Her2-Positive and Microsatellite Instability Status in Gastric Cancer—Clinicopathological Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection
2.2. Clinical and Pathological Data
2.3. Molecular Data
2.4. Sample Size and Statistical Analysis
3. Results
3.1. Study Group. Clinicopathological Features
3.2. HER2-Positive Status and Clinicopathological Features
3.3. MSI Status and Clinicopathological Features
4. Discussion
4.1. HER2-Positive Status and Clinicopathological Features
4.2. MSI Status and Clinicopathological Features
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garattini, S.K.; Basile, D.; Cattaneo, M.; Fanotto, V.; Ongaro, E.; Bonotto, M.; Negri, F.V.; Berenato, R.; Ermacora, P.; Cardellino, G.G.; et al. Molecular classifications of gastric cancers: Novel insights and possible future applications. World J. Gastrointest. Oncol. 2017, 9, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Birkman, E.M.; Mansuri, N.; Kurki, S.; Ålgars, A.; Lintunen, M.; Ristamäki, R.; Sundström, J.; Carpén, O. Gastric cancer: Immunohistochemical classification of molecular subtypes and their association with clinicopathological characteristics. Virchows Arch. 2018, 472, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Polom, K.; Marano, L.; Marrelli, D.; De Luca, R.; Roviello, G.; Savelli, V.; Tan, P.; Roviello, F. Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer. Br. J. Surg. 2018, 105, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, L.; Manchi, M.; De Re, V.; Dolcetti, R.; Canzonieri, V. Proposed Molecular and miRNA Classification of Gastric Cancer. Int. J. Mol. Sci. 2018, 19, 1683. [Google Scholar] [CrossRef]
- Gullo, I.; Carneiro, F.; Oliveira, C.; Almeida, G.M. Heterogeneity in Gastric Cancer: From Pure Morphology to Molecular Classifications. Pathobiology 2017, 85, 50–63. [Google Scholar] [CrossRef]
- Matsuoka, T.; Yashiro, M. Biomarkers of gastric cancer: Current topics and future perspective. World J. Gastroenterol. 2018, 24, 2818–2832. [Google Scholar] [CrossRef]
- Cisło, M.; Filip, A.A.; Arnold Offerhaus, G.J.; Ciseł, B.; Rawicz-Pruszyński, K.; Skierucha, M.; Polkowski, W.P. Distinct molecular subtypes of gastric cancer: From Laurén to molecular pathology. Oncotarget 2018, 9, 19427–19442. [Google Scholar] [CrossRef]
- Owen, G.I.; Pinto, M.P.; Retamal, I.N.; Fernádez, M.F.; Cisternas, B.; Mondaca, S.; Sanchez, C.; Galindo, H.; Nervi, B.; Ibañez, C.; et al. Chilean Gastric Cancer Task Force. A study protocol to obtain a clinical and molecular classification of a cohort of gastric cancer patients. Medicine 2018, 97, 16. [Google Scholar] [CrossRef]
- Kankeu Fonkoua, L.; Yee, N.S. Molecular Characterization of Gastric Carcinoma: Therapeutic Implications for Biomarkers and Targets. Biomedicines 2018, 6, 32. [Google Scholar] [CrossRef]
- Serra, O.; Galán, M.; Ginesta, M.M.; Calvo, M.; Salad, N.; Salazar, R. Comparison and applicability of molecular classifications for gastric cancer. Cancer Treat. Rev. 2019, 77, 29–34. [Google Scholar] [CrossRef]
