Predicting Polysomnography Parameters from Anthropometric Features and Breathing Sounds Recorded during Wakefulness
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Procedure and Measurements
Tracheal Breathing Sounds
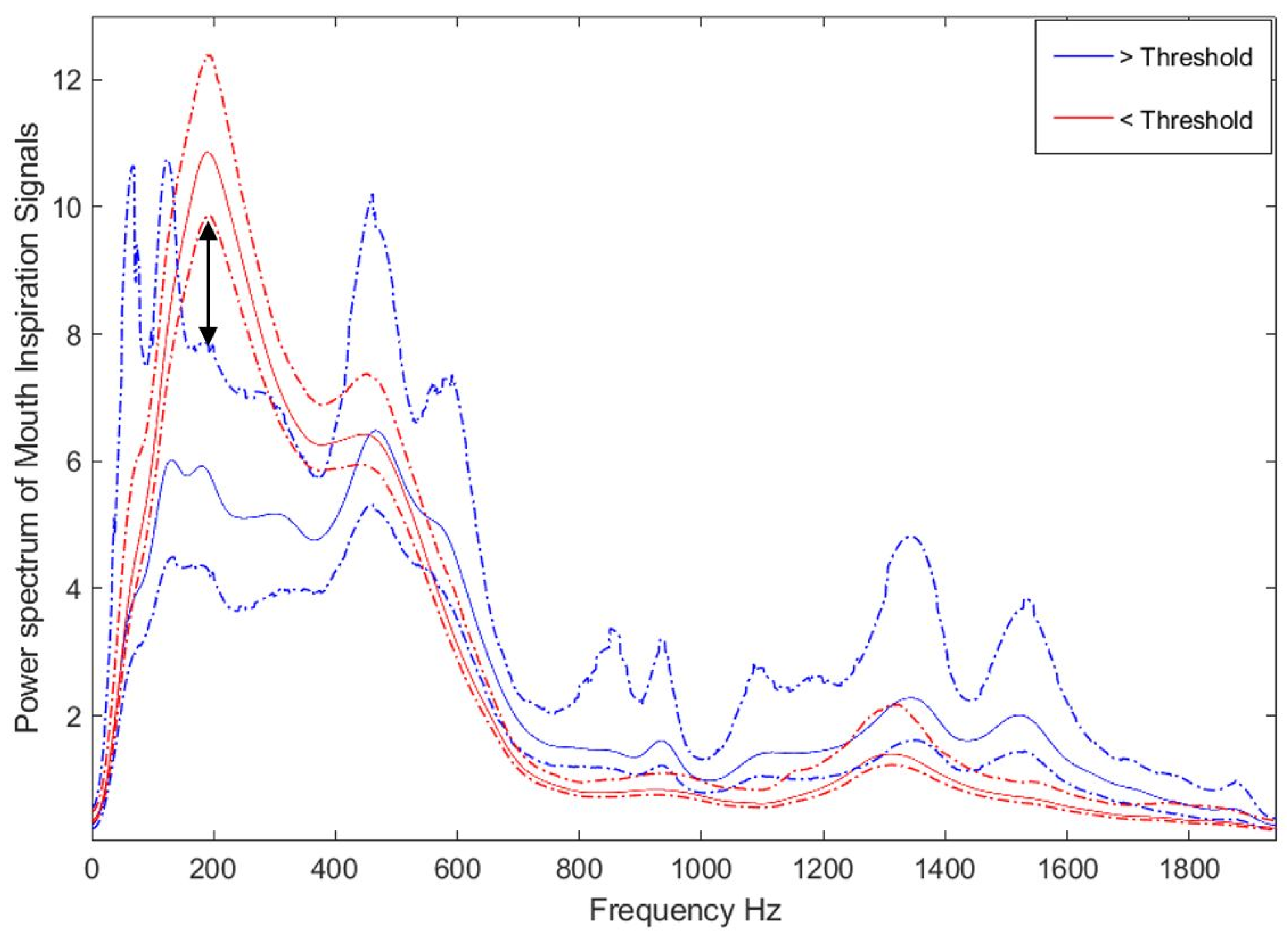
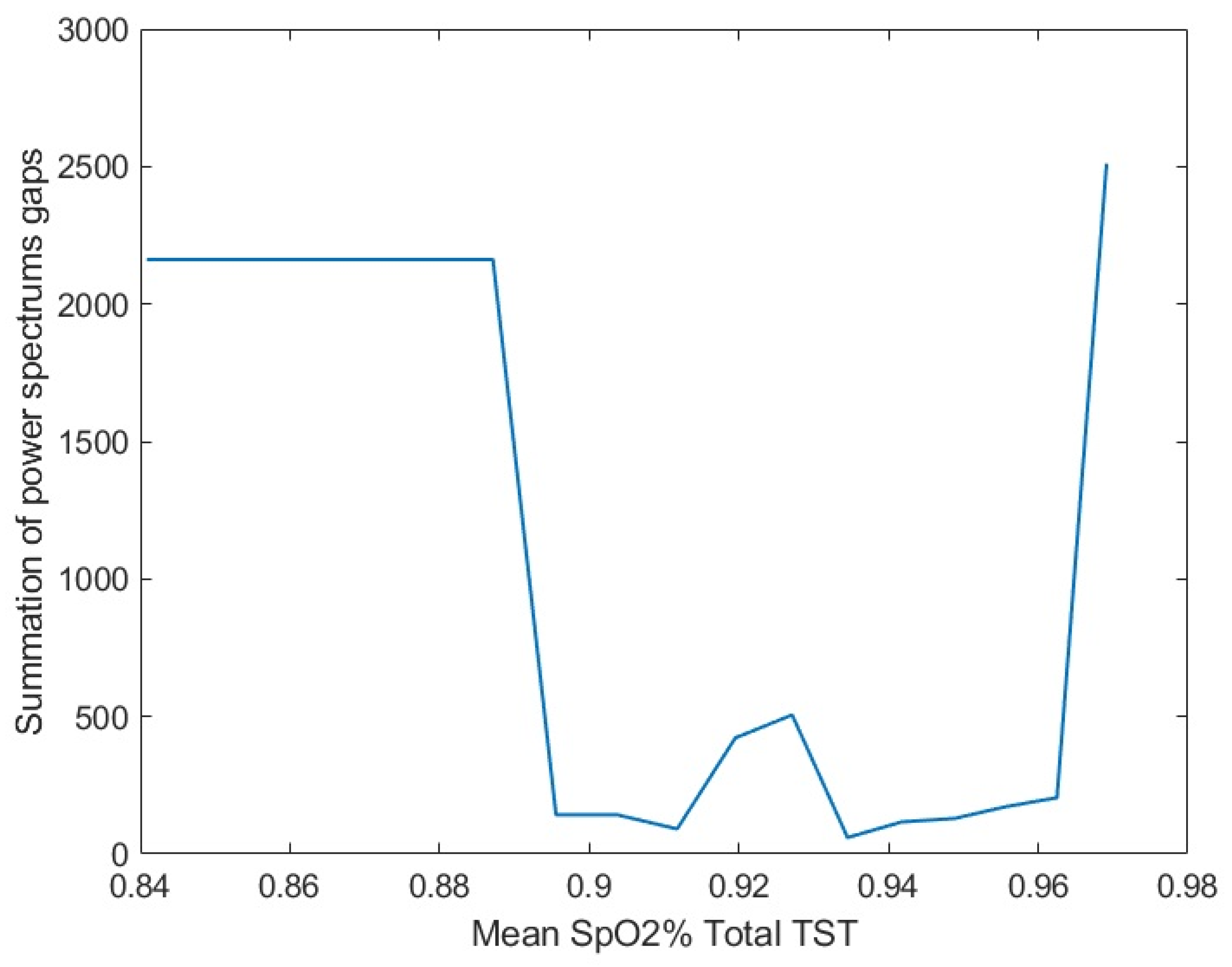
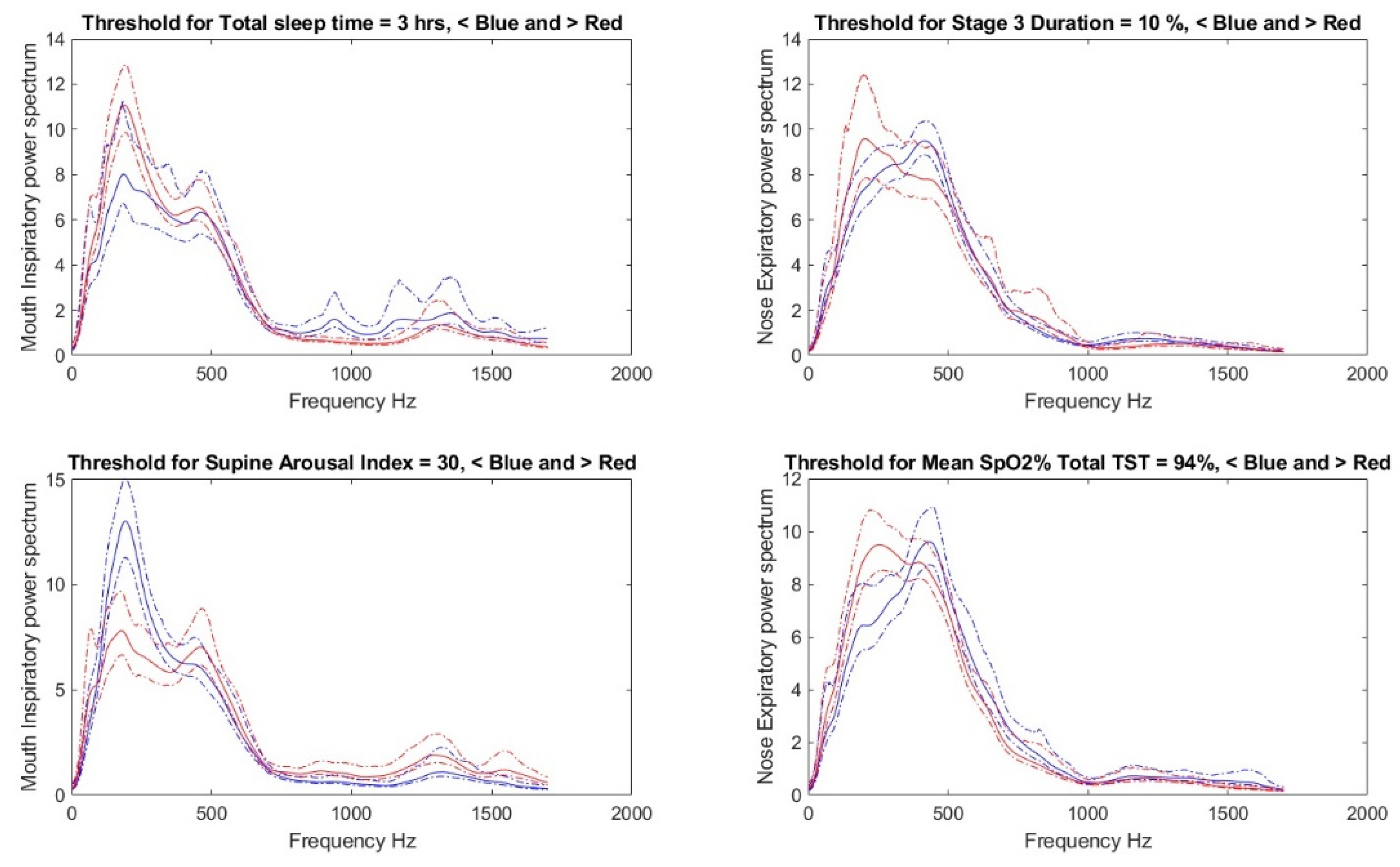
2.3. Threshold Determination, Data Preparation, and Feature Selection
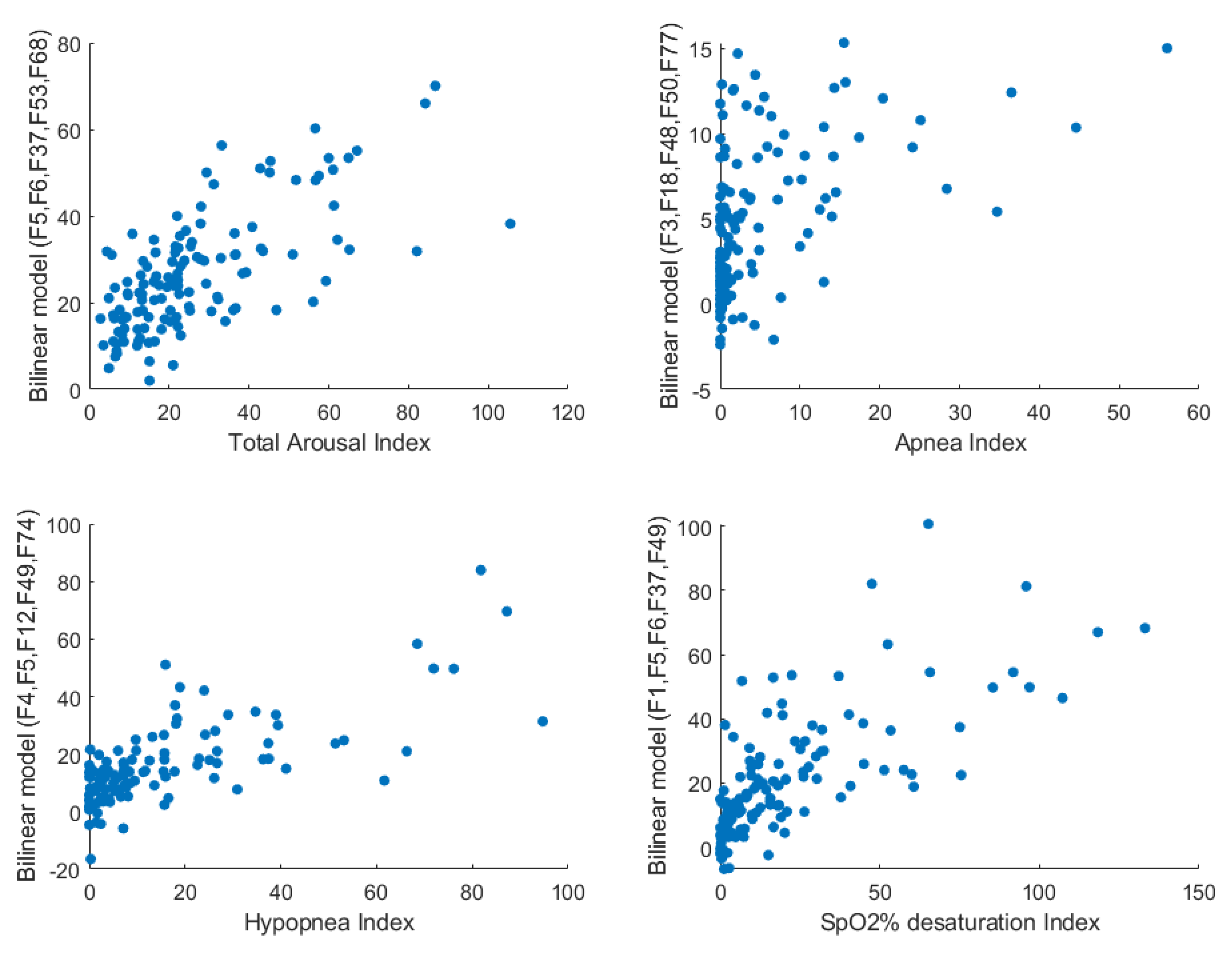
2.4. Bilinear Modeling and Correlation Analysis
2.5. Classification and Prediction of PSG Parameters
3. Results
4. Discussion
Limitations and Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| PSG Parameter |
|---|
| Total sleep time TST |
| Sleep Efficiency |
| Total Awakenings |
| Total Awakenings Index |
| Stage 1 Duration % |
| Stage 2 Duration % |
| Stage 3 Duration % |
| Stage 4 Duration % |
| REM Duration % |
| Respiratory-Related Total Arousal Index |
| PLM-Related Total Arousal Index |
| EEG Total Arousal Index |
| Total Arousal Index |
| NREM Total Arousal Index |
| REM Arousal Index |
| Supine Arousal Index |
| Non-Supine Arousal Index |
| Total Arousal + Awakenings Index |
| Supine Sleep % |
| Prone Sleep % |
| Left-Side Sleep % |
| Right-Side Sleep |
| Apnea Total Index |
| Apnea REM Index |
| Apnea Non-REM Index |
| Apnea Total Index (No Central) |
| Apnea REM Index (No Central) |
| Apnea Non-REM Index (No Central) |
| Hypopnea Total Index |
| Hypopnea REM Index |
| Hypopnea Non-REM Index |
| Hypopnea Total Index (No Central) |
| Hypopnea REM Index (No Central) |
| Hypopnea Non-REM Index (No Central) |
| Supine Sleep Obs. Apnea |
| Supine Sleep Mix. Apnea |
| Supine Sleep Cen. Apnea |
| Supine Sleep Obs. Hypopnea |
| Supine Sleep A+H (with central) |
| Supine Sleep A+H (without central) |
| Prone Sleep Obs. Apnea |
| Prone Sleep Mix. Apnea |
| Prone Sleep Cen. Apnea |
| Prone Sleep Obs. Hypopnea |
| Prone Sleep A+H (with central) |
| Prone Sleep A+H (without central) |
| Left-side Sleep Obs. Apnea |
| Left-side Sleep Mix. Apnea |
| Left-side Sleep Cen. Apnea |
| Left-side Sleep Obs. Hypopnea |
| Left-side Sleep A+H (with central) |
| Left-side Sleep A+H (without central) |
| Right-side Sleep Obs. Apnea |
| Right-side Sleep Mix. Apnea |
| Right-side Sleep Cen. Apnea |
| Right-side Sleep Obs. Hypopnea |
| Right-side Sleep A+H (with central) |
| Right-side Sleep A+H (without central) |
| Total AHI (With Central) |
| Total AHI (Without Central) |
| De-SpO2 Index Total |
| De-SpO2 Index Non-REM |
| De-SpO2 Index REM |
| Mean SpO2% Wake |
