Influencing Factors on the Oncuria™ Urinalysis Assay: An Experimental Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Sampling and Processing
2.2. Cell Lines and Culture
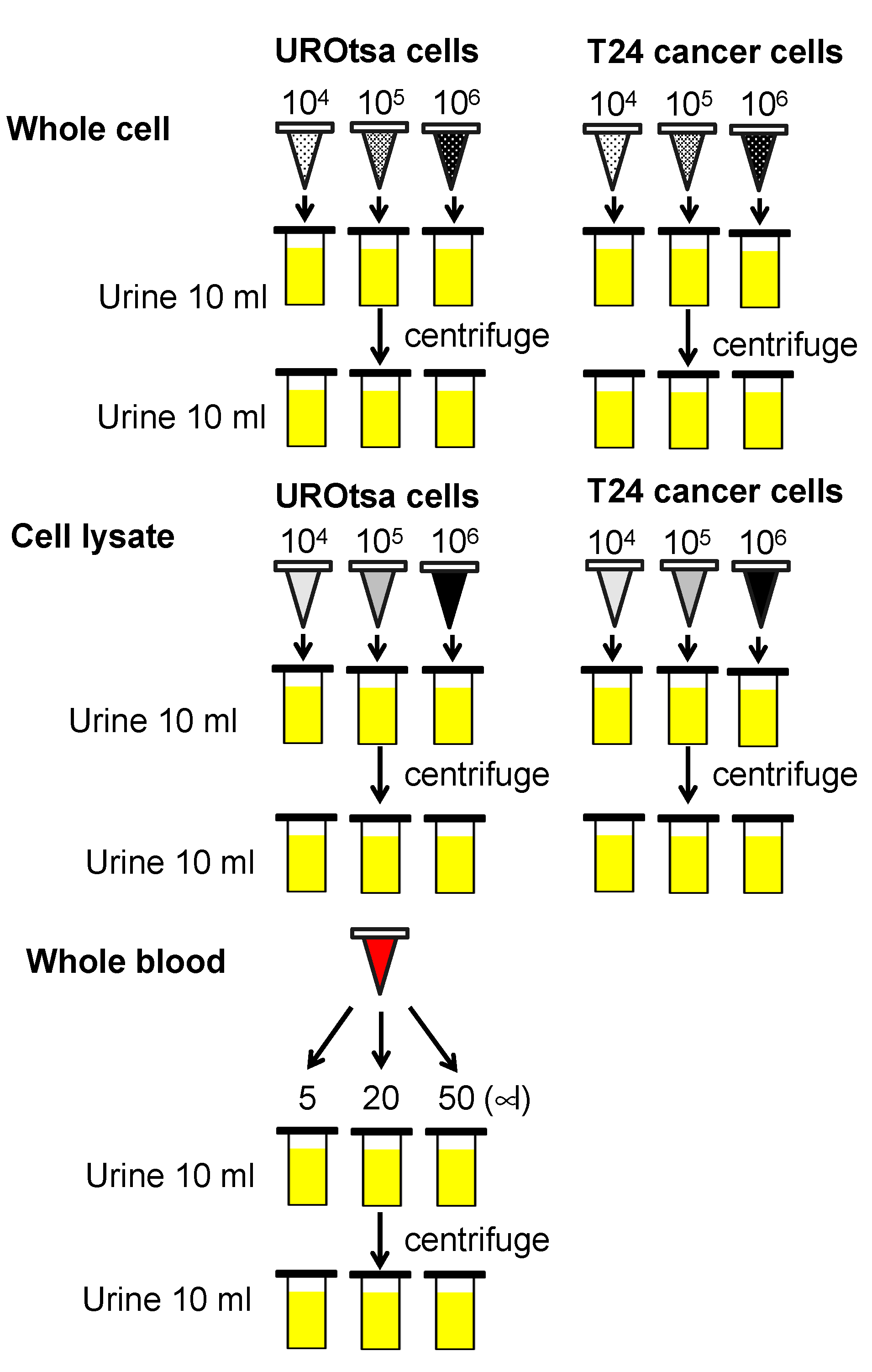
2.3. Experimental Model
2.4. Multiplex Immunoassay
2.5. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rife, C.C.; Farrow, G.M.; Utz, D.C. Urine cytology of transitional cell neoplasms. Urol. Clin. N. Am. 1979, 6, 599–612. [Google Scholar]
- Van Rhijn, B.W.; van der Poel, H.G.; van der Kwast, T.H. Urine Markers for Bladder Cancer Surveillance: A Systematic Review. Eur. Urol. 2005, 47, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Heicappell, R.; Wettig, I.C.; Schostak, M.; Müller, M.; Steiner, U.; Sauter, T.; Miller, K. Quantitative detection of human complement factor H-related protein in transitional cell carcinoma of the urinary bladder. Eur. Urol. 1999, 35, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Leyh, H.; Marberger, M.; Bombardieri, E.; Bassi, P.; Pagano, F.; Pansadoro, V.; Sternberg, C.N.; Boccon-Gibod, L.; Ravery, V.; et al. Multicenter trial of the quantitative BTA TRAK assay in the detection of bladder cancer. Clin. Chem. 1999, 45, 472–477. [Google Scholar] [PubMed]
- Soloway, M.S.; Briggman, J.V.; Carpinito, G.A.; Chodak, G.W.; Church, P.A.; Lamm, D.L.; Lange, P.; Messing, E.; Pasciak, R.M.; Reservitz, G.B.; et al. Use of a New Tumor Marker, Urinary NMP22, in the Detection of Occult or Rapidly Recurring Transitional Cell Carcinoma of the Urinary Tract Following Surgical Treatment. J. Urol. 1996, 156, 363–367. [Google Scholar] [CrossRef]