- Lu, S.; Wang, L.J.; Lombardo, K.; Kwak, Y.; Kim, W.H.; Resnick, M.B. Expression of Indoleamine 2, 3-dioxygenase 1 (IDO1) and Tryptophanyl-tRNA Synthetase (WARS) in Gastric Cancer Molecular Subtypes. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 360–368. [Google Scholar] [CrossRef]
- Magalhães, H.; Fontes-Sousa, M.; Machado, M. Immunotherapy in Advanced Gastric Cancer: An Overview of the Emerging Strategies. Can. J. Gastroenterol. Hepatol. 2018, 27, 32408. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.S.; Röcken, C. Chromosomal Instability in Gastric Cancer. Neoplasia 2017, 19, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Chia, N.Y.; Tan, P. Molecular classification of gastric cancer. Ann. Oncol. 2016, 27, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Díaz del Arco, C.; Estrada Muñoz, L.; Molina Roldán, E.; Cerón Nieto, M.A.; Ortega Medina, L.; García Gómez de las Heras, S.; Fernández Aceñero, M.J. Immunohistochemical classification of gastric cancer based on new molecular biomarkers: A potential predictor of survival. Virchows Arch. 2018, 473, 687–695. [Google Scholar] [CrossRef]
- Carlomagno, N.; Incollingo, P.; Tammaro, V.; Peluso, G.; Rupealta, N.; Chiacchio, G.; Sandoval Sotelo, M.L.; Minieri, G.; Pisani, A.; Riccio, E.; et al. Diagnostic, Predictive, Prognostic, and Therapeutic Molecular Biomarkers in Third Millennium: A Breakthrough in Gastric Cancer. Biomed. Res. Int. 2017, 2017, 7869802. [Google Scholar] [CrossRef]
- Fanotto, V.; Ongaro, E.; Rihawi, K.; Avallone, A.; Silvestris, N.; Fornaro, L.; Vasile, E.; Antonuzzo, L.; Leone, F.; Rosati, G.; et al. HER-2 inhibition in gastric and colorectal cancers: Tangible achievements, novel acquisitions and future perspectives. Oncotarget 2016, 7, 60060–60074. [Google Scholar] [CrossRef]
- Chivu-Economescu, M.; Matei, L.; Necula, L.G.; Dragu, D.L.; Bleotu, C.; Diaconu, C.C. New therapeutic options opened by the molecular classification of gastric cancer. World J. Gastroenterol. 2018, 24, 1942–1961. [Google Scholar] [CrossRef]
- Boku, N. HER2-positive gastric cancer. Gastric Cancer 2014, 17, 423–430. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Bang, Y.J.; Feng-Yi, F.; Xu, J.M.; Lee, K.W.; Jiao, S.C.; Chong, J.L.; López-Sanchez, R.I.; Price, T.; Gladkov, O.; et al. HER2 screening data from ToGA: Targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 2015, 18, 476–484. [Google Scholar] [CrossRef]
- Tan, I.B.; Ivanova, T.; Lim, K.H.; Ong, C.W.; Deng, N.; Lee, J.; Tan, S.H.; Wu, J.; Lee, M.H.; Ooi, C.H.; et al. Intrinsic Subtypes of Gastric Cancer, Based on Gene Expression Pattern, Predict Survival and Respond Differently to Chemotherapy. Gastroenterology 2011, 141, 476–485. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, M.Y.; Kim, J.; Kim, S.N.; Oh, S.C.; Lee, K.A. Profiling cancer-associated genetic alterations and molecular classification of cancer in Korean gastric cancer patients. Oncotarget 2017, 8, 69888–69905. [Google Scholar] [CrossRef]
