The Role of CX3CL1 and ADAM17 in Pathogenesis of Diffuse Parenchymal Lung Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Procedures and Sample Processing
2.3. Statistical Analysis
3. Results
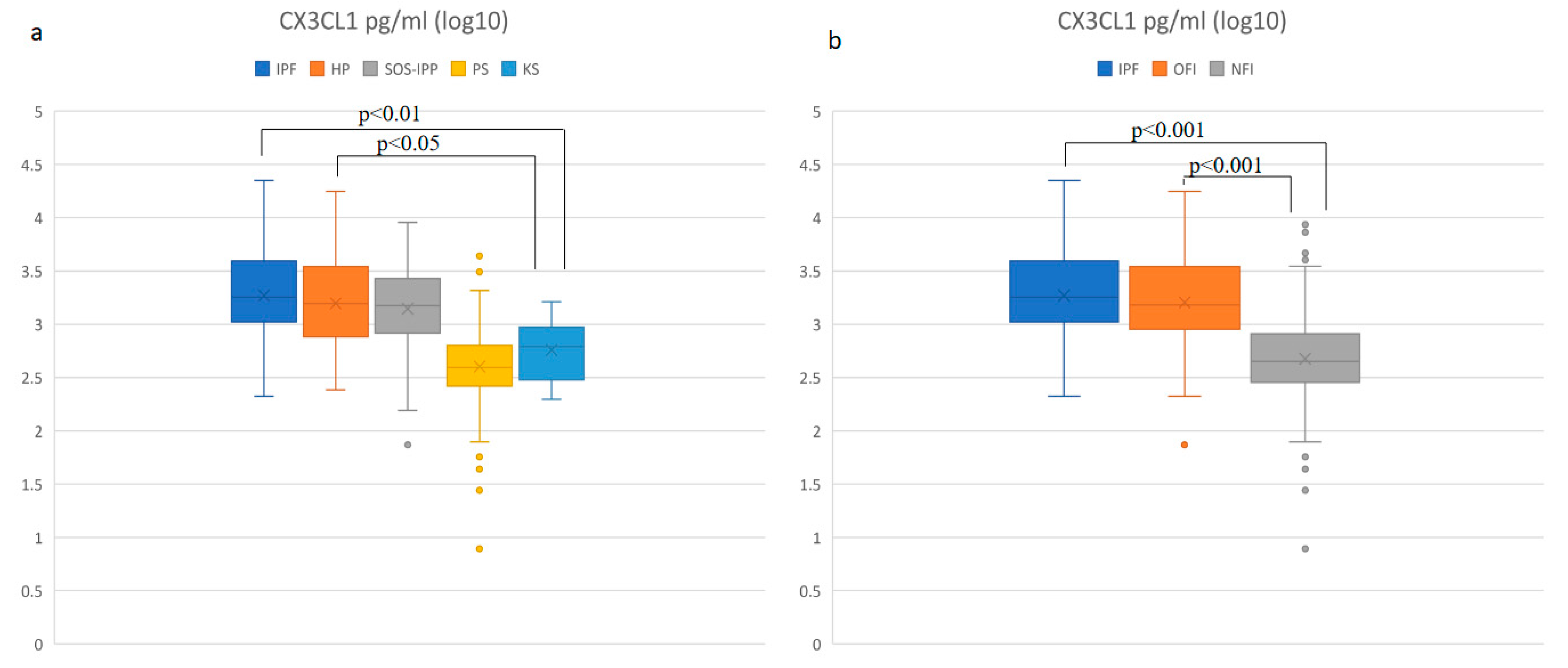
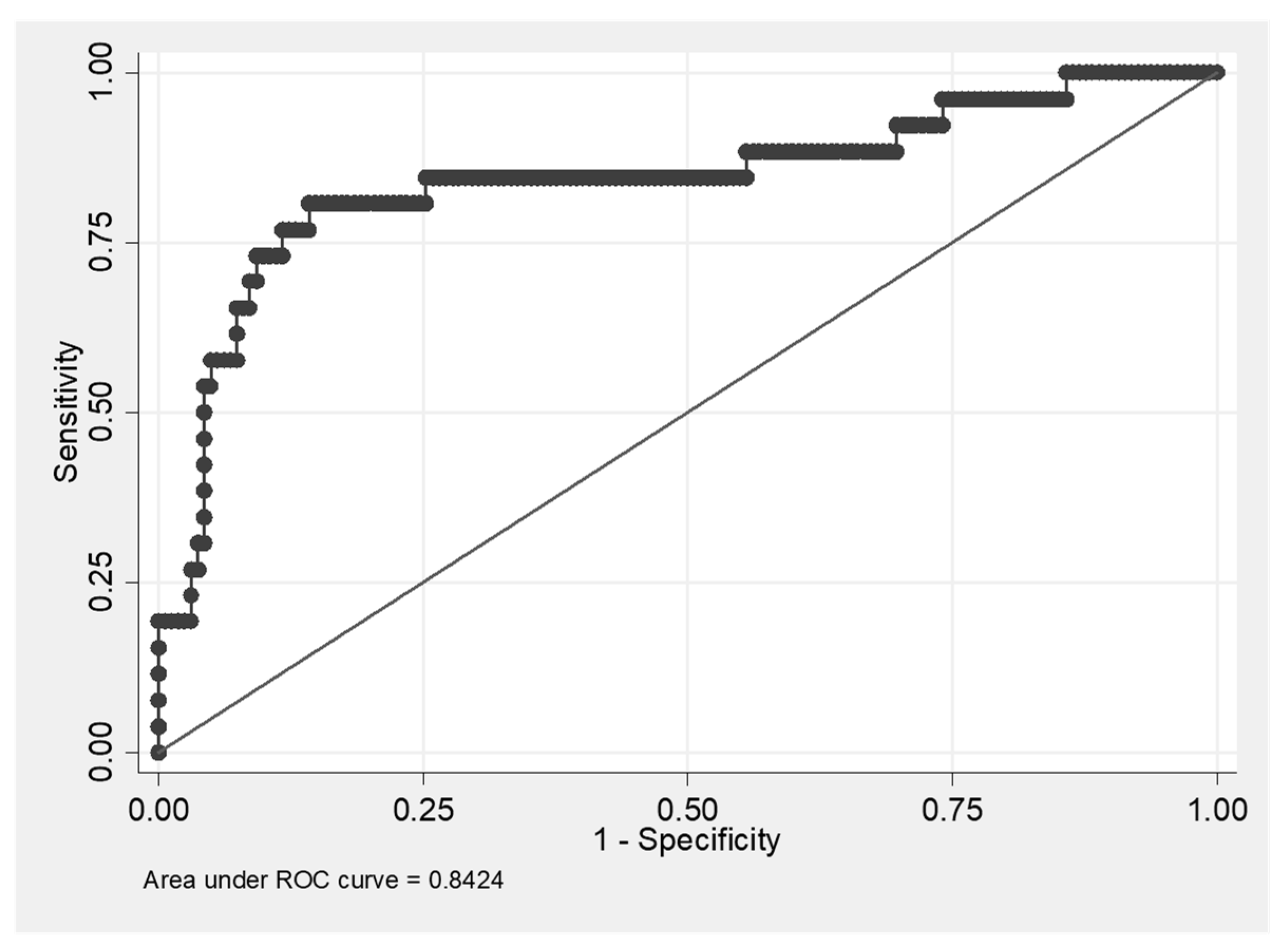
3.1. Increased Concentration of CX3CL1 in Patients with IPF and HP
3.2. Positive Correlation of the Level of CX3CL1 with Number of Macrophages, Neutrophils and CD8+ T Cells, and a Negative Correlation with the Number of CD4+ T Cells and with DLCO Values
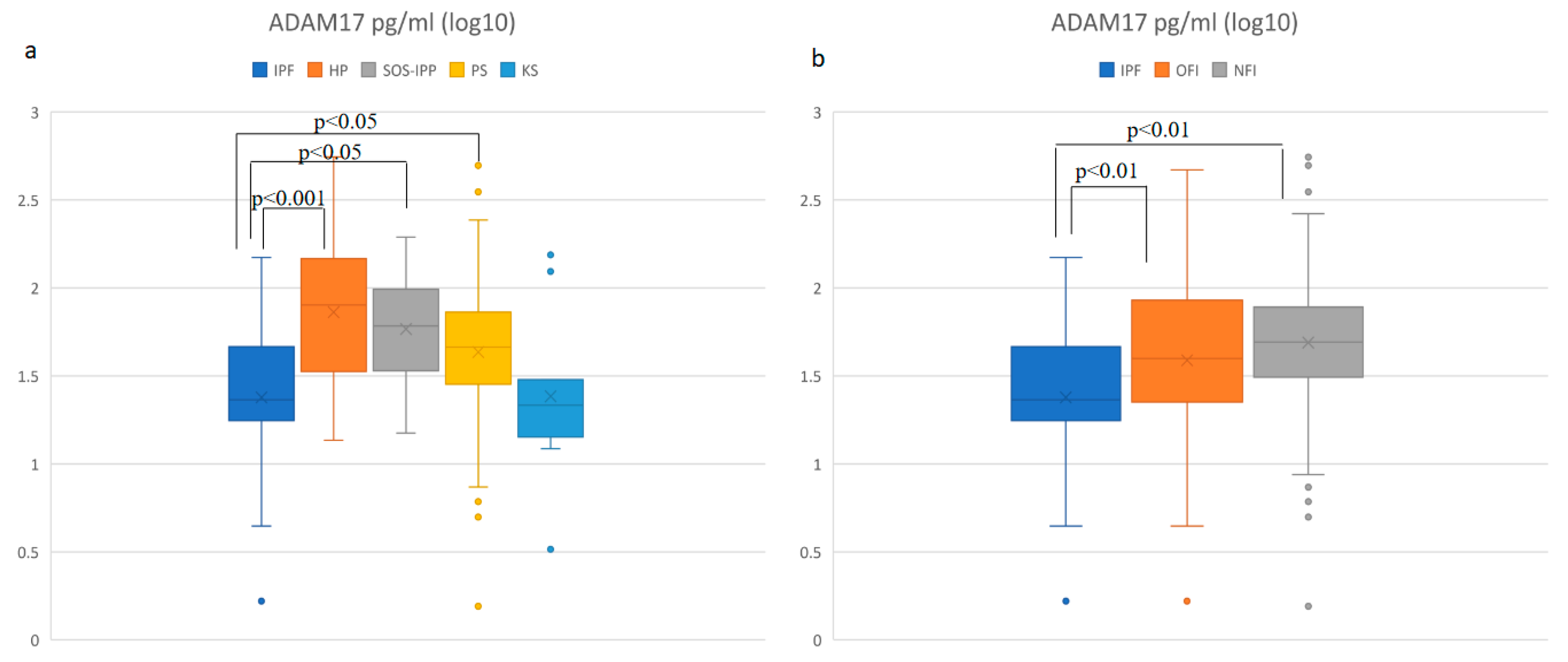
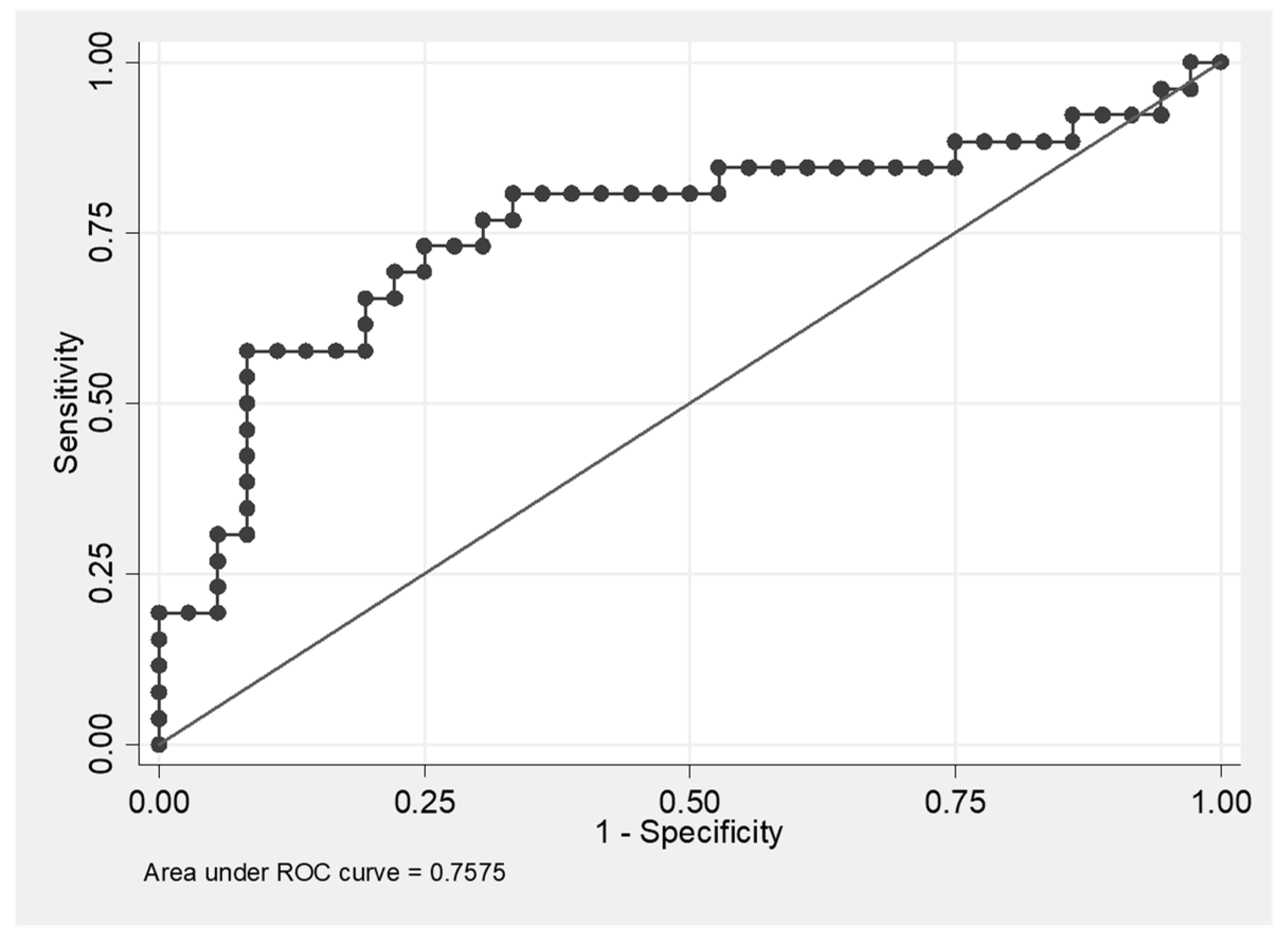
3.3. Soluble ADAM-17 Concentrations in BALF Are Elevated in HP and Decreased in IPF Patients
3.4. Positive Correlation of the Level of ADAM17 with Absolute Cell Count in BALF
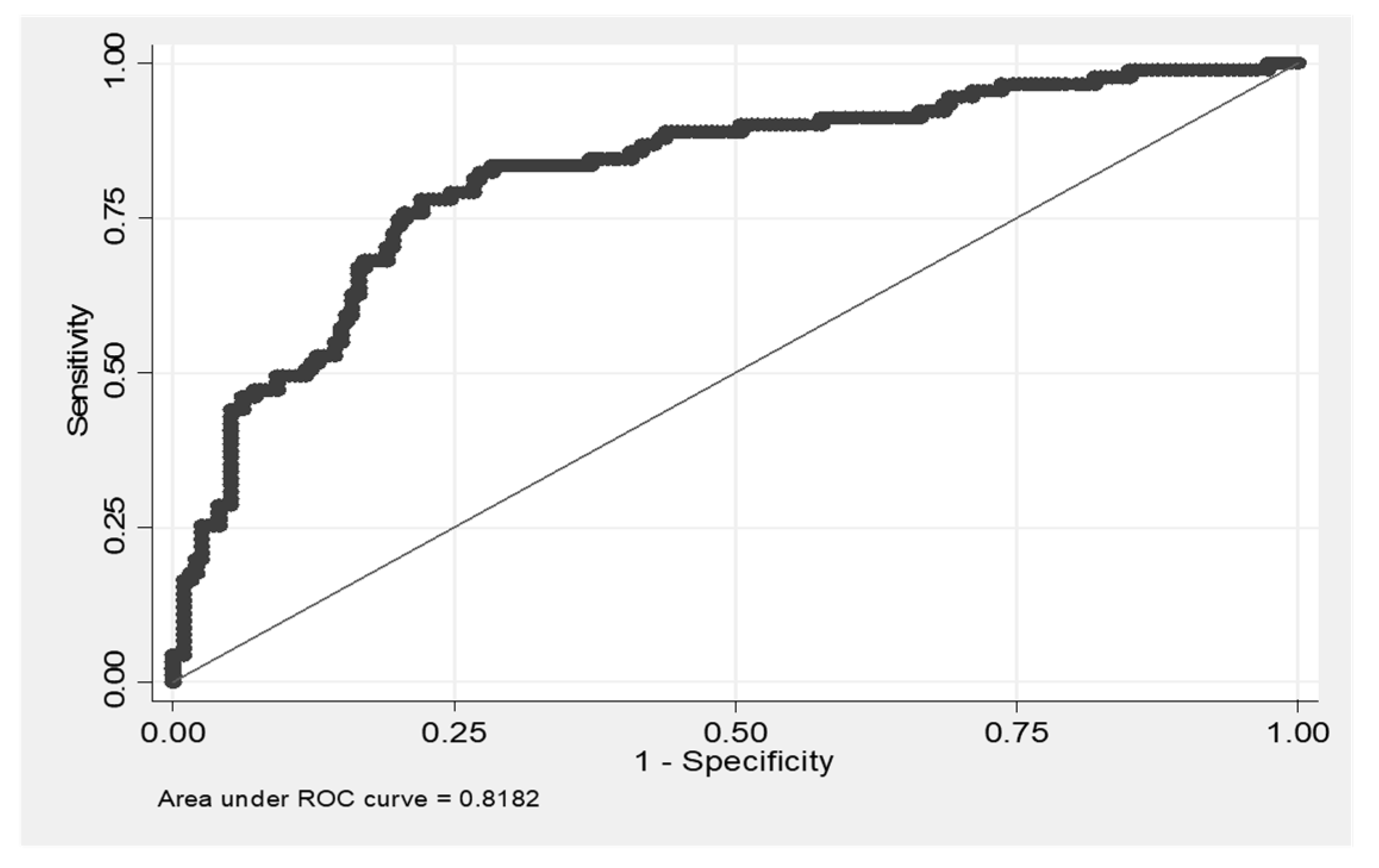
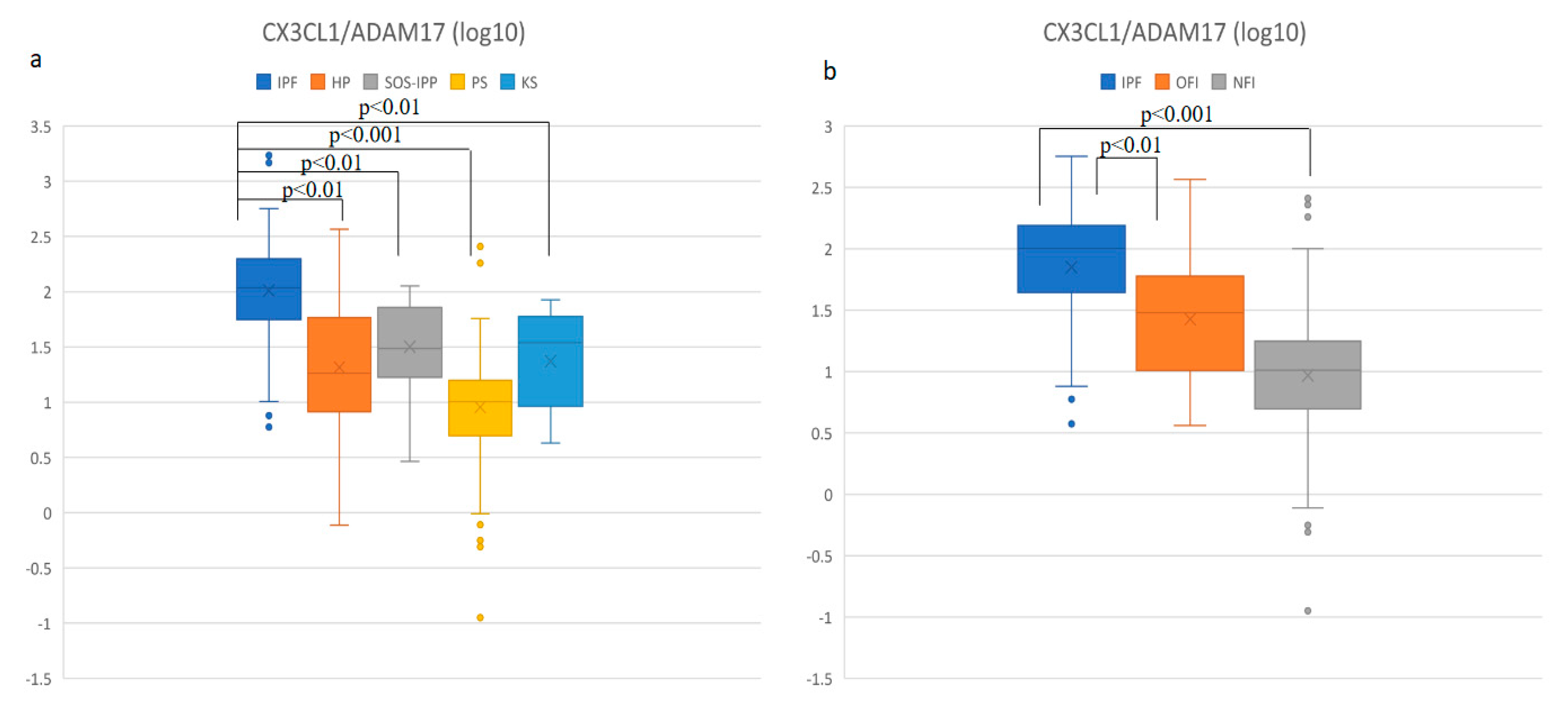
3.5. IPF Patients Had the Highest CX3CL1/ADAM17 Ratio
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Bagnato, G.; Harari, S. Cellular interactions in the pathogenesis of interstitial lung diseases. Eur. Respir. Rev. 2015, 24, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, W.A.; Agostini, C.; Antoniou, K.M.; Bouros, D.; Chambers, R.C.; Cottin, V.; Egan, J.J.; Lambrecht, B.N.; Lories, R.; Parfrey, H.; et al. The pathogenesis of pulmonary fibrosis: A moving target. Eur. Respir. J. 2013, 41, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.W.; Ryerson, C.J.; Guler, S.A. Progression of fibrosing interstitial lung disease. Respir. Res. 2020, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Lioyd, C.; Zhou, H.; Dolich, S.; Deeds, J.; Gonzalo, J.A.; Vath, J.; Gosselin, M.; Ma, J.; Dussault, B.; et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature 1997, 387, 611–617. [Google Scholar] [CrossRef]