| Mean SpO2% NREM |
| Mean SpO2% REM |
| Mean SpO2% Total TST |
| Mean SpO2% Supine |
| Mean SpO2% Non-Supine |
| SpO2% ≤ 85% percentage of TST |
| Sleep Onset |
| REM Latency |
| REM Latency Less wake |
| Wake after sleep Onset |
| Sleep Onset/TST |
| REM Latency/TST |
| REM Latency Less wake/TST |
| Wake after sleep Onset/TST |
| PSG Parameters | Threshold | VCA2_Total | VCA2_<15 | VCA2_>15 | TCA2_Total | TCA2_<15 | TCA2_>15 |
|---|---|---|---|---|---|---|---|
| Stage 3 Duration % | 10% | 85.71% | 85.00% | 84.44% | 75.00% | 83.33% | 25.00% |
| Total Arousal Index | 25 | 70.87% | 72.88% | 63.64% | 80.95% | 85.00% | 0.00% |
| Apnea Total Index | 1 | 79.46% | 75.00% | 84.09% | 82.61% | 81.82% | 100.00% |
| Apnea Non-REM Index | 1 | 83.33% | 86.57% | 78.72% | 90.00% | 92.59% | 66.67% |
| Hypopnea Total Index | 10 | 90.09% | 96.83% | 81.25% | 85.71% | 82.61% | 100.00% |
| Hypopnea Non-REM Index | 7.5 | 81.74% | 81.82% | 75.51% | 85.71% | 80.00% | 100.00% |
| Hypopnea Total Index (No Central) | 5 | 83.04% | 79.03% | 86.00% | 93.33% | 91.67% | 100.00% |
| Supine Sleep Obs. Apnea | 1 | 75.24% | 70.97% | 79.07% | 86.96% | 86.36% | 100.00% |
| Supine Sleep Obs. Hypopnea | 15 | 81.31% | 80.65% | 82.22% | 77.78% | 76.19% | 83.33% |
| Supine Sleep A+H (with central) | 15 | 86.67% | 86.89% | 86.36% | 88.89% | 86.96% | 100.00% |
| Total AHI (With Central) | 15 | 83.48% | 89.23% | 76.00% | 86.67% | 87.50% | 83.33% |
| Total AHI (Without Central) | 15 | 86.84% | 93.75% | 76.00% | 85.71% | 85.71% | 85.71% |
| De-SpO2 Index Total | 15 | 81.42% | 84.13% | 74.00% | 92.86% | 100.00% | 75.00% |
| Mean SpO2% Total TST | 94% | 69.09% | 63.93% | 75.51% | 72.00% | 68.42% | 83.33% |
| Mean SpO2% Supine | 94% | 81.40% | 78.00% | 80.56% | 81.25% | 91.67% | 50.00% |
| Mean SpO2% Non-Supine | 93% | 86.09% | 87.10% | 84.91% | 76.19% | 72.22% | 100.00% |
| Wake after sleep Onset/TST | 0.15 | 73.53% | 67.80% | 76.74% | 69.23% | 66.67% | 50.00% |
References
- American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual; American Academy of Sleep Medicine: Westchester, NY, USA, 2005. [Google Scholar]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Young, T.; Finn, L.; Peppard, P.E.; Szklo-Coxe, M.; Austin, D.; Nieto, F.J.; Stubbs, R.; Hla, K.M. Sleep disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008, 31, 1071–1078. [Google Scholar]
