardiovascular morbidity and mortality in patients with seropositive rheumatoid arthritis in Northern Sweden. J. Rheumatol. 1997, 24, 445–451. [Google Scholar]
- Ridker, P.M. From C-reactive protein to interleukin-6 to interleukin-1. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef]
- Slatter, D.A.; Bolton, C.H.; Bailey, A.J.J.D. The importance of lipid-derived malondialdehyde in diabetes mellitus. Diabetologia 2000, 43, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Hadj Ahmed, S.; Kharroubi, W.; Kaoubaa, N.; Zarrouk, A.; Batbout, F.; Gamra, H.; Najjar, M.F.; Lizard, G.; Hininger-Favier, I.; Hammami, M. Correlation of trans fatty acids with the severity of coronary artery disease lesions. Lipids Health Dis. 2018, 17, 52. [Google Scholar] [CrossRef]
- Amaki, T.; Suzuki, T.; Nakamura, F.; Hayashi, D.; Imai, Y.; Morita, H.; Fukino, K.; Nojiri, T.; Kitano, S.; Hibi, N.; et al. Circulating malondialdehyde modified LDL is a biochemical risk marker for coronary artery disease. Heart 2004, 90, 1211–1213. [Google Scholar] [CrossRef]
- Abolhasani, S.; Shahbazloo, S.V.; Saadati, H.M.; Mahmoodi, N.; Khanbabaei, N. Evaluation of serum levels of inflammation, fibrinolysis and oxidative stress markers in coronary artery disease prediction: A cross-sectional study. Arq. Bras. Cardiol. 2019, 113, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Miller, Y.I.; Choi, S.H.; Wiesner, P.; Fang, L.; Harkewicz, R.; Hartvigsen, K.; Boullier, A.; Gonen, A.; Diehl, C.J.; Que, X.; et al. Oxidation-Specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011, 108, 235–248. [Google Scholar] [CrossRef]
- Canton, J.; Neculai, D.; Grinstein, S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 2013, 13, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Elkon, K.; Casali, P. Nature and functions of autoantibodies. Nat. Clin. Pract. Rheumatol. 2008, 4, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Holodick, N.E.; Rodríguez-Zhurbenko, N.; Hernández, A.M. Defining natural antibodies. Front. Immunol. 2017, 8, 872. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.M.; Holodick, N.E. Editorial: Natural antibodies in health and disease. Front. Immunol. 2017, 8, 1795. [Google Scholar] [CrossRef]
- Hörkkö, S.; Bird, D.A.; Miller, E.; Itabe, H.; Leitinger, N.; Subbanagounder, G.; Berliner, J.A.; Friedman, P.; Dennis, E.A.; Curtiss, L.K.; et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J. Clin. Investig. 1999, 103, 117–128. [Google Scholar] [CrossRef]
- Binder, C.J. Natural IgM antibodies against oxidation-specific epitopes. J. Clin. Immunol. 2010, 30 (Suppl. 1), S56–S60. [Google Scholar] [CrossRef]
- Björkbacka, H.; Alm, R.; Persson, M.; Hedblad, B.; Nilsson, J.; Fredrikson, G.N. Low levels of apolipoprotein B-100 autoantibodies are associated with increased risk of coronary events. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 765–771. [Google Scholar] [CrossRef]