- Marano, L.; Roviello, F. The distinctive nature of HER2-positive gastric cancers. Eur. J. Surg. Oncol. 2015, 41, 271–273. [Google Scholar] [CrossRef]
- Ho, S.W.T.; Tan, P. Dissection of gastric cancer heterogeneity for precision oncology. Cancer Sci. 2019, 110, 3405–3414. [Google Scholar] [CrossRef]
- Fiorillo, C.; Laterza, V.; Quero, G.; Menghi, R.; Cinaa, C.; Rosa, F.; Tortorelli, A.P.; Boskoski, I.; Alfieri, S. From biology to surgery: One step beyond histology for tailored surgical treatments of gastric cancer. Surg. Oncol. 2020, 34, 86–95. [Google Scholar] [CrossRef]
- Lei, Z.; Tan, I.B.; Das, K.; Deng, N.; Zouridis, H.; Pattison, S.; Chua, C.; Feng, Z.; Guan, Y.K.; Ooi, C.H.; et al. Identification of molecular subtypes of gastric cancer with different responses to PI3-kinase inhibitors and 5-fluorouracil. Gastroenterology 2013, 145, 554–565. [Google Scholar] [CrossRef]
- Bass, A.J.; Thorsson, V.; Shmulevich, I.; Reynolds, S.M.; Miller, M.; Bernard, B.; Hinoue, T. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Ichikawa, H.; Nagahashi, M.; Shimada, Y.; Hanyu, T.; Ishikawa, T.; Kameyama, H.; Kobayashi, T.; Sakata, J.; Yabusaki, H.; Nakagawa, S.; et al. Actionable gene-based classification toward precision medicine in gastric cancer. Genome Med. 2017, 9, 93. [Google Scholar] [CrossRef]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, W.; Guan, W.; Cai, L.; Qiao, M.; Zheng, L.; Jiang, R.; Wang, R.; Wang, L. Integrated assessment of PD-L1 expression and molecular classification facilitates therapy selection and prognosis prediction in gastric cancer. Cancer Manag. Res. 2019, 11, 6397–6410. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Zhu, G.Q.; Lu, X.F.; Zheng, K.Y.; Wang, Q.W.; Chen, J.N.; Zhang, Q.W.; Yan, F.R.; Li, X.B. Identification and validation of tumour microenvironment-based immune molecular subgroups for gastric cancer: Immunotherapeutic implications. Cancer Immunol. Immunother. 2020, 69, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Hang, X.; Li, D.; Wang, J.; Wang, G. Prognostic significance of microsatellite instability-associated pathways and genes in gastric cancer. Int. J. Mol. Med. 2018, 42, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y. Molecular classification and precision therapy of cancer: Immune checkpoint inhibitors. Front. Med. 2018, 12, 229–235. [Google Scholar] [CrossRef]
- Di Pinto, F.; Armentano, R.; Arborea, G.; Schena, N.; Donghia, R.; Valentini, A.M. Are Immunohistochemical Markers Useful in Phenotypic Gastric Cancer Classification? Oncology 2020, 98, 566–574. [Google Scholar] [CrossRef]
- Irkkan, C.; Balci, S.; Güler Tezel, G.; Akinci, B.; Yalcin, B.; Güler, G. Comparison of Clinicopathologic Parameters and Survivals Between Epstein-Barr Virus–positive and Her2-positive Gastric Cancers. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 609–614. [Google Scholar] [CrossRef]