- Bazan, J.F.; Bacon, K.B.; Hardiman, G.; Wang, W.; Soo, K.; Ross, D.; Greaves, D.R.; Zlotnik, A.; Schall, T.J. A new class of membrane-bound chemokine with a CX3C motif. Nature 1997, 385, 640–644. [Google Scholar] [CrossRef]
- Umehara, H.; Bloom, E.T.; Okazaki, T.; Domae, N.; Imai, T. Fractalkine and vascular injury. Trends Immunol. 2001, 22, 602–607. [Google Scholar] [CrossRef]
- Umehara, H.; Imai, T. The role of fractalkine in leukocyte adhesion and migration, and vascular injury. Drug News Perspect. 2001, 14, 460–464. [Google Scholar] [CrossRef]
- Lucas, A.D.; Chadwick, N.; Warren, B.F.; Jewell, D.P.; Gordon, S.; Powrie, F.; Greaves, D.R. The transmembrane form of the CX3CL1 chemokine fractalkine is expressed predominantly by epithelial cells in vivo. Am. J. Pathol. 2001, 158, 855–866. [Google Scholar] [CrossRef]
- Kim, K.W.; Vallon-Eberhard, A.; Zigmond, E.; Farache, J.; Shezen, E.; Shakhar, G.; Ludwig, A.; Lira, S.A.; Jung, S. In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood 2011, 118, 156–167. [Google Scholar] [CrossRef]
- Garton, K.J.; Gough, P.J.; Blobel, C.P.; Murphy, G.; Greaves, D.R.; Dempsey, P.J.; Raines, E.W. Tumor necrosis factor α-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1). J. Biol. Chem. 2001, 276, 37993–38001. [Google Scholar] [CrossRef]
- Hundhausen, C.; Misztela, D.; Berkhout, T.A.; Broadway, N.; Saftig, P.; Reiss, K.; Hartmann, D.; Fahrenholz, F.; Postina, R.; Matthews, V.; et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood 2003, 102, 1186–1195. [Google Scholar] [CrossRef]
- Imai, T.; Hieshima, K.; Haskell, C.; Baba, M.; Nagira, M.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Nomiyama, H.; Schall, T.J.; et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 1997, 91, 521–530. [Google Scholar] [CrossRef]