- Grossman, H.B.; Messing, E.; Soloway, M.; Tomera, K.; Katz, G.; Berger, Y.; Shen, Y. Detection of Bladder Cancer Using a Point-of-Care Proteomic Assay. JAMA 2005, 293, 810–816. [Google Scholar] [CrossRef]
- Hajdinjak, T. UroVysion FISH test for detecting urothelial cancers: Meta-analysis of diagnostic accuracy and comparison with urinary cytology testing. Urol. Oncol. Semin. Orig. Investig. 2008, 26, 646–651. [Google Scholar] [CrossRef]
- Li, H.-X.; Li, M.; Li, C.-L.; Ma, J.-H.; Wang, M.-R.; Rao, J.; Pan, Q.-J. ImmunoCyt and cytokeratin 20 immunocytochemistry as adjunct markers for urine cytologic detection of bladder cancer: A prospective study. Anal. Quant. Cytol. Histol. 2010, 32, 45–52. [Google Scholar]
- Miyake, M.; Goodison, S.; Giacoia, E.G.; Rizwani, W.; Ross, S.; Rosser, C.J. Influencing factors on the NMP-22 urine assay: An experimental model. BMC Urol. 2012, 12, 23. [Google Scholar] [CrossRef]
- Miyake, M.; Goodison, S.; Rizwani, W.; Ross, S.J.; Grossman, H.B.; Rosser, C.J. Urinary BTA: Indicator of bladder cancer or of hematuria. World J. Urol. 2012, 30, 869–873. [Google Scholar] [CrossRef]
- PR Newswire. Global Urothelial Carcinoma Market Insights Epidemiology and Market Forecasts Report 2019–2028. Available online: https://www.prnewswire.com/news-releases/global-urothelial-carcinoma-market-insights-epidemiology-and-market-forecasts-report-2019-2028-300955460.html (accessed on 5 March 2021).