- Nagappa, M.; Liao, P.; Wong, J.; Auckley, D.; Ramachandran, S.K.; Memtsoudis, S.; Mokhlesi, B.; Chung, F. Validation of the STOP-Bang questionnaire as a screening tool for obstructive sleep apnea among different populations: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0143697. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, I.H. Comparison of four sleep questionnaires for screening obstructive sleep apnea. Egypt. J. Chest Dis. Tuberc. 2012, 61, 433–441. [Google Scholar] [CrossRef]
- Gross, J.B.; Apfelbaum, J.L.; Caplan, R.A.; Connis, R.T.; Cote, C.J.; Nickinovich, D.G.; Ward, D.S.; Weaver, E.M.; Ydens, L. Practice guidelines for the perioperative management of patients with obstructive sleep apnea an updated report by the American society of anesthesiologists task force on perioperative management of patients with obstructive sleep apnea. Anesthesiology 2014, 120, 268–286. [Google Scholar]
- Elwali, A.; Meza-Vargas, S.; Moussavi, Z. Using tracheal breathing sounds and anthropometric information for screening obstructive sleep apnoea during wakefulness. J. Med. Eng. Technol. 2019, 43, 111–123. [Google Scholar] [CrossRef]
- Elwali, A.; Moussavi, Z. A novel Decision Making procedure during Wakefulness for Screening obstructive Sleep Apnea using Anthropometric information and tracheal Breathing Sounds. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Elwali, A.; Moussavi, Z. Determining Breathing Sound Features Representative of Obstructive Sleep Apnea During Wakefulness with Least Sensitivity to Other Risk Factors. J. Med. Biol. Eng. 2019, 39, 230–237. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Epstein, L.J.; Kristo, D.; Strollo, P.J., Jr.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 2009, 5, 263–276. [Google Scholar]
- Otero, A.; Félix, P.; Presedo, J.; Zamarrón, C. An evaluation of indexes as support tools in the diagnosis of sleep apnea. Ann. Biomed. Eng. 2012, 40, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, Y.; Wolf, L.; Nachmani, A.; Lipowezky, U.; Rub, M.; Berger, G. Velopharyngeal anatomy in patients with obstructive sleep apnea versus normal subjects. J. Oral Maxillofac. Surg. 2014, 72, 1350–1372. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Itoi, A.; Takashima, M.; Oda, M.; Tomoda, K. Difference of pharyngeal morphology and mechanical property between OSAHS patients and normal subjects. Auris Nasus Larynx 2006, 33, 433–439. [Google Scholar] [CrossRef]
- Elwali, A.; Moussavi, Z. Obstructive sleep apnea screening and airway structure characterization during wakefulness using tracheal breathing sounds. Ann. Biomed. Eng. 2017, 45, 839–850. [Google Scholar] [CrossRef]