- Nishimura, M.; Umehara, H.; Nakayama, T.; Yoneda, O.; Hieshima, K.; Kakizaki, M.; Dohmae, N.; Yoshie, O.; Imai, T. Dual functions of fractalkine/ CX3CR1 in trafficking of circulating cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J. Immunol. 2002, 168, 6173–6180. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, O.; Imai, T.; Gouda, S.; Inoue, H.; Yamauchi, A.; Okazaki, T.; Imai, H.; Yoshie, O.; Bloom, E.T.; Domae, N.; et al. NK cell-mediated vascular injury. J. Immunol. 2000, 164, 4055–4062. [Google Scholar] [CrossRef] [PubMed]
- Lesnik, P.; Haskell, C.A.; Charo, I.F. Decreased atherosclerosis in CX3CR1−/− mice reveals a role for fractalkine in atherogenesis. J. Clin. Investig. 2003, 111, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Odai, T.; Matsunawa, M.; Takahashi, R.; Wakabayashi, K.; Isozaki, T.; Yajima, N.; Miwa, Y.; Kasama, T. Correlation of CX3CL1 and CX3CR1 levels with response to infliximab therapy in patients with rheumatoid arthritis. J. Rheumatol. 2009, 36, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- El-Shazly, A.; Berger, P.; Girodet, P.O.; Ousova, O.; Fayon, M.; Vernejoux, J.M.; Marthan, R.; Tunon-de-Lara, J.M. Fraktalkine produced by airway smooth muscle cells contributes to mast cell recruitment in asthma. J. Immunol. 2006, 176, 1860–1868. [Google Scholar] [CrossRef]
- McComb, J.G.; Ranganathan, M.; Liu, X.H.; Pilewski, J.M.; Ray, P.; Watkins, S.C.; Choi, A.M.; Lee, J.S. CX3CL1 up-regulation is associated with recruitment of CX3CR1+ mononuclear phagocytes and T lymphocytes in the lungs during cigarette smoke-induced emphysema. Am. J. Pathol. 2008, 173, 949–961. [Google Scholar] [CrossRef]