- Faiena, I.; Rosser, C.J.; Chamie, K.; Furuya, H. Diagnostic biomarkers in non-muscle invasive bladder cancer. World J. Urol. 2019, 37, 2009–2016. [Google Scholar] [CrossRef]
- Witjes, J.A.; Morote, J.; Cornel, E.B.; Gakis, G.; van Valenberg, F.J.P.; Lozano, F.; Sternberg, I.A.; Willemsen, E.; Hegemann, M.L.; Paitan, Y.; et al. Performance of the Bladder EpiCheck™ Methylation Test for Patients Under Surveillance for Non–muscle-invasive Bladder Cancer: Results of a Multicenter, Prospective, Blinded Clinical Trial. Eur. Urol. Oncol. 2018, 1, 307–313. [Google Scholar] [CrossRef]
- Wolfs, J.R.E.; Hermans, T.J.N.; Koldewijn, E.L.; van de Kerkhof, D. Novel urinary biomarkers ADXBLADDER and bladder EpiCheck for diagnostics of bladder cancer: A review. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 161–170. [Google Scholar] [CrossRef]
- Schutte, E.; Gansevoort, R.T.; Benner, J.; Lutgers, H.L.; Heerspink, H.J.L. Will the future lie in multitude? A critical appraisal of biomarker panel studies on prediction of diabetic kidney disease progression. Nephrol. Dial. Transplant. 2015, 30, iv96–iv104. [Google Scholar] [CrossRef] [PubMed]
- Mahnert, B.; Tauber, S.; Kriegmair, M.; Nagel, D.; Holdenrieder, S.; Hofmann, K.; Reiter, W.; Schmeller, N.; Stieber, P. Measurements of Complement Factor H-Related Protein (BTA-TRAK™ Assay) and Nuclear Matrix Protein (NMP22 Assay)—Useful Diagnostic Tools in the Diagnosis of Urinary Bladder Cancer? Clin. Chem. Lab. Med. 2003, 41, 104–110. [Google Scholar] [CrossRef][Green Version]
- Yang, N.; Feng, S.; Shedden, K.; Xie, X.; Liu, Y.; Rosser, C.J.; Lubman, D.M.; Goodison, S. Urinary Glycoprotein Biomarker Discovery for Bladder Cancer Detection Using LC/MS-MS and Label-Free Quantification. Clin. Cancer Res. 2011, 17, 3349–3359. [Google Scholar] [CrossRef] [PubMed]
- Kreunin, P.; Zhao, J.; Rosser, C.; Urquidi, V.; Lubman, D.M.; Goodison, S. Bladder Cancer Associated Glycoprotein Signatures Revealed by Urinary Proteomic Profiling. J. Proteome Res. 2007, 6, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Rosser, C.J.; Liu, L.; Sun, Y.; Villicana, P.; McCullers, M.; Porvasnik, S.; Young, P.R.; Parker, A.S.; Goodison, S. Bladder Cancer–Associated Gene Expression Signatures Identified by Profiling of Exfoliated Urothelia. Cancer Epidemiol. Biomark. Prev. 2009, 18, 444–453. [Google Scholar] [CrossRef]
- Urquidi, V.; Goodison, S.; Cai, Y.; Sun, Y.; Rosser, C.J. A Candidate Molecular Biomarker Panel for the Detection of Bladder Cancer. Cancer Epidemiol. Biomark. Prev. 2012, 21, 2149–2158. [Google Scholar] [CrossRef]
- Goodison, S.; Chang, M.; Dai, Y.; Urquidi, V.; Rosser, C.J. A Multi-Analyte Assay for the Non-Invasive Detection of Bladder Cancer. PLoS ONE 2012, 7, e47469. [Google Scholar] [CrossRef]
- Rosser, C.J.; Ross, S.; Chang, M.; Dai, Y.; Mengual, L.; Zhang, G.; Kim, J.; Urquidi, V.; Alcaraz, A.; Goodison, S. Multiplex Protein Signature for the Detection of Bladder Cancer in Voided Urine Samples. J. Urol. 2013, 190, 2257–2262. [Google Scholar] [CrossRef]