- Proakis, J. DG Monolakis-Digital Signal Processing; Prentice Hall: Upper Saddle River, NJ, USA, 2006; p. 911. [Google Scholar]
- Nikias, C.L.; Raghuveer, M.R. Bispectrum estimation: A digital signal processing framework. Proc. IEEE 1987, 75, 869–891. [Google Scholar] [CrossRef]
- Ohayon, M.M.; Carskadon, M.A.; Guilleminault, C.; Vitiello, M.V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep 2004, 27, 1255–1273. [Google Scholar] [CrossRef] [PubMed]
- Boselli, M.; Parrino, L.; Smerieri, A.; Terzano, M.G. Effect of age on EEG arousals in normal sleep. Sleep 1998, 21, 361–367. [Google Scholar]
- Majumdar, S.R.; Eurich, D.T.; Gamble, J.; Senthilselvan, A.; Marrie, T.J. Oxygen saturations less than 92% are associated with major adverse events in outpatients with pneumonia: A population-based cohort study. Clin. Infect. Dis. 2011, 52, 325–331. [Google Scholar] [CrossRef]
- Chung, F.; Liao, P.; Elsaid, H.; Islam, S.; Shapiro, C.M.; Sun, Y. Oxygen desaturation index from nocturnal oximetry: A sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesth. Analg. 2012, 114, 993–1000. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hadi, A.S. Influential observations, high leverage points, and outliers in linear regression. Stat. Sci. 1986, 1, 379–393. [Google Scholar]
- Spearman, C. The proof and measurement of association between two things. Int. J. Epidemiol. 2010, 39, 1137–1150. [Google Scholar] [CrossRef]
- Elwali, A. Fast and Accurate Screening of Obstructive Sleep Apnea Disorder by Analyzing a Few Tracheal Breathing Sounds Recorded While the Individuals Are Awake. Ph.D. Thesis, University of Manitoba, Winnipeg, MB, Canada, May 2020. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Hajipour, F.; Jozani, M.J.; Elwali, A.; Moussavi, Z. Regularized logistic regression for obstructive sleep apnea screening during wakefulness using daytime tracheal breathing sounds and anthropometric information. Med. Biol. Eng. Comput. 2019, 57, 2641–2655. [Google Scholar] [CrossRef] [PubMed]
- Petrofsky, J.S.; Laymon, M.S.; Khowailed, I.A.; Fisher, S.; Mills, A. The Effect of BMI on Oxygen Saturation at Rest and During Mild Walking. J. Appl. Med. Sci. 2015, 4, 1–8. [Google Scholar]
- Simply, R.M.; Dafna, E.; Zigel, Y. Obstructive sleep apnea (OSA) classification using analysis of breathing sounds during speech. In Proceedings of the 2018 26th European Signal Processing Conference (EUSIPCO), Rome, Italy, 3–7 September 2018; pp. 1132–1136. [Google Scholar]