- Ishida, Y.; Kimura, A.; Nosaka, M.; Kuninaka, Y.; Hemmi, H.; Sasaki, I.; Kaisho, T.; Mukaida, N.; Kondo, T. Essential involvement of the CX3CL1-CX3CR1 axis in bleomycin-induced pulmonary fibrosis via regulation of fibrocyte and M2 macrophage migration. Sci. Rep. 2017, 7, 16833. [Google Scholar] [CrossRef]
- Hoffmann-Vold, A.M.; Weig, S.S.; Palchevskiy, V.; Volkmann, E.; Saggar, R.; Li, N.; Midtvedt, Ø.; Lund, M.B.; Garen, T.; Fishbein, M.C.; et al. Augmented concentrations of CX3CL1 are associated with interstitial lung disease in systemic sclerosis. PLoS ONE 2018, 13, e0206545. [Google Scholar] [CrossRef]
- Greiffo, F.R.; Viteri-Alvarez, V.; Frankenberger, M.; Dietel, D.; Ortega-Gomez, A.; Lee, J.S.; Hilgendorff, A.; Behr, J.; Soehnlein, O.; Eickelberg, O.; et al. CX3CR1–fractalkine axis drives kinetic changes of monocytes in fibrotic interstitial lung diseases. Eur. Respir. J. 2020, 55, 1900460. [Google Scholar] [CrossRef]
- Hunninghake, G.W.; Costabel, U.; Ando, M.; Baughman, R.; Cordier, J.F.; Du, B.R.; Eklund, A.; Kitaichi, M.; Lynch, J.; Rizzato, G.; et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc. Diffus. Lung Dis. 1999, 16, 149–173. [Google Scholar]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2018, 198, 44–68. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Ryerson, C.J.; Myers, J.L.; Kreuter, M.; Vasakova, M.; Bargagli, E.; Chung, J.H.; Collins, B.F.; Bendstrup, E.; et al. Diagnosis of Hypersensitivity Pneumonitis in Adults An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2020, 202, 36–69. [Google Scholar] [CrossRef]