- Chen, L.M.; Chang, M.; Dai, Y.; Chai, K.X.; Dyrskjot, L.; Sanchez-Carbayo, M.; Szarvas, T.; Zwarthoff, E.C.; Lokeshwar, V.; Jeronimo, C.; et al. External Validation of a Multiplex Urinary Protein Panel for the Detection of Bladder Cancer in a Multicenter Cohort. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Rosser, C.J.; Chang, M.; Dai, Y.; Ross, S.; Mengual, L.; Alcaraz, A.; Goodison, S. Urinary Protein Biomarker Panel for the Detection of Recurrent Bladder Cancer. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Goodison, S.; Ogawa, O.; Matsui, Y.; Kobayashi, T.; Miyake, M.; Ohnishi, S.; Fujimoto, K.; Dai, Y.; Shimizu, Y.; Tsukikawa, K.; et al. A multiplex urinary immunoassay for bladder cancer detection: Analysis of a Japanese cohort. J. Transl. Med. 2016, 14, 287. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Furuya, H.; Greenwood, P.B.; Chan, O.; Dai, Y.; Thornquist, M.D.; Goodison, S.; Rosser, C.J. A multiplex immunoassay for the non-invasive detection of bladder cancer. J. Transl. Med. 2016, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Kou, L.; Furuya, H.; Yu, C.; Goodison, S.; Kattan, M.; Garmire, L.; Rosser, C.J. A Nomogram Derived by Combination of Demographic and Biomarker Data Improves the Noninvasive Evaluation of Patients at Risk for Bladder Cancer. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1361–1366. [Google Scholar] [CrossRef]
- Masuda, N.; Ogawa, O.; Park, M.; Liu, A.Y.; Goodison, S.; Dai, Y.; Kozai, L.; Furuya, H.; Lotan, Y.; Rosser, C.J.; et al. Meta-analysis of a 10-plex urine-based biomarker assay for the detection of bladder cancer. Oncotarget 2018, 9, 7101–7111. [Google Scholar] [CrossRef]
- Furuya, H.; Tabula, L.; Lee, R.; Kralovec, P.; Ramsden, M.; Wong, R.; Rosser, C.J. Analytical validation of ONCURIA™ a multiplex bead-based immunoassay for the non-invasive bladder cancer detection. Pract. Lab. Med. 2020, 22, e00189. [Google Scholar] [CrossRef]
- Furuya, H.; Pagano, I.; Chee, K.; Kobayashi, T.; Wong, R.S.; Lee, R.; Rosser, C.J. Comparison of Commercial ELISA Kits, a Prototype Multiplex Electrochemoluminescent Assay, and a Multiplex Bead-Based Immunoassay for Detecting a Urine-Based Bladder-Cancer-Associated Diagnostic Signature. Diagnostics 2019, 9, 166. [Google Scholar] [CrossRef]
- Motulsky, H.; Christopoulos, A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Hirasawa, Y.; Pagano, I.; Chen, R.; Sun, Y.; Dai, Y.; Gupta, A.; Tikhonenkov, S.; Goodison, S.; Rosser, C.J.; Furuya, H. Diagnostic performance of Oncuria™, a urinalysis test for bladder cancer. J. Transl. Med. 2021, 19, 1–10. [Google Scholar] [CrossRef]
- Atsü, N.; Ekici, S.; Öge, Ö.; Ergen, A.; Hasçelik, G.; Özen, H. False-Positive Results of the NMP22 Test Due to Hematuria. J. Urol. 2002, 167, 555–558. [Google Scholar] [CrossRef]
- Öge, Ö.; Kozaci, D.; Gemalmaz, H. The bta stat test is nonspecific for hematuria: An experimental hematuria model. J. Urol. 2002, 167, 1318–1320. [Google Scholar] [CrossRef]
- Miyake, M.; Ross, S.; Lawton, A.; Chang, M.; Dai, Y.; Mengual, L.; Alcaraz, A.; Giacoia, E.G.; Goodison, S.; Rosser, C.J. Investigation of CCL18 and A1AT as potential urinary biomarkers for bladder cancer detection. BMC Urol. 2013, 13, 42. [Google Scholar] [CrossRef]