- Wu, Y.; Liu, J.; He, B.; Zhang, X.; Yu, L. Adaptive Filtering Improved Apnea Detection Performance Using Tracheal Sounds in Noisy Environment: A Simulation Study. BioMed Res. Int. 2020, 2020, 7429345. [Google Scholar] [CrossRef]
- Solà-Soler, J.; Fiz, J.A.; Torres, A.; Jané, R. Identification of obstructive sleep apnea patients from tracheal breath sound analysis during wakefulness in polysomnographic studies. In Proceedings of the 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Chicago, IL, USA, 26–30 August 2014; pp. 4232–4235. [Google Scholar]


| FL | BM | Feature’s Definition |
|---|---|---|
| F1 | - | Body mass index (BMI) |
| F2 | - | Age |
| F3 | - | Sex |
| F4 | - | Weight |
| F5 | - | Neck circumference (NC) |
| F6 | - | Mallampati score (MpS) |
| F7 | InsN | |
| F8 | ExpM | |
| F9 | InsN | |
| F10 | InsM | |
| F11 | InsM | |
| F12 | ExpM | |
| F13 | InsM | using zero-crossing |
| F14 | InsM | |
| F15 | InsM | |
| F16 | InsM | |
| F17 | ExpM | |
| F18 | InsN | |
| F19 | InsN | |
| F20 | InsN | |
| F21 | InsN | |
| F22 | InsN | |
| F23 | InsN | |
| F24 | InsN | |
| F25 | InsM | Higuchi fractal dimension |
| F26 | InsM | |
| F27 | InsM | Total |
| F28 | InsM | |
| F29 | ExpM | Total |
| F30 | InsN | |
| F31 | InsN | |
| F32 | InsM | |
| F33 | InsN | |
| F34 | InsN | |
| F35 | InsN | |
| F36 | InsN | |
| F37 | InsM | using zero-crossing |
| F38 | InsM | Total |
| F39 | InsM | |
| F40 | InsM | |
| F41 | InsN | |
| F42 | InsN | |
| F43 | InsN | |
| F44 | ExpM | |
| F45 | InsN | |
| F46 | InsN | |
| F47 | InsN | |
| F48 | ExpN | |
| F49 | ExpM | |
| F50 | InsN | |
| F51 | ExpN | |
| F52 | InsM | |
| F53 | ExpM | |
| F54 | ExpM | |
| F55 | ExpN | |
| F56 | InsN | |
| F57 | InsN | |
| F58 | InsM | |
| F59 | InsN | using zero-crossing |
| F60 | InsM | |
| F61 | InsM | |
| F62 | InsN | |
| F63 | InsN | |
| F64 | ExpN | |
| F65 | InsN | |
| F66 | InsM | |
| F67 | InsN | |
| F68 | InsM | |
| F69 | InsM | |
| F70 | InsN | |
| F71 | InsN | |
| F72 | ExpN | |
| F73 | InsM | |
| F74 | InsM | |
| F75 | InsM | |
| F76 | InsM | |
| F77 | InsM | |
| F78 | ExpM | |
| F79 | InsN |
| Non-OSA (AHI < 15) n = 80 | OSA (AHI ≥ 15) n = 65 | |
|---|---|---|
| AHI, events/h, median (IQR) | 2.9 (0.33–6.68) | 29.4 (20.1–57.3) |
| Age, years, median (IQR) | 50 (41–57) | 51 (43–59) |
| Sex, n (%) | ||
| Female | 43 (53.8) | 17 (26.2) |
| Male | 37 (46.3) | 48 (73.8) |
| BMI, kg/m2, median (IQR) | 31.2 (26.9–35.7) | 34.8 (30.2–39.8) |
| NC, cm, mean (SD) | 39.87 (4.98) | 44.37 (3.74) |
| MpS, n (%) | ||
| I | 45 (56.3) | 15 (23.1) |
| II | 21 (26.3) | 23 (35.4) |
| III | 11 (13.8) | 19 (29.2) |