- Mira-Avendano, I.; Abril, A.; Burger, C.D.; Dellaripa, P.F.; Fischer, A.; Gotway, M.B.; Lee, A.S.; Lee, J.S.; Matteson, E.L.; Yi, E.S.; et al. Interstitial lung disease and other pulmonary manifestations in connective tissue diseases. Mayo Clin. Proc. 2019, 94, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Geerts, S.; Wuyts, W.; Langhe, E.; Lenaerts, J.; Yserbyt, J. Connective tissue disease associated interstitial pneumonia: A challenge for both rheumatologists and pulmonologists. Sarcoidosis Vasc. Diffus. Lung Dis. 2017, 34, 326–335. [Google Scholar]
- Meyer, K.C.; Raghu, G.; Baughman, R.P.; Brown, K.K.; Costabel, U.; Du Bois, R.M.; Drent, M.; Haslam, P.L.; Kim, D.S.; Nagai, S.; et al. An official American Thoracic Society clinical practice guideline: The clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef] [PubMed]
- Coward, W.R.; Saini, G.; Jenkins, G. The pathogenesis of idiopathic pulmonary fibrosis. Adv. Respir. Dis. 2010, 4, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Wollin, L.; Fischer, A.; Quaresma, M.; Stowasser, S.; Harari, S. Fibrosing interstitial lung diseases: Knowns and unknowns. Eur. Respir. Rev. 2019, 28, 180100. [Google Scholar] [CrossRef]
- Daniil, Z.; Kitsanta, P.; Kapotsis, G.; Mathioudaki, M.; Kollintza, A.; Karatza, M.; Milic-Emili, J.; Roussos, C.; Papiris, S.A. CD8+ T lymphocytes in lung tissue from patients with idiopathic pulmonary fibrosis. Respir. Res. 2005, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, T.Y.; Robidoux, T.E.; Weinstein, J.S.; Craft, J.; Swain, S.L.; Marshak-Rothstein, A. IL-21 Promotes Pulmonary Fibrosis through the Induction of Profibrotic CD8+ T Cells. J. Immunol. 2015, 195, 5251–5260. [Google Scholar] [CrossRef] [PubMed]
- Pruessmeyer, J.; Hess, F.M.; Alert, H.; Groth, E.; Pasqualon, T.; Schwarz, N.; Nyamoya, S.; Kollert, J.; van der Vorst, E.; Donners, M.; et al. Leukocytes require ADAM10 but not ADAM17 for their migration and inflammatory recruitment into the alveolar space. Blood 2014, 123, 4077–4088. [Google Scholar] [CrossRef]