- Su, J.; Georgiades, A.; Wu, R.; Thulin, T.; de Faire, U.; Frostegård, J. Antibodies of IgM subclass to phosphorylcholine and oxidized LDL are protective factors for atherosclerosis in patients with hypertension. Atherosclerosis 2006, 188, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, D.; Frostegård, A.G.; Singh, S.; Rahman, M.; Liu, A.; Vikström, M.; Leander, K.; Gigante, B.; Hellenius, M.L.; Zhang, B.; et al. Human IgM antibodies to malondialdehyde conjugated with albumin are negatively associated with cardiovascular disease among 60-year-olds. J. Am. Heart Assoc. 2016, 5, e004415. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.; Tokarz-Deptuła, B.; Deptuła, J.; Deptuła, W. Natural antibodies—Facts known and unknown. Cent. Eur. J. Immunol. 2018, 43, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Yoon, J.G.; Park, H.; Kim, Y.D.; Nam, H.S.; Heo, J.H. Machine learning-based model for prediction of outcomes in acute stroke. Stroke 2019, 50, 1263–1265. [Google Scholar] [CrossRef]
- Ambale-Venkatesh, B.; Yang, X.; Wu, C.O.; Liu, K.; Hundley, W.G.; McClelland, R.; Gomes, A.S.; Folsom, A.R.; Shea, S.; Guallar, E.; et al. Cardiovascular event prediction by machine learning: The multi-ethnic study of atherosclerosis. Circ. Res. 2017, 121, 1092–1101. [Google Scholar] [CrossRef]
- Yang, L.; Wu, H.; Jin, X.; Zheng, P.; Hu, S.; Xu, X.; Yu, W.; Yan, J. Study of cardiovascular disease prediction model based on random forest in eastern China. Sci. Rep. 2020, 10, 5245. [Google Scholar] [CrossRef]
- Vabalas, A.; Gowen, E.; Poliakoff, E.; Casson, A.J. Machine learning algorithm validation with a limited sample size. PLoS ONE 2019, 14, e0224365. [Google Scholar] [CrossRef]
- Esmaily, H.; Tayefi, M.; Doosti, H.; Ghayour-Mobarhan, M.; Nezami, H.; Amirabadizadeh, A. A comparison between decision tree and random forest in determining the risk factors associated with type 2 diabetes. J. Res. Health Sci. 2018, 18, e00412. [Google Scholar]
- Tsai, K.L.; Chang, C.C.; Chang, Y.S.; Lu, Y.Y.; Tsai, I.J.; Chen, J.H.; Lin, S.H.; Tai, C.C.; Lin, Y.F.; Chang, H.W.; et al. Isotypes of autoantibodies against novel differential 4-hydroxy-2-nonenal-modified peptide adducts in serum is associated with rheumatoid arthritis in Taiwanese women. BMC Med. Inform. Decis. Mak. 2021, 21, 49. [Google Scholar] [CrossRef]
| Variables | Shuang-Ho Hospital | Luodong Poh-Ai Hospital | ||||
|---|---|---|---|---|---|---|
| Stenosis Rate of Patients | ||||||
| RA (n = 30) | RA with CAD (n = 30) | HC (n = 40) | <30% (n = 46) | 30–70% (n = 47) | >70% (n = 79) | |
| Age (yr) | 56.43 ± 8.29 | 56.26 ± 8.29 | 38.41 ± 10.42 | 62.72 ± 10.32 ** | 63.57 ± 9.55 ** | 62.79 ± 9.27 ** |