| IV | 3 (3.8) | 8 (12.3) |
| PSG# | PSG Parameter | Anthropometric and Sound Feature | Correlation Coefficient |
|---|---|---|---|
| PSG1 | Stage 3 Duration % | F2 | −0.35 |
| PSG2 | EEG Total Arousal Index | F11/F37/F8 | 0.35/0.33/−0.31 |
| PSG3 | Total Arousal Index | F5/F11/F37 | 0.41/0.35/0.33 |
| PSG4 | Supine Arousal Index | F5/F37/F46/F53 | 0.46/0.36/0.31/0.31 |
| PSG5 | Non-Supine Arousal Index | F1/F4/F10/F69 | 0.37/0.36/0.36/0.36 |
| PSG6 | Supine Sleep % | F1/F17 | −0.29/0.29 |
| PSG7 | Apnea Total Index | F3/F5/F51 | −0.5/0.41/−0.38 |
| PSG8 | Apnea Non-REM Index | F3/F5/F49 | −0.51/0.46/−0.39 |
| PSG9 | Apnea Total Index (No Central) | F3/F5/F51 | −0.44/0.38/−0.33 |
| PSG10 | Apnea Non-REM Index (No Central) | F3/F5/F49/F37 | −0.45/0.43/−0.37/0.36 |
| PSG11 | Hypopnea Total Index | F5/F26/F4/F37/F49 | 0.54/−0.45/0.41/0.4/−0.4 |
| PSG12 | Hypopnea REM Index | F31 | −0.34 |
| PSG13 | Hypopnea Non-REM Index | F5/F4/F37/F57 | 0.53/0.41/0.41/0.4 |
| PSG14 | Hypopnea Total Index (No Central) | F5/F32/F4/F49 | 0.54/−0.43/0.41/−0.4 |
| PSG15 | Hypopnea REM Index (No Central) | F1/F20/F4/F6 | 0.3/0.28/0.27/0.26 |
| PSG16 | Hypopnea Non-REM Index (No Central) | F5/F4/F37/F49 | 0.53/0.41/0.41/−0.4 |
| PSG17 | Supine Sleep Obs. Apnea | F5/F3/49 | 0.43/−0.43/−0.36 |
| PSG18 | Supine Sleep Obs. Hypopnea | F5/F26/F49 | 0.51/−0.38/−0.37 |
| PSG19 | Supine Sleep A + H (with central) | F5/F37/F57/F10 | 0.6/0.41/0.38/0.36 |
| PSG20 | Supine Sleep A + H (without central) | F5/F57/F26/F49 | 0.55/0.42/−0.4/−0.41 |
| PSG21 | Left-side Sleep Obs. Hypopnea | F5/F1/61/F32 | 0.45/0.42/−0.38/−0.38 |
| PSG22 | Left-side Sleep A + H (with central) | F5/F1/F32/F4 | 0.45/0.4/−0.39/0.36 |
| PSG23 | Left-side Sleep A + H (without central) | F1/F32/F4 | 0.4/−0.4/0.37 |
| PSG24 | Right-side Sleep Obs. Hypopnea | F26/F69/F1/F38 | −0.44/−0.43/0.41/0.41 |
| PSG25 | Right-side Sleep A + H (with central) | F8/F5/F67/F27 | −0.43/0.43/0.41/0.4 |
| PSG26 | Right-side Sleep A + H (without central) | F21/F26/F5/F8/F9 | −0.43/−0.44/0.42/−0.43/−0.42 |
| PSG27 | Total AHI (With Central) | F5/F21/F25/F54 | 0.56/−0.41/0.4/0.4 |
| PSG28 | Total AHI (Without Central) | F5/F37/F57/F49 | 0.54/0.43/0.43/−0.42 |
| PSG29 | De-SpO2 Index Total | F5/F60/F37/F1 | 0.56/−0.44/0.42/0.4 |
| PSG30 | De-SpO2 Index Non-REM | F5/F4/F21/F37 | 0.55/0.43/−0.41/0.42 |
| PSG31 | De-SpO2 Index REM | F1/F5/F8/F23 | 0.41/0.39/−0.38/0.38 |
| PSG32 | Mean SpO2% Total TST | F1/F47/F4 | −0.4/−0.39/−0.36 |
| PSG33 | Mean SpO2% Supine | F47 | −0.32 |