- Arndt, P.G.; Strahan, B.; Wang, Y.; Long, C.; Horiuchi, K.; Walcheck, B. Leukocyte ADAM17 regulates acute pulmonary inflammation. PLoS ONE 2011, 6, e19938. [Google Scholar] [CrossRef]
- Long, C.; Hosseinkhani, M.R.; Wang, Y.; Sriramarao, P.; Walcheck, B. ADAM17 activation in circulating neutrophils following bacterial challenge impairs their recruitment. J. Leukoc. Biol. 2012, 92, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Verma, V.; Maskos, K.; Nath, D.; Knauper, V.; Dodds, P.; Amour, A.; Murphy, G. Engineering N-terminal domain of tissue inhibitor of metalloproteinase (TIMP)-3 to be a better inhibitor against tumour necrosis factor-alpha-converting enzyme. Biochem. J. 2002, 364, 227–234. [Google Scholar] [CrossRef]
- García-Alvarez, J.; Ramirez, R.; Checa, M.; Nuttall, R.K.; Sampieri, C.L.; Edwards, D.R.; Selman, M.; Pardo, A. Tissue inhibitor of metalloproteinase-3 is up-regulated by transforming growth factor-beta1 in vitro and expressed in fibroblastic foci in vivo in idiopathic pulmonary fibrosis. Exp. Lung Res. 2006, 32, 201–214. [Google Scholar] [CrossRef] [PubMed]
| PS | IPF | HP | CTD-ILD | CG | |
|---|---|---|---|---|---|
| Subjects * | 156 | 46 | 46 | 22 | 16 |
| Age (mean) | 46 | 69 | 49 | 63 | 49 |
| Sex (Male/Female) * | 90/66 | 30/16 | 30/16 | 7/15 | 12/4 |
| Smoking status * (current/former/never) | 20/27/109 | 10/22/14 | 3/10/33 | 4/7/11 | 3/4/9 |
| DLCO (% pred) † | 87 (15) | 53 (12) | 62 (16) | 58 (15) | 94 (21) |
| Fibrotic stage (Yes/No) * | 7/149 | 46/0 | 21/25 | 18/4 | 0/16 |
| BALF—cell count/μL ‡ | 120 (84) | 102 (82) | 319 (238) | 110 (122) | 79 (67) |
| BALF—Mac (%) ‡ | 58 (32) | 75 (18) | 28 (25) | 61 (31) | 87 (9) |