- Fujimoto, M.; Matsuzaki, I.; Nishino, M.; Iwahashi, Y.; Warigaya, K.; Kojima, F.; Ono, K.; Murata, S.Y. HER2 is frequently overexpressed in hepatoid adenocarcinoma and gastric carcinoma with enteroblastic differentiation: A comparison of 35 cases to 334 gastric carcinomas of other histological types. J. Clin. Pathol. 2018, 71, 600–607. [Google Scholar] [CrossRef]
- Gravalos, C.; Jimeno, A. HER2 in gastric cancer: A new prognostic factor and a novel therapeutic target. Ann. Oncol. 2008, 19, 1523–1529. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, G.; Hu, C. Molecular Classification of Gastric Adenocarcinoma. Gastroenterol. Res. 2019, 12, 275–282. [Google Scholar] [CrossRef]
- Mathiak, M.; Warneke, V.S.; Behrens, H.M.; Haag, J.; Böger, C.; Krüger, S.; Röcken, C. Clinicopathologic Characteristics of Microsatellite Instable Gastric Carcinomas Revisited: Urgent Need for Standardization. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 12–24. [Google Scholar] [CrossRef]
- Smyth, E.C.; Wotherspoon, A.; Peckitt, C.; Gonzalez, D.; Hulkki-Wilson, S.; Eltahir, Z.; Fassan, M.; Rugge, M.; Valeri, N.; Okines, A.; et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC). JAMA Oncol. 2017, 3, 1197–1203. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Kim, H.; Shin, S.J.; Kim, H.Y.; Lee, J.; Yang, H.K.; Kim, W.H.; Kim, Y.W.; Kook, M.C.; Park, Y.K.; et al. Microsatellite Instability and Programmed Cell Death-Ligand 1 Expression in Stage II/III Gastric Cancer: Post Hoc Analysis of the CLASSIC Randomized Controlled study. Ann. Surg. 2019, 270, 309–316. [Google Scholar] [CrossRef]
- Martinson, H.A.; Mallari, D.; Richter, C.; Wu, T.T.; Tiesinga, J.; Alberts, S.R.; Olnes, M.J. Molecular Classification of Gastric Cancer among Alaska Native People. Cancers 2020, 12, 198. [Google Scholar] [CrossRef]
- Laurén, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef]
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. WHO Classification of Tumours of the Digestive System, 4th ed.; World Health Organization: Geneva, Switzerland, 2010; ISBN 9789283224327. [Google Scholar]
- Martín-Richard, M.; Carmona-Bayonas, A.; Custodio, A.B.; Gallego, J.; Jiménez-Fonseca, P.; Reina, J.J.; Richart, P.; Rivera, F.; Alsina, M.; Sastre, J. SEOM clinical guideline for the diagnosis and treatment of gastric cancer (GC) and gastroesophageal junction adenocarcinoma (GEJA) (2019). Clin. Transl. Oncol. 2020, 22, 236–244. [Google Scholar] [CrossRef]
- Mariette, C.; Carneiro, F.; Grabsch, H.I.; van der Post, R.S.; Allum, W.; de Manzoni, G. Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer 2019, 22, 1–9. [Google Scholar] [CrossRef]
- Li, G.C.; Jia, X.C.; Zhao, Q.C.; Zhang, H.W.; Yang, P.; Xu, L.L.; Pang, F.N.; Sun, J.B. The expression of epidermal growth factor receptor 1 and human epidermal growth factor receptor 2 based on tumor location affect survival in gastric cancer. Medicine 2020, 99, e20460. [Google Scholar] [CrossRef]