| PSG34 | Mean SpO2% Non-Supine | F1/F4/F47/F37 | −0.47/−0.43/−0.4/−0.39 |
| PSG35 | REM Latency/TST | F28/F29 | −0.29/0.25 |
| PSG36 | Wake after sleep Onset/TST | F2 | 0.39 |
| PSG# | Feature Combination | CC | F1-Score | ACC% |
|---|---|---|---|---|
| PSG1 | F2 F3(0.31) F46(−0.14) F74 F77 | 0.63 | 0.63 | 63 |
| PSG2 | F8(7.3) F12 F46(110) F49 F56 | 0.7 | 0.82 | 80 |
| PSG3 | F5(−31) F6(246) F37 F53 F68 | 0.74 | 0.76 | 81 |
| PSG4 | F5 F6(−21) F37 F46(86) F74 | 0.8 | 0.62 | 64 |
| PSG5 | F1 F10 F49(0.73) F56(−3.2) F72 | 0.71 | 0.62 | 74 |
| PSG6 | F2 F4 F33(0.07) F35 F46(2.3) | 0.64 | 0.64 | 64 |
| PSG7 | F3(106) F18 F48(−1367) F50 F77 | 0.75 | 0.74 | 71 |
| PSG8 | F3(8.4) F37 F48(−562) F49 F51 | 0.72 | 0.67 | 71 |
| PSG9 | F3 F19 F46(30.7) F48(−342) F77 | 0.69 | 0.6 | 67 |
| PSG10 | F3(30.7) F6(64.4) F19 F53 F78 | 0.72 | 0.63 | 68 |
| PSG11 | F4(2.6) F5(13.4) F12 F26 F38 | 0.82 | 0.83 | 82 |
| PSG12 | F4(−5.5) F5 F12(−14) F49 F74 | 0.72 | 0.67 | 79 |
| PSG13 | F4 F5(17.1) F11 F49(−5.5) F52 | 0.81 | 0.6 | 62 |
| PSG14 | F1(−7.2) F4(5.5) F5 F12 F15 | 0.76 | 0.78 | 78 |
| PSG15 | F1(−0.96) F29 F30 F49(−1.3) | 0.59 | 0.6 | 78 |
| PSG16 | F5 F6(36.3) F10 F46(288) F53 | 0.78 | 0.8 | 79 |
| PSG17 | F3 F46(11.1) F57(79.1) F66 F77 | 0.75 | 0.73 | 77 |
| PSG18 | F4 F5(9.9) F10 F17 F49(2.1) | 0.79 | 0.62 | 71 |
| PSG19 | F5(3.1) F38 F57(−730) F68 F71 | 0.79 | 0.95 | 92 |
| PSG20 | F5(32.3) F6(−99) F11 F50 F74 | 0.83 | 0.86 | 85 |
| PSG21 | F4(3.9) F5(−10.8) F24 F42 | 0.64 | 0.78 | 74 |
| PSG22 | F4 F5(−3.3) F6(33.48) F32 F66 | 0.73 | 0.67 | 69 |
| PSG23 | F1 F6(473) F32(−80.5) F49 F53 | 0.73 | 0.63 | 67 |
| PSG24 | F6(−15.3) F8(3.9) F10 F38 F41 | 0.81 | 0.77 | 73 |
| PSG25 | F5(−76) F6(113) F19 F54 F77 | 0.79 | 0.67 | 60 |
| PSG26 | F6 F9(−18) F26 F38 F46(−23.5) | 0.84 | 0.73 | 72 |
| PSG27 | F5(−161) 21 F25(−2550) F38 F51 | 0.81 | 0.67 | 75 |
| PSG28 | F5 F11 F25(−1889) F57(−4046) F71 | 0.8 | 0.63 | 67 |
| PSG29 | F1 F5(4.4) F6(−8.4) F37 F49 | 0.77 | 0.63 | 73 |
| PSG30 | F5(12.73) F21 F53 F65(−89.5) F77 | 0.78 | 0.84 | 80 |
| PSG31 | F6(44) F16 F29 F30 F65(24.7) | 0.8 | 0.89 | 92 |
| PSG32 | F1(−0.005) F24(0.0009) F29 F51 | 0.65 | 0.62 | 58 |
| PSG33 | F18 F28 F29 F47(0.15) F68(−0.0004) | 0.63 | 0.72 | 68 |
| PSG34 | F1(0.0157) F2(−0.006) F31 F37 F53 | 0.71 | 0.8 | 72 |
| PSG35 | F1(0.22) F2 F28 F32(−0.13) F36 | 0.63 | 0.74 | 74 |
| PSG36 | F2(−0.75) F22 F39 F46(1.78) F76 | 0.55 | 0.7 | 65 |
| PSG# | Threshold | VF1 | VCA% | VSpec% | VSens% | TF1 | TCA% | TSpec% | TSens% |