| BALF—Neu (%) ‡ | 3 (6) | 10 (17) | 5 (7) | 6 (11) | 4 (8) |
| BALF—Ly (%) ‡ | 36 (31) | 7 (6) | 61 (34) | 21 (20) | 7 (6) |
| BALF—CD3 (%) ‡ | 93 (4) | 87 (9) | 92 (6) | 88 (11) | 81 (10) |
| BALF—CD4 (%) ‡ | 72 (21) | 49 (23) | 42 (37) | 43 (36) | 49 (19) |
| BALF—CD8 (%) ‡ | 19 (19) | 36 (22) | 43 (41) | 42 (37) | 33 (12) |
| BALF—CD4/CD8 ‡ | 3.7 (5.0) | 1.3 (1.5) | 1.0 (4.6) | 1.0 (1.7) | 1.3 (1.1) |
| Model (1) | Model (2) | |
|---|---|---|
| Fibrotic process (IPF + OFI) | ||
| CX3CL1 | 0.000762 *** | 0.000586 *** |
| (p = 0.000) | (p = 0.000) | |
| Age | 0.120 *** | |
| (p = 0.000) | ||
| Sex | 0.694 | |
| (p = 0.083) | ||
| Ex-smoker | 0.221 | |
| (p = 0.617) | ||
| Current smoker | 0.490 | |
| (p = 0.404) | ||
| N | 285 | 273 |
| pseudo R2 | 0.179 | 0.404 |
| CX3CL1 (pg/mL) | ||
|---|---|---|
| r * | p Value | |
| DLCO % pred | −0.45475 | <0.0001 |
| BALF—cell count/μL | 0.20252 | 0.0003 |
| BALF—Mac (%) | −0.05081 | 0.3718 |
| BALF—Mac (abs) | 0.17195 | 0.0023 |
| BALF—Neu (%) | 0.27287 | <0.0001 |
| BALF—Neu (abs) | 0.35674 | <0.0001 |
| BALF—Ly (%) | −0.13920 | 0.0140 |
| BALF—Ly (abs) | −0.01664 | 0.7704 |
| BALF—CD3 (%) | −0.14968 | 0.0082 |
| BALF—CD3 (abs) | −0.01713 | 0.7635 |
| BALF—CD4 (%) | −0.35272 | <0.0001 |
| BALF—CD4 (abs) | −0.11397 | 0.0446 |
| BALF—CD8 (%) | 0.34571 | <0.0001 |
| BALF—CD8 (abs) | 0.14061 | 0.0131 |
| BALF—CD4/CD8 | −0.35010 | <0.0001 |
| ADAM17 (pg/mL) | ||
|---|---|---|
| r * | p Value | |
| DLCO % pred | −0.00945 | 0.8904 |
| BALF—cell count/μL | 0.49927 | <0.0001 |
| BALF—Mac (%) | −0.29936 | <0.0001 |
| BALF—Mac (abs) | 0.29944 | <0.0001 |
| BALF—Neu (%) | −0.14157 | 0.0354 |
| BALF—Neu (abs) | 0.13762 | 0.0405 |
| BALF—Ly (%) | 0.39393 | <0.0001 |
| BALF—Ly (abs) | 0.51110 | <0.0001 |