- Rice, T.W.; Kelsen, D.P.; Blackstone, E.H.; Ishwaran, H.; Patil, D.T.; Bass, A.J.; Erasmus, J.J.; Gerdes, H.; Hofstetter, W.L. Esophagus and esophagogastric junction. In AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Ed.; Springer: New York, NY, USA, 2017; pp. 185–202. ISBN 9783319406176. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.J.; Iwasa, S.; Sugimoto, N.; Ryu, M.H.; Sakai, D.; Chung, H.C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 18, 2419–2430. [Google Scholar] [CrossRef]
- Polom, K.; Das, K.; Marrelli, D.; Roviello, G.; Pascale, V.; Voglino, C.; Rho, H.; Tan, P.; Roviello, F. KRAS Mutation in Gastric Cancer and Prognostication Associated with Microsatellite Instability Status. Pathol. Oncol. Res. 2019, 25, 333–340. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, Q.; Iu, Y.; Guo, H.; Ren, Y.; Li, J.; Zhao, Q. Clinical characteristics and prognostic significance of TCGA and ACRG classification in gastric cancer among the Chinese population. Mol. Med. Rep. 2020, 22, 828–840. [Google Scholar] [CrossRef]
- Cai, L.; Sun, Y.; Wang, K.; Guan, W.; Yue, J.; Li, J.; Wang, R.; Wang, L. The Better Survival of MSI Subtype Is Associated With the Oxidative Stress Related Pathways in Gastric Cancer. Front. Oncol. 2020, 10, 1269. [Google Scholar] [CrossRef]
- Vrána, D.; Matzenauer, M.; Neoral, C.; Aujeský, R.; Vrba, R.; Melichar, B.; Rušarová, N.; Bartoušková, M.; Jankowski, J. From Tumor Immunology to Immunotherapy in Gastric and Esophageal Cancer. Int. J. Mol. Sci. 2018, 20, 13. [Google Scholar] [CrossRef]
- Ramos, M.F.K.P.; Pereira, M.A.; Amorim, L.C.; De Mello, E.S.; Faraj, S.F.; Ribeiro, U.; Hoff, P.M.G.; Cecconello, I.; De Castria, T.B. Gastric cancer molecular classification and adjuvanttherapy: Is there a different benefit according to the subtype? J. Surg. Oncol. 2020, 121, 804–813. [Google Scholar] [CrossRef]
- Pinto, M.P.; Córdova-Delgado, M.; Retamal, I.N.; Muñoz-Medel, M.; Bravo, M.L.; Durán, D.; Villanueva, F.; Sanchez, C.; Acevedo, F.; Mondaca, S.; et al. A Molecular Stratification of Chilean Gastric Cancer Patients with Potential Clinical Applicability. Cancers 2020, 12, 1863. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; Van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.P.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018, 10, e180013. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Faggian, A.; Sintali, D.N.; Khan, G.J.; Naeem, S.; Shi, M.; Dingding, C. Current and future biomarkers in gastric cancer. Biomed. Pharmacother. 2018, 103, 1688–1700. [Google Scholar] [CrossRef]
- Pereira, M.A.; Ramos, M.F.K.P.; Dias, A.R.; Faraj, S.F.; Ribeiro, R.R.E.; de Castria, T.B.; Zilberstein, B.; Alves, V.A.F.; Ribeiro, U., Jr.; de Mello, E.S. Expression Profile of Markers for Targeted Therapy in Gastric Cancer Patients: HER-2, Microsatellite Instability and PD-L1. Mol. Diagn. Ther. 2019, 23, 761–771. [Google Scholar] [CrossRef]
- Ross, J.S.; Fakih, M.; Ali, S.M.; Elvin, J.A.; Schrock, A.B.; Suh, J.; Vergilio, J.A.; Ramkissoon, S.; Severson, E.; Daniel, S.; et al. Targeting HER2 in colorectal cancer: The landscape of amplification and short variant mutations in ERBB2 and ERBB3. Cancer 2018, 1, 1358–1373. [Google Scholar] [CrossRef]