| Male | 12 (40%) | 9 (30%) | 24 (60%) | 31 (67%) | 33 (70%) | 60 (75%) |
| Drinker | - | - | 9 (22%) | 7 (15%) | 7 (14%) | 9 (11%) |
| Used to smoke | - | - | 0 | 16 (34%) * | 8 (17%) | 19 (24%) |
| Current smoker | - | - | 13 (32%) | 2 (4%) * | 10 (21%) | 28 (35%) |
| Diabetes | - | - | - | 13 (28%) | 17 (36%) | 31 (39%) |
| Hypertension | - | - | - | 28 (60%) | 40 (85%) | 51 (64%) |
| Use of lipid-lowering agents | - | - | - | 14(30%) | 18 (38%) | 44 (55%) |
| TC (mg/dL) | - | - | 160.03 ± 35.22 | 144.52 ± 37.35 | 142.92 ± 31.35 * | 140.67 ± 45.49 * |
| HDL-c (mg/dL) | - | - | 50.83 ± 15.41 | 45.83 ± 15.74 | 46.46 ± 16.07 | 39.16 ± 12.41 ** |
| LDL-c (mg/dL) | - | - | 94.09 ± 35.92 | 84.29 ± 30.81 | 85.46 ± 33.19 | 89.28 ± 38.42 |
| TG (mg/dL) | - | - | 77.26 ± 28.46 | 117.06 ± 116.06 | 109.25 ± 113.86 | 123.94 ± 104.88 * |
| MDA (μM) | - | - | 10.1 ± 4.7 | 11.37 ± 3.75 | 12.63 ± 5.49 | 12.81 ± 7.64 |
| MDA-protein adducts (μg/mL) | - | - | 0.208 ± 0.016 | 0.219 ± 0.023 * | 0.215 ± 0.021 | 0.216 ± 0.021 * |
| Variables | Cut Off | Stenosis Rate | Multivariate Logistic Regression Model $ | ||
|---|---|---|---|---|---|
| <30% | >30% | ||||
| n = 86 | n = 126 | ORs (95% C.I.) | p-Value | ||
| MDA | 8.453 | 29 | 25 | Ref. | 0.046 |
| 8.453 | 57 | 101 | 2.149 (1.012, 4.561) | ||
| MDA adduct | 0.202 | 18 | 38 | Ref. | 0.129 |
| 0.202 | 68 | 88 | 0.562 (0.267, 1.183) | ||
| IgG anti A2M824–841 | 0.706 | 26 | 27 | Ref. | 0.054 |
| 0.706 | 60 | 99 | 2.022 (0.986, 4.15) | ||
| IgG anti A2M824–841 MDA | 3.118 | 21 | 32 | Ref. | 0.842 |
| 3.118 | 65 | 94 | 1.076 (0.522, 2.219) | ||
| IgG anti ApoB1004022–4040 | 0.990 | 13 | 40 | Ref. | 0.004 |
| 0.990 | 73 | 86 | 0.315 (0.142, 0.701) | ||
| IgG anti ApoB1004022–4040 MDA | 0.582 | 18 | 35 | Ref. | 0.360 |
| 0.582 | 68 | 91 | 0.705 (0.333, 1.492) | ||
| IgG anti A1AT284–298 | 1.260 | 23 | 29 | Ref. | 0.304 |
| 1.260 | 63 | 97 | 1.446 (0.716, 2.922) | ||
| IgG anti A1AT284–298 MDA | 2.033 | 25 | 28 | Ref. | 0.127 |
| 2.033 | 61 | 98 | 1.739 (0.854, 3.539) | ||
| IgG anti IGKC76–99 | 0.766 | 17 | 36 | Ref. | 0.149 |
| 0.766 | 69 | 90 | 0.578 (0.274, 1.217) | ||
| IgG anti IGKC76–99 MDA | 0.677 | 14 | 38 | Ref. | 0.266 |
| 0.677 | 72 | 88 | 0.663 (0.321, 1.37) | ||
| IgM anti A2M824–841 | 0.386 | 10 | 42 | Ref. | 0.004 |
| 0.386 | 76 | 84 | 0.311 (0.139, 0.699) | ||
| IgM anti A2M824–841 MDA | 0.694 | 14 | 38 | Ref. | 0.105 |
| 0.694 | 72 | 88 | 0.533 (0.249, 1.141) | ||
| IgM anti ApoB1004022–4040 | 0.559 | 15 | 38 | Ref. | 0.157 |
| 0.559 | 71 | 88 | 0.580 (0.272, 1.234) | ||
| IgM anti ApoB1004022–4040 MDA | 0.581 | 12 | 41 | Ref. | 0.002 |
| 0.581 | 74 | 85 | 0.288 (0.127, 0.652) | ||
| IgM anti A1AT284–298 | 0.345 | 11 | 43 | Ref. | 0.010 |