- Miyake, M.; Lawton, A.; Dai, Y.; Chang, M.; Mengual, L.; Alcaraz, A.; Goodison, S.; Rosser, C.J. Clinical implications in the shift of syndecan-1 expression from the cell membrane to the cytoplasm in bladder cancer. BMC Cancer 2014, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Chan, O.T.; Furuya, H.; Pagano, I.; Shimizu, Y.; Hokutan, K.; Dyrskjøt, L.; Jensen, J.B.; Malmstrom, P.-U.; Segersten, U.; Janku, F.; et al. Association of MMP-2, RB and PAI-1 with decreased recurrence-free survival and overall survival in bladder cancer patients. Oncotarget 2017, 8, 99707–99721. [Google Scholar] [CrossRef]
- Furuya, H.; Chan, O.T.; Hokutan, K.; Tsukikawa, Y.; Chee, K.; Kozai, L.; Chan, K.S.; Dai, Y.; Wong, R.S.; Rosser, C.J. Prognostic Significance of Lymphocyte Infiltration and a Stromal Immunostaining of a Bladder Cancer Associated Diagnostic Panel in Urothelial Carcinoma. Diagnostics 2019, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Gomes-Giacoia, E.; Dai, Y.; Lawton, A.; Miyake, M.; Furuya, H.; Goodison, S.; Rosser, C.J. Validation and clinicopathologic associations of a urine-based bladder cancer biomarker signature. Diagn. Pathol. 2014, 9, 1–10. [Google Scholar] [CrossRef]
| Condition | Spike | Centrifuge | Level | Category | MMP9 | CXCL8_IL8 | VEGFA | IX_CA9 | SDC1 | PAI1 | APOE | A1AT | ANG | MMP10 | Risk Scorre |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pooled Urine | 16.7 | 5.62 | 38.2 | 0.629 | 7818 | 6.83 | 516.4 | 13,139 | 49.5 | 3.8 | 12.8 | |||
| 2 | Blood | No | Low | 26.2 ^ | 6.07 | 36.9 | 0.659 | 7704 | 6.49 | 801.7 | 25,052 | 43.1 | 9.1 | 18.8 | |
| 3 | Blood | No | Med | 42.1 ^ | 5.82 | 38.9 | 0.523 | 7907 | 7.96 | 1449.7 ^ | 52,793 | 62.0 | 10.8 | 28.6 | |
| 4 | Blood | No | High | 77.8 ^ | 5.75 | 47.9 # | 0.546 | 8556 | 13.5 ^ | 3610.1 ^ | 215,761.2 # | 139.6 ^ | 11.9 * | 51.7 | |
| 5 | Blood | Yes | Low | 25.2 ^ | 5.49 | 31.4 ~ | 0.318 | 7991 | 4.81 | 725.3 | 25,554 | 28.5 | 7.0 | 10.8 | |
| 6 | Blood | Yes | Med | 43.1 ^ | 5.58 | 32.4 * | 0.477 | 7560 | 4.70 | 1083.6 ~ | 58,279 | 26.5 * | 13.9 * | 17.5 | |
| 7 | Blood | Yes | High | 69.9 ^ | 5.57 | 33.5 | 0.318 | 8324 | 6.21 | 2387.7 ^ | 127,200.2 * | 34.0 | 10.0 | 28 | |
| 8 | Cell Lysate | No | Low | Benign | 20.3 * | 5.84 | 44.7 * | 1.023 | 7218 | 7.56 | 892.2 ~ | 15,936 | 88.5 ~ | 17.6 ~ | 29.6 |
| 9 | Cell Lysate | No | Med | Benign | 18.9 | 5.56 | 40.3 | 0.432 | 6972 | 7.72 | 904.6 ~ | 15,544 | 110.7 ^ | 86.6 ^ | 27.4 |
| 10 | Cell Lysate | No | High | Benign | 16.4 | 5.65 | 42.6 | 1.023 | 7350 | 8.06 | 958.9 ~ | 16,179 | 104.9 # | 469.9 ^ | 37.6 |
| 14 | Cell Lysate | Yes | Low | Benign | 19.4 * | 5.72 | 43.6 * | 0.864 | 7503 | 8.31 | 870.6 ~ | 16,263.8 * | 122.8 ^ | 25.4 ~ | 29 |
| 15 | Cell Lysate | Yes | Med | Benign | 19.9 * | 5.74 | 44.8 * | 0.796 | 7138 | 7.84 | 839.3 ~ | 16,500.6 * | 123.5 ^ | 123.4 ^ | 30.3 |
| 16 | Cell Lysate | Yes | High | Benign | 18.4 | 5.73 | 42.9 | 0.455 | 7606 | 8.68 | 931.7 ~ | 162,96.6 * | 131.6 ^ | 1169.0 ^ | 31.7 |
| 11 | Cell Lysate | No | Low | Cancer | 20.3 * | 5.39 | 40.9 | 0.568 | 7837 | 8.14 | 887.1 ~ | 16,845.5* | 88.5 ~ | 10.9 | 23.4 |
| 12 | Cell Lysate | No | Med | Cancer | 20.6 ~ | 5.49 | 41.3 | 1.9 * | 7566 | 8.45 | 921.5 ~ | 15,523 | 95.6 # | 15.5 ~ | 37 |