- Refolo, M.G.; Lotesoriere, C.; Messa, C.; Caruso, M.G.; D’Alessandro, R. Integrated immune gene expression signature and molecular classification in gastric cancer: New insights. J. Leukoc. Biol. 2020, 108, 633–646. [Google Scholar] [CrossRef]
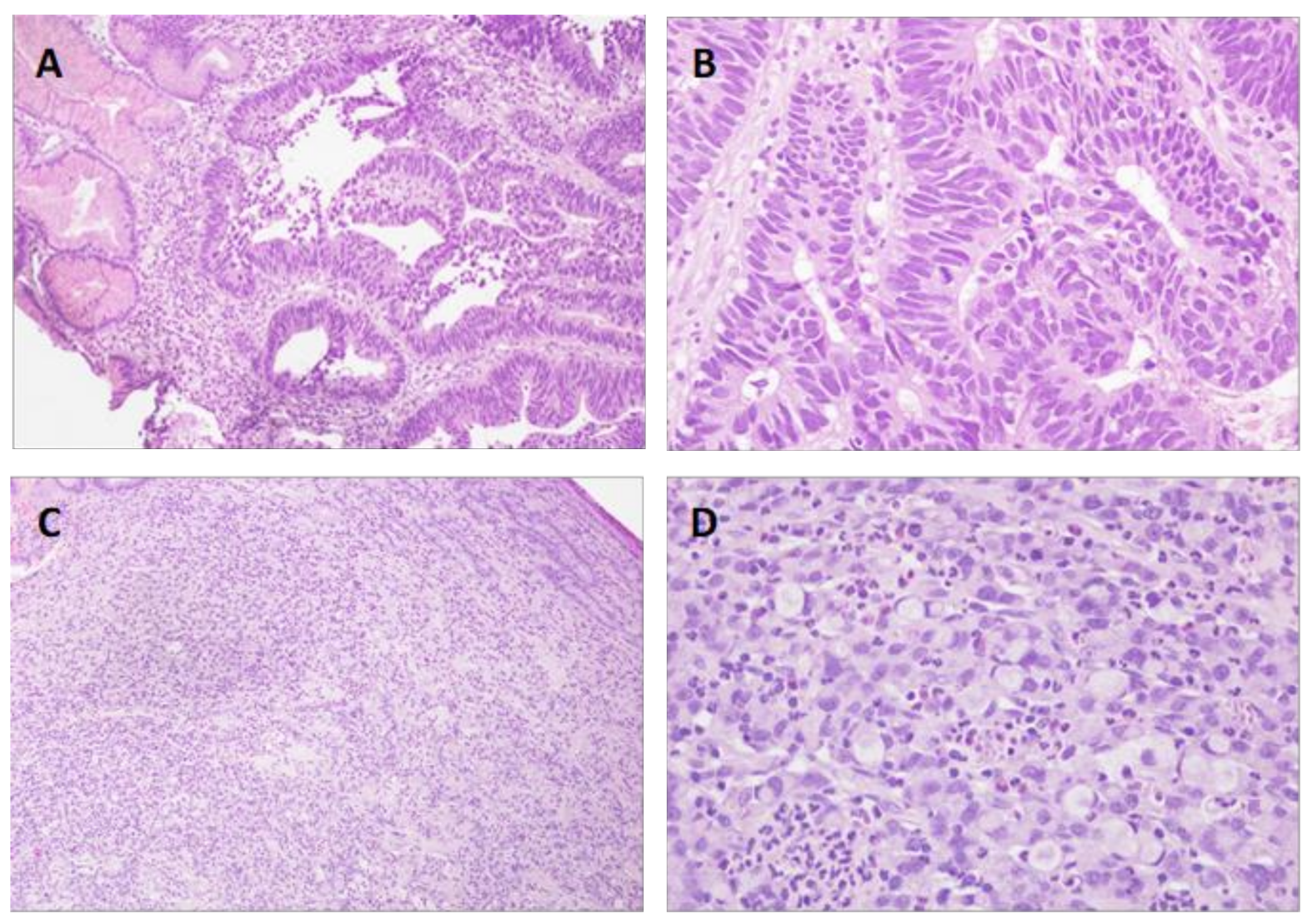
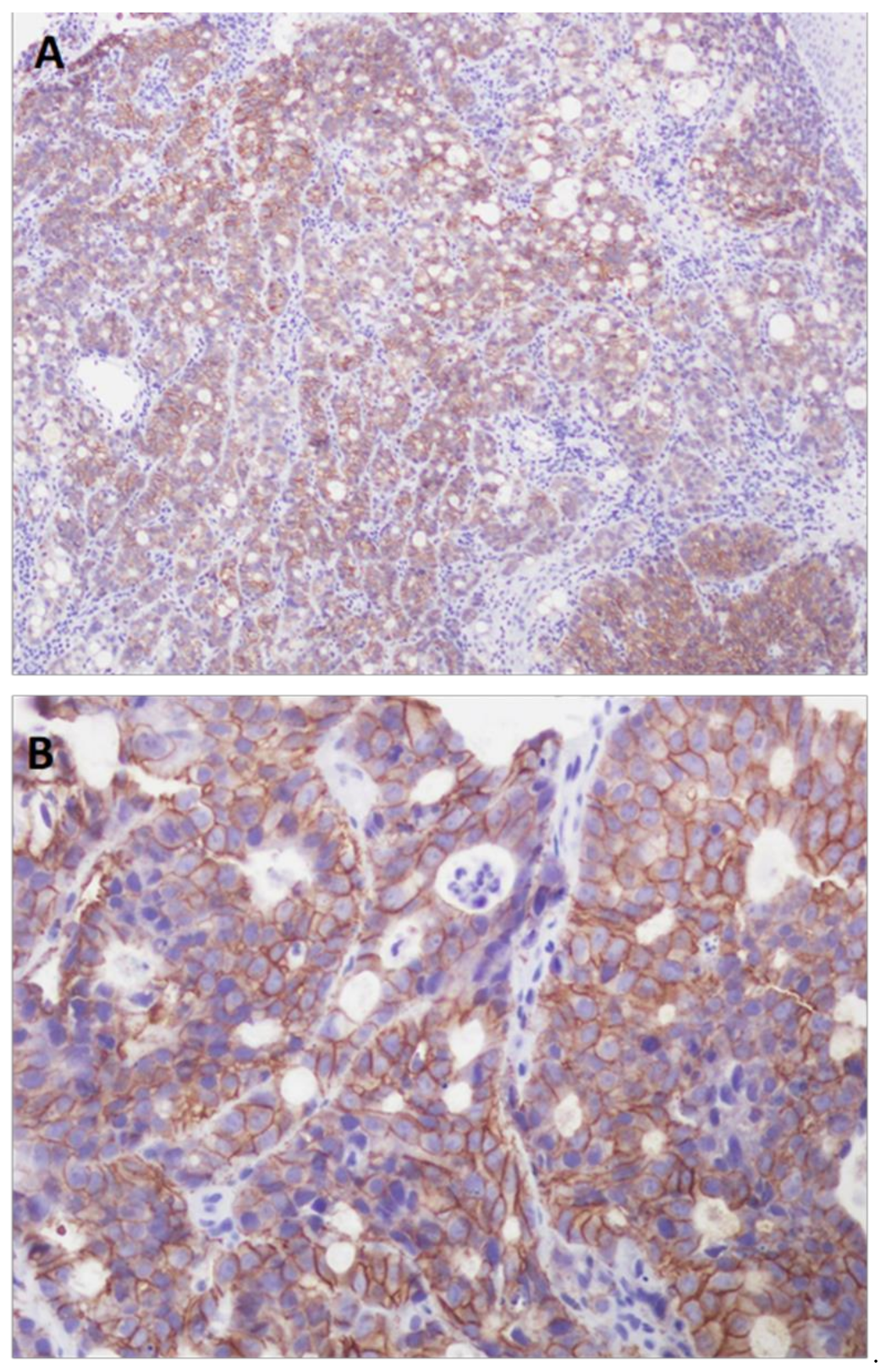
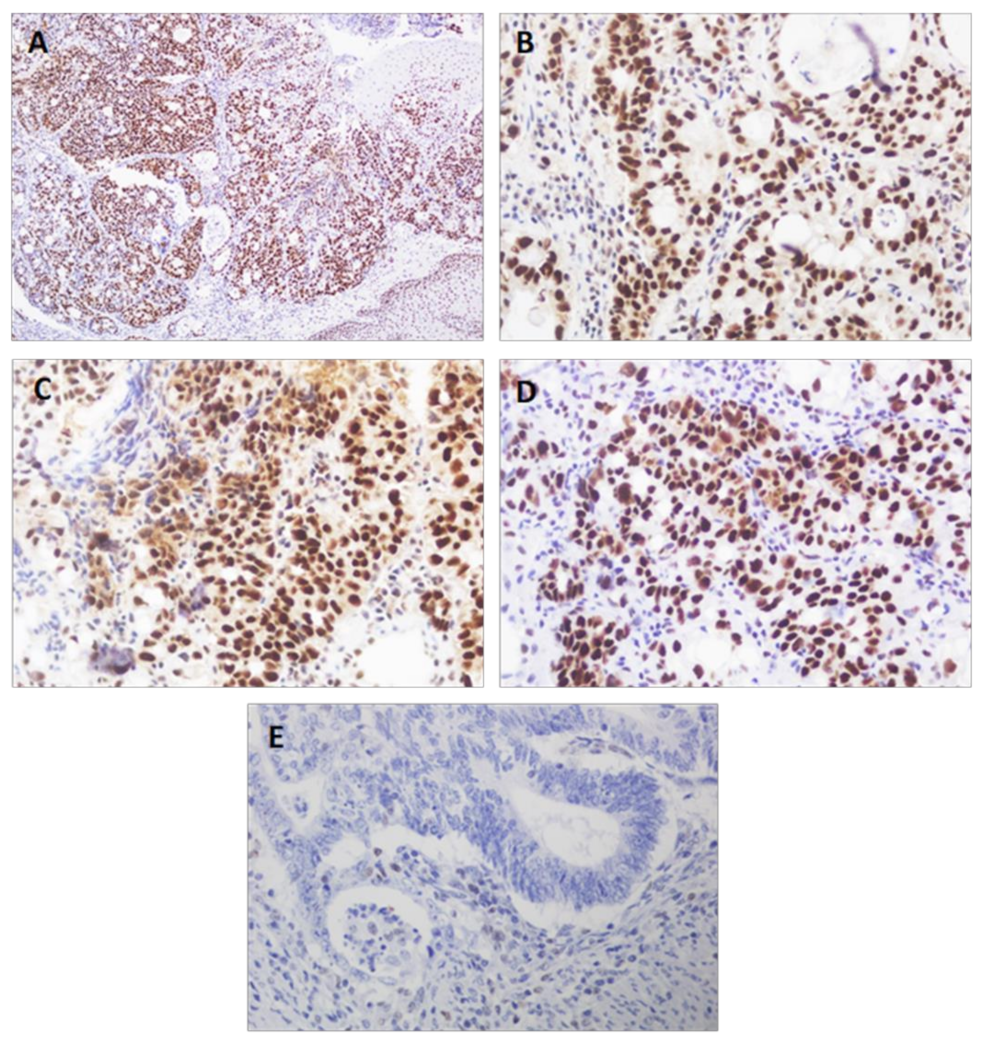
| Features | Cases | Percentage% |
|---|---|---|
| Age (mean and range) | 65.41 | 34–86 |
| ≤70 | 83 | 58.45% |
| ≥71 | 59 | 41.55% |
| Molecular characteristics | ||
| HER2-positive | 19 | 13.40% |
| MSI | 23 | 16.20% |
| Sex | ||
| Male | 89 | 62.70% |
| Female | 53 | 37.30% |
| Location | ||
| Cardia | 23 | 16.20% |
| Fundus | 1 | 0.70% |
| Corpus | 58 | 40.80% |
| Antrum | 59 | 41.50% |
| Histological type | ||
| Intestinal | 76 | 53.50% |
| Diffuse | 57 | 40.10% |
| Mixed | 9 | 6.40% |
| Pathological diagnosis | ||
| Adenocarcinoma | 104 | 73.20% |
| Signet ring cell carcinoma | 34 | 23.90% |
| Small cell carcinoma | 2 | 1.40% |
| Undifferentiated carcinoma | 1 | 0.70% |
| Squamous cell carcinoma | 1 | 0.70% |
| Degree of differentiation | ||
| G1 | 18 | 13.00% |
| G2 | 46 | 33.30% |
| G3 | 74 | 53.60% |
| T | ||
| Tis | 6 | 4.20% |
| T1a/T1b | 15 | 10.60% |
| T2 | 21 | 14.80% |
| T3 | 57 | 40.10% |
| T4a/T4b | 43 | 30.30% |
| N | ||
| N0 | 54 | 38.00% |
| N1 | 38 | 26.80% |
| N2 | 23 | 16.20% |
| N3a/N3b | 27 | 19.00% |
| M | ||
| M0 | 129 | 90.80% |
| M1 | 13 | 9.20% |
| Stage | ||
| IA/IB | 33 | 23.24% |
| IIA/IIB | 47 | 33.10% |
| IIIA/IIIB/IIIC | 46 | 32.40% |
| IV | 16 | 11.26% |
| Lymphatic involvement | ||
| Yes | 64 | 45.10% |
| No | 78 | 54.90% |
| Vascular involvement | ||
| Yes | 59 | 41.50% |
| No | 83 | 58.50% |
| Perineural involvement | ||
| Yes | 48 | 33.80% |
| No | 94 | 66.20% |
| Adjuvant chemotherapy | ||
| Yes | 43 | 30.30% |
| No | 99 | 69.70% |
| Survival | ||
| Living | 56 | 39.40% |
| Deceased | 86 | 60.60% |
| HER2-Positive (n = 19) | HER2-Negative (n = 123) | ||||
|---|---|---|---|---|---|
| Features | Cases | Percentage% | Cases | Percentage% | p |
| Age | 0.085 | ||||
| Mean | 61 | 66 | |||
| ≤70 | 14 | 16.86% | 70 | 84.33% | |
| ≥71 | 5 | 8.47% | 53 | 91.53% | |
| Sex | 0.578 | ||||
| Male | 13 | 14.60% | 76 | 85.40% | |
| Female | 6 | 11.30% | 47 | 88.70% | |
| Location | 0.041 | ||||
| Cardia | 1 | 4.30% | 22 | 95.70% | |
| Fundus | 1 | 100% | 0 | 0.0% | |
| Corpus | 8 | 13.80% | 50 | 86.20% | |
| Antrum | 9 | 15.30% | 50 | 84.70% | |
| Histological type | 0.013 | ||||
| Intestinal | 16 | 21.10% | 60 | 78.90% | |
| Diffuse | 2 | 3.50% | 55 | 96.50% | |
| Mixed | 1 | 11.10% | 9 | 88.90% | |
| Pathological diagnosis | 0.267 | ||||
| Adenocarcinoma | 18 | 17.30% | 86 | 82.70% | |
| Signet ring cell carcinoma | 1 | 2.90% | 33 | 97.10% | |
| Small cell carcinoma | 0 | 0.00% | 2 | 100% | |
| Undifferentiated carcinoma | 0 | 0.00% | 1 | 100% | |