| BALF—CD3 (%) | 0.23604 | 0.0004 |
| BALF—CD3 (abs) | 0.51137 | <0.001 |
| BALF—CD4 (%) | 0.01233 | 0.8550 |
| BALF—CD4 (abs) | 0.47316 | <0.0001 |
| BALF—CD8 (%) | 0.02179 | 0.7468 |
| BALF—CD8 (abs) | 0.48314 | <0.0001 |
| BALF—CD4/CD8 | −0.01211 | 0.8576 |
| Model (1) | Model (2) | |
|---|---|---|
| IPF | ||
| CX3CL1/ADAM17 | 0.0119 *** | 0.0109 * |
| (p = 0.000) | (p = 0.017) | |
| Age | 0.220 *** | |
| (p = 0.000) | ||
| Sex | 0.690 | |
| (p = 0.419) | ||
| Ex-smoker | 1.826 * | |
| (p = 0.045) | ||
| Current smoker | 5.141 ** | |
| (p = 0.002) | ||
| N | 199 | 191 |
| pseudo R2 | 0.218 | 0.635 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urban, J.; Suchankova, M.; Ganovska, M.; Leksa, V.; Sandor, F.; Tedlova, E.; Konig, B.; Bucova, M. The Role of CX3CL1 and ADAM17 in Pathogenesis of Diffuse Parenchymal Lung Diseases. Diagnostics 2021, 11, 1074. https://doi.org/10.3390/diagnostics11061074
Urban J, Suchankova M, Ganovska M, Leksa V, Sandor F, Tedlova E, Konig B, Bucova M. The Role of CX3CL1 and ADAM17 in Pathogenesis of Diffuse Parenchymal Lung Diseases. Diagnostics. 2021; 11(6):1074. https://doi.org/10.3390/diagnostics11061074
Chicago/Turabian StyleUrban, Jan, Magda Suchankova, Martina Ganovska, Vladimir Leksa, Frantisek Sandor, Eva Tedlova, Brian Konig, and Maria Bucova. 2021. "The Role of CX3CL1 and ADAM17 in Pathogenesis of Diffuse Parenchymal Lung Diseases" Diagnostics 11, no. 6: 1074. https://doi.org/10.3390/diagnostics11061074
APA StyleUrban, J., Suchankova, M., Ganovska, M., Leksa, V., Sandor, F., Tedlova, E., Konig, B., & Bucova, M. (2021). The Role of CX3CL1 and ADAM17 in Pathogenesis of Diffuse Parenchymal Lung Diseases. Diagnostics, 11(6), 1074. https://doi.org/10.3390/diagnostics11061074