| 13 | Cell Lysate | No | High | Cancer | 19.9 * | 5.94 | 43.2 | 13.9 ^ | 7098 | 10.8 ~ | 864.9 ~ | 14,751 | 92.4 # | 15.3 ~ | 61.4 |
| 17 | Cell Lysate | Yes | Low | Cancer | 21.5 # | 5.23 | 63.3 ^ | 1.652 | 10,358.7 * | 7.70 | 690.1 | 15,801 | 107.9 # | 33.6 # | 19.4 |
| 18 | Cell Lysate | Yes | Med | Cancer | 20.0 ~ | 5.49 | 40.4 | 2.5 ~ | 7504 | 8.35 | 852.1 ~ | 15,237 | 96.4 ~ | 11.4 | 37.8 |
| 19 | Cell Lysate | Yes | High | Cancer | 18.0 | 5.53 | 41.1 | 11.9^ | 7488 | 11.1 # | 824.0 * | 15,701 | 84.5 * | 14.8 | 56.8 |
| 20 | Whole Cell | No | Low | Benign | 19.1 * | 5.38 | 41.9 | 0.546 | 7119 | 7.49 | 828.0 ~ | 14,936 | 91.9 ~ | 20.7 * | 24 |
| 21 | Whole Cell | No | Med | Benign | 20.0 ~ | 5.49 | 41.7 | 1.251 | 7436 | 7.87 | 927.5 ~ | 17,132 | 101.5 # | 56.5 ^ | 34.5 |
| 22 | Whole Cell | No | High | Benign | 19.4 * | 5.81 | 46.4 ~ | 0.591 | 7429 | 8.58 | 983.9 # | 16,529 | 122.8 ^ | 666.9 ^ | 34.1 |
| 26 | Whole Cell | Yes | Low | Benign | 18.2 | 5.58 | 42.1 | 0.364 | 7832 | 8.17 | 808.4 ~ | 16,903. 8 * | 116.6 ^ | 16.6 | 19.2 |
| 27 | Whole Cell | Yes | Med | Benign | 20.0 ~ | 5.53 | 43.8 * | 0.637 | 7129 | 7.71 | 820.1 ~ | 15,723 | 112.5 ^ | 70.5 ^ | 27.1 |
| 28 | Whole Cell | Yes | High | Benign | 19.6 * | 5.64 | 43.2 | 1.387 | 7392 | 8.08 | 829.4 ~ | 15,956 | 123.7 ^ | 837.6 ^ | 38.6 |
| 23 | Whole Cell | No | Low | Cancer | 24.0 ^ | 5.79 | 71.7 ^ | 1.538 | 11,688.9 # | 9.0 * | 796.8 * | 16,460 | 137.2 ^ | 23.3 * | 19.9 |
| 24 | Whole Cell | No | Med | Cancer | 25.1 ^ | 6.17 | 71.9 ^ | 2.922 | 11,278.3 ~ | 8.89 | 790.2 * | 16,320 | 115.7 ^ | 24.5 ~ | 24.9 |
| 25 | Whole Cell | No | High | Cancer | 18.3 | 5.12 | 56.7 ^ | 5.9 ^ | 10,854.3 ~ | 10.8 # | 734.7 | 12,662 | 122.9 ^ | 24.6 ~ | 35.4 |
| 29 | Whole Cell | Yes | Low | Cancer | 21.2 # | 5.81 | 65.5 ^ | 2.238 | 9888 | 6.09 | 551.5 | 15,600 | 70.0 | 28.2~ | 15.5 |
| 30 | Whole Cell | Yes | Med | Cancer | 17.5 | 4.80 | 51.8 ^ | 1.954 | 9533 | 5.25 | 519.2 | 14,850 | 47.6 | 26.3 * | 13.9 |
| 31 | Whole Cell | Yes | High | Cancer | 18.2 | 5.39 | 49.9 # | 6.6 ^ | 9589 | 4.74 | 459.4 | 14,629 | 32.9 | 28.9 ~ | 17.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murakami, K.; Pagano, I.; Chen, R.; Sun, Y.; Goodison, S.; Rosser, C.J.; Furuya, H. Influencing Factors on the Oncuria™ Urinalysis Assay: An Experimental Model. Diagnostics 2021, 11, 1023. https://doi.org/10.3390/diagnostics11061023
Murakami K, Pagano I, Chen R, Sun Y, Goodison S, Rosser CJ, Furuya H. Influencing Factors on the Oncuria™ Urinalysis Assay: An Experimental Model. Diagnostics. 2021; 11(6):1023. https://doi.org/10.3390/diagnostics11061023
Chicago/Turabian StyleMurakami, Kaoru, Ian Pagano, Runpu Chen, Yijun Sun, Steve Goodison, Charles J. Rosser, and Hideki Furuya. 2021. "Influencing Factors on the Oncuria™ Urinalysis Assay: An Experimental Model" Diagnostics 11, no. 6: 1023. https://doi.org/10.3390/diagnostics11061023
APA StyleMurakami, K., Pagano, I., Chen, R., Sun, Y., Goodison, S., Rosser, C. J., & Furuya, H. (2021). Influencing Factors on the Oncuria™ Urinalysis Assay: An Experimental Model. Diagnostics, 11(6), 1023. https://doi.org/10.3390/diagnostics11061023