| Squamous cell carcinoma | 0 | 0.00% | 1 | 100% | |
| Degree of differentiation | 0.178 | ||||
| G1 | 0 | 0.00% | 18 | 100% | |
| G2 | 8 | 17.40% | 38 | 82.60% | |
| G3 | 11 | 14.90% | 63 | 85.10% | |
| T | 0.25 | ||||
| Tis | 0 | 0.00% | 6 | 100% | |
| T1a/T1b | 0 | 0.00% | 15 | 100% | |
| T2 | 1 | 4.80% | 20 | 95.20% | |
| T3 | 11 | 19.30% | 46 | 80.70% | |
| T4a/T4b | 7 | 16.27% | 36 | 83.73% | |
| N | 0.455 | ||||
| N0 | 5 | 9.30% | 49 | 90.70% | |
| N1 | 5 | 13.20% | 33 | 86.80% | |
| N2 | 3 | 13.00% | 20 | 87.00% | |
| N3a/N3b | 6 | 22.20% | 21 | 77.80% | |
| M | 0.005 | ||||
| M0 | 14 | 10.90% | 115 | 89.10% | |
| M1 | 5 | 38.50% | 8 | 61.50% | |
| Stage | 0.012 | ||||
| IA/IB | 1 | 3.10% | 32 | 96.90% | |
| IIA/IIB | 6 | 12.80% | 41 | 87.20% | |
| IIIA/IIIB/IIIC | 6 | 13.00% | 40 | 87.00% | |
| IV | 6 | 37.50% | 10 | 62.50% | |
| Lymphatic involvement | 0.227 | ||||
| Yes | 11 | 17.20% | 53 | 82.80% | |
| No | 8 | 10.30% | 70 | 89.70% | |
| Vascular involvement | 0.292 | ||||
| Yes | 10 | 16.90% | 49 | 83.10% | |
| No | 9 | 10.80% | 74 | 89.20% | |
| Perineural involvement | 0.763 | ||||
| Yes | 7 | 14.60% | 41 | 85.40% | |
| No | 12 | 12.80% | 82 | 87.20% | |
| Adjuvant chemotherapy | 0.005 | ||||
| Yes | 11 | 25.60% | 32 | 74.40% | |
| No | 8 | 8.10% | 91 | 91.90% | |
| Survival | 0.209 | ||||
| Living | 5 | 8.90% | 51 | 91.10% | |
| Deceased | 14 | 16.30% | 72 | 83.70% | |
| MSI (n = 23) | MSS (n = 119) | ||||
|---|---|---|---|---|---|
| Features | Cases | Percentage% | Cases | Percentage% | p |
| Age | <0.0001 | ||||
| Mean | 75.43 | 63.48 | |||
| ≤70 | 2 | 2.40% | 81 | 97.60% | |
| ≥71 | 21 | 35.59% | 38 | 64.41% | |
| Sex | 0.038 | ||||
| Male | 10 | 11.20% | 79 | 88.80% | |
| Female | 13 | 24.50% | 40 | 75.50% | |
| Location | 0.015 | ||||
| Cardia | 0 | 0.00% | 23 | 100.00% | |
| Fundus | 0 | 0.00% | 1 | 100.00% | |
| Corpus | 7 | 12.10% | 51 | 89.70% | |
| Antrum | 16 | 27.10% | 43 | 72.90% | |
| Histological type | 0.033 | ||||
| Intestinal | 18 | 23.70% | 58 | 76.30% | |
| Diffuse | 4 | 7.00% | 53 | 93.00% | |
| Mixed | 1 | 11.10% | 8 | 88.90% | |
| Pathological diagnosis | 0.324 | ||||
| Adenocarcinoma | 21 | 20.20% | 83 | 79.80% | |
| Signet ring cell carcinoma | 2 | 5.90% | 32 | 94.10% | |
| Small cell carcinoma | 0 | 0.00% | 2 | 100.00% | |
| Undifferentiated carcinoma | 0 | 0.00% | 1 | 100.00% | |
| Squamous cell carcinoma | 0 | 0.00% | 1 | 100.00% | |
| Degree of differentiation | 0.122 | ||||
| G1 | 6 | 33.30% | 12 | 66.70% | |
| G2 | 6 | 13.00% | 40 | 87.00% | |
| G3 | 11 | 14.90% | 63 | 85.10% | |
| T | 0.593 | ||||
| Tis | 2 | 33.30% | 4 | 66.70% | |
| T1a/T1b | 2 | 13.30% | 13 | 86.70% | |
| T2 | 5 | 23.80% | 16 | 76.20% | |
| T3 | 7 | 12.30% | 50 | 87.70% | |
| T4a/T4b | 7 | 16.27% | 36 | 83.73% | |
| N | 0.647 | ||||
| N0 | 11 | 20.40% | 43 | 79.60% | |
| N1 | 4 | 10.50% | 34 | 89.50% | |
| N2 | 4 | 17.40% | 19 | 82.60% | |
| N3a/N3b | 4 | 14.80% | 23 | 85.20% | |
| M | 0.383 | ||||
| M0 | 22 | 17.10% | 107 | 82.90% | |