| 0.345 | 75 | 83 | 0.356 (0.162, 0.785) | ||
| IgM anti A1AT284–298 MDA | 0.466 | 8 | 45 | Ref. | <0.001 |
| 0.466 | 78 | 81 | 0.191 (0.077, 0.474) | ||
| IgM anti IGKC76–99 | 0.589 | 17 | 36 | Ref. | 0.790 |
| 0.589 | 69 | 90 | 0.905 (0.434, 1.890) | ||
| IgM anti IGKC76–99 MDA | 0.252 | 11 | 41 | Ref. | 0.072 |
| 0.252 | 75 | 85 | 0.485 (0.221, 1.067) | ||
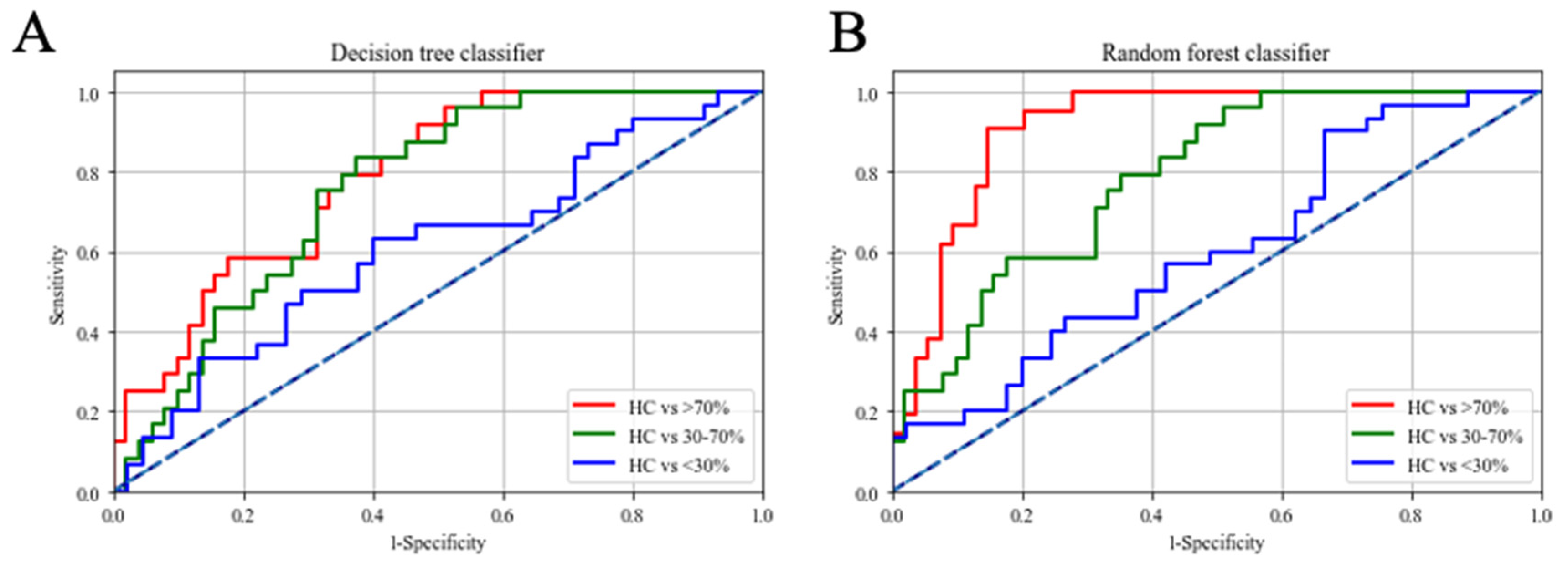
| Decision Tree Classifier | |||
| IgM anti-ApoB1004022–4040 MDA, IgM anti-IGKC76–99 MDA, IgM anti-A1AT284–298 MDA | |||
| Sensitivity (95% C.I.) | Specificity (95% C.I.) | AUC (95% C.I.) | |
| HC v.s. <30% | 68.7% (59.3–77.6%) | 61.9% (55.4–75.5%) | 0.67 (0.55–0.73) |
| HC v.s. 30–70% | 77.4% (66.7–84.5%) | 66.4% (58.7–80.9%) | 0.76 (0.65–0.82) |
| HC v.s. >70% | 85.7% (73.3–90.1%) | 71.7% (68.1–80.6%) | 0.81 (0.76–0.86) |
| Random Forest Classifier | |||
| IgG anti-IGKC76–99, IgM anti-IGKC76–99 MDA, IgM anti-A1AT284–298 MDA | |||
| Sensitivity (95% C.I.) | Specificity (95% C.I.) | AUC (95% C.I.) | |
| HC v.s. <30% | 74.6% (68.0–79.3%) | 64.5% (58.1–72.4%) | 0.76 (0.72–0.82) |
| HC v.s. 30–70% | 90.2% (84.5–93.5%) | 82.7% (77.9–88.1%) | 0.91 (0.87–0.94) |
| HC v.s. >70% | 88.7% (82.7–92.3%) | 85.8% (81.0–89.7%) | 0.94 (0.88–0.96) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-C.; Tsai, I.-J.; Hsu, H.; Hsu, P.-W.; Cheng, M.-H.; Huang, Y.-L.; Chen, J.-H.; Lei, M.-H.; Lin, C.-Y. Using Anti-Malondialdehyde Modified Peptide Autoantibodies to Import Machine Learning for Predicting Coronary Artery Stenosis in Taiwanese Patients with Coronary Artery Disease. Diagnostics 2021, 11, 961. https://doi.org/10.3390/diagnostics11060961
Hsu Y-C, Tsai I-J, Hsu H, Hsu P-W, Cheng M-H, Huang Y-L, Chen J-H, Lei M-H, Lin C-Y. Using Anti-Malondialdehyde Modified Peptide Autoantibodies to Import Machine Learning for Predicting Coronary Artery Stenosis in Taiwanese Patients with Coronary Artery Disease. Diagnostics. 2021; 11(6):961. https://doi.org/10.3390/diagnostics11060961
Chicago/Turabian StyleHsu, Yu-Cheng, I-Jung Tsai, Hung Hsu, Po-Wen Hsu, Ming-Hui Cheng, Ying-Li Huang, Jin-Hua Chen, Meng-Huan Lei, and Ching-Yu Lin. 2021. "Using Anti-Malondialdehyde Modified Peptide Autoantibodies to Import Machine Learning for Predicting Coronary Artery Stenosis in Taiwanese Patients with Coronary Artery Disease" Diagnostics 11, no. 6: 961. https://doi.org/10.3390/diagnostics11060961
APA StyleHsu, Y.-C., Tsai, I.-J., Hsu, H., Hsu, P.-W., Cheng, M.-H., Huang, Y.-L., Chen, J.-H., Lei, M.-H., & Lin, C.-Y. (2021). Using Anti-Malondialdehyde Modified Peptide Autoantibodies to Import Machine Learning for Predicting Coronary Artery Stenosis in Taiwanese Patients with Coronary Artery Disease. Diagnostics, 11(6), 961. https://doi.org/10.3390/diagnostics11060961