| M1 | 1 | 7.70% | 12 | 92.30% | |
| Stage | 0.555 | ||||
| IA/IB | 5 | 15.20% | 28 | 84.80% | |
| IIA/IIB | 10 | 21.30% | 37 | 78.72% | |
| IIIA/IIIB/IIIC | 7 | 15.20% | 39 | 84.80% | |
| IV | 1 | 6.30% | 15 | 93.70% | |
| Lymphatic involvement | 0.532 | ||||
| Yes | 9 | 14.10% | 55 | 85.90% | |
| No | 14 | 17.90% | 64 | 82.10% | |
| Vascular involvement | 0.797 | ||||
| Yes | 9 | 15.30% | 50 | 84.70% | |
| No | 14 | 16.90% | 69 | 83.10% | |
| Perineural involvement | 0.914 | ||||
| Yes | 8 | 16.70% | 40 | 83.30% | |
| No | 15 | 16.00% | 79 | 84.00% | |
| Adjuvant chemotherapy | 0.33 | ||||
| Yes | 5 | 11.60% | 38 | 88.40% | |
| No | 18 | 18.20% | 81 | 81.80% | |
| Survival | 0.618 | ||||
| Living | 8 | 14.30% | 48 | 85.70% | |
| Deceased | 15 | 17.40% | 71 | 82.60% | |
| MSI | MSS | ||||
|---|---|---|---|---|---|
| Status | Living | Deceased | Living | Deceased | p |
| Chemotherapy | 1 (20%) | 4 (80%) | 15 (39.5%) | 23 (60.5%) | 0.397 |
| Surgery alone | 7 (38.9%) | 11 (61.1%) | 33 (40.7%) | 48 (59.3%) | 0.885 |
| Features | HER2-Positive and MSI (n = 2) | |
|---|---|---|
| Case 1 | Case 2 | |
| Age | 76 | 74 |
| Sex | Female | Male |
| Location | Antrum | Antrum |
| Histological type | Intestinal | Intestinal |
| Pathological diagnosis | Adenocarcinoma | Adenocarcinoma |
| Degree of differentiation | G3 | G3 |
| T | T3 | T3 |
| N | N3a | N0 |
| M | M1 | M0 |
| Stage | IV | IIA |
| Lymphatic involvement | Yes | Yes |
| Vascular involvement | Yes | Yes |
| Perineural involvement | Yes | Yes |
| Adjuvant chemotherapy | No | No |
| Survival | Deceased | Deceased |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bermúdez, A.; Arranz-Salas, I.; Mercado, S.; López-Villodres, J.A.; González, V.; Ríus, F.; Ortega, M.V.; Alba, C.; Hierro, I.; Bermúdez, D. Her2-Positive and Microsatellite Instability Status in Gastric Cancer—Clinicopathological Implications. Diagnostics 2021, 11, 944. https://doi.org/10.3390/diagnostics11060944
Bermúdez A, Arranz-Salas I, Mercado S, López-Villodres JA, González V, Ríus F, Ortega MV, Alba C, Hierro I, Bermúdez D. Her2-Positive and Microsatellite Instability Status in Gastric Cancer—Clinicopathological Implications. Diagnostics. 2021; 11(6):944. https://doi.org/10.3390/diagnostics11060944
Chicago/Turabian StyleBermúdez, Ana, Isabel Arranz-Salas, Silvia Mercado, Juan A. López-Villodres, Virginia González, Francisca Ríus, María V. Ortega, Carmen Alba, Isabel Hierro, and Diego Bermúdez. 2021. "Her2-Positive and Microsatellite Instability Status in Gastric Cancer—Clinicopathological Implications" Diagnostics 11, no. 6: 944. https://doi.org/10.3390/diagnostics11060944
APA StyleBermúdez, A., Arranz-Salas, I., Mercado, S., López-Villodres, J. A., González, V., Ríus, F., Ortega, M. V., Alba, C., Hierro, I., & Bermúdez, D. (2021). Her2-Positive and Microsatellite Instability Status in Gastric Cancer—Clinicopathological Implications. Diagnostics, 11(6), 944. https://doi.org/10.3390/diagnostics11060944