|---|---|---|---|---|---|---|---|---|---|
| PSG1 | 10% | 0.66 | 80.7 | 88.9 | 61.9 | 0.73 | 78.2 | 80.1 | 73.8 |
| PSG2 | 15 | 0.80 | 75.3 | 69.1 | 79.2 | 0.83 | 81.6 | 78.0 | 83.8 |
| PSG3 | 25 | 0.65 | 74.6 | 83.9 | 60.6 | 0.66 | 73.6 | 81.6 | 61.7 |
| PSG4 | 30 | 0.75 | 76.8 | 78.7 | 74.5 | 0.72 | 73.4 | 73.7 | 73.7 |
| PSG5 | 20 | 0.70 | 76.2 | 81.8 | 67.9 | 0.76 | 84.7 | 97.8 | 63.3 |
| PSG6 | 30% | 0.79 | 74.9 | 63.1 | 83.7 | 0.75 | 72.4 | 76.4 | 70.5 |
| PSG7 | 1 | 0.81 | 80.8 | 80.6 | 81.1 | 0.83 | 82.9 | 82.1 | 84.0 |
| PSG8 | 1 | 0.79 | 82.3 | 88.7 | 74.1 | 0.73 | 80.7 | 85.4 | 70.1 |
| PSG9 | 3.5 | 0.70 | 83.4 | 90.4 | 66.6 | 0.76 | 85.6 | 89.7 | 78.1 |
| PSG10 | 0.5 | 0.76 | 77.9 | 80.7 | 74.8 | 0.79 | 80.6 | 79.6 | 82.6 |
| PSG11 | 10 | 0.78 | 83.0 | 88.5 | 75.0 | 0.74 | 80.4 | 82.2 | 76.4 |
| PSG12 | 20 | 0.70 | 77.8 | 84.6 | 67.0 | 0.68 | 79.5 | 91.3 | 60.6 |
| PSG13 | 7.5 | 0.83 | 84.5 | 86.7 | 81.9 | 0.78 | 80.4 | 80.1 | 80.2 |
| PSG14 | 5 | 0.87 | 82.8 | 66.9 | 91.6 | 0.90 | 88.8 | 82.5 | 94.7 |
| PSG15 | 20 | 0.67 | 76.2 | 82.0 | 66.3 | 0.68 | 80.6 | 89.6 | 62.5 |
| PSG16 | 5 | 0.83 | 80.7 | 77.2 | 83.5 | 0.83 | 82.6 | 77.7 | 90.3 |
| PSG17 | 1 | 0.77 | 77.1 | 80.1 | 74.1 | 0.77 | 79.9 | 85.6 | 74.5 |
| PSG18 | 15 | 0.86 | 86.1 | 85.3 | 86.9 | 0.78 | 81.6 | 82.1 | 79.8 |
| PSG19 | 15 | 0.87 | 85.7 | 83.9 | 86.9 | 0.82 | 79.7 | 71.8 | 86.3 |
| PSG20 | 20 | 0.84 | 83.4 | 80.6 | 85.8 | 0.81 | 81.4 | 79.4 | 83.4 |
| PSG21 | 2.5 | 0.76 | 78.8 | 82.1 | 74.1 | 0.78 | 80.4 | 78.5 | 84.8 |
| PSG22 | 7 | 0.76 | 84.4 | 92.1 | 70.3 | 0.71 | 80.2 | 85.9 | 69.8 |
| PSG23 | 4 | 0.74 | 80.1 | 87.1 | 69.6 | 0.75 | 79.5 | 78.0 | 79.2 |
| PSG24 | 1 | 0.84 | 81.6 | 80.3 | 82.6 | 0.82 | 80.4 | 77.5 | 82.5 |
| PSG25 | 0.5 | 0.87 | 82.5 | 71.9 | 88.2 | 0.91 | 88.4 | 94.3 | 86.9 |
| PSG26 | 0.5 | 0.86 | 82.2 | 75.6 | 86.1 | 0.87 | 83.7 | 85.0 | 84.2 |
| PSG27 | 15 | 0.77 | 78.7 | 81.2 | 75.8 | 0.78 | 82.4 | 85.0 | 79.3 |
| PSG28 | 15 | 0.76 | 80.5 | 87.1 | 71.7 | 0.75 | 80.5 | 83.9 | 74.5 |
| PSG29 | 15 | 0.75 | 77.2 | 81.1 | 72.8 | 0.78 | 82.0 | 84.5 | 78.7 |
| PSG30 | 5 | 0.87 | 83.0 | 70.7 | 89.5 | 0.89 | 86.5 | 77.9 | 91.6 |
| PSG31 | 25 | 0.78 | 83.3 | 91.5 | 71.5 | 0.77 | 82.5 | 83.9 | 80.0 |
| PSG32 | 94% | 0.72 | 70.5 | 68.7 | 72.0 | 0.74 | 71.5 | 68.6 | 75.4 |
| PSG33 | 94% | 0.69 | 72.3 | 77.0 | 66.6 | 0.78 | 77.8 | 74.7 | 81.9 |
| PSG34 | 93% | 0.88 | 81.8 | 60.1 | 90.9 | 0.92 | 87.8 | 77.1 | 92.3 |
| PSG35 | 0.45 | 0.78 | 72.4 | 64.3 | 77.2 | 0.88 | 86.5 | 100 | 80.6 |
| PSG36 | 0.15 | 0.78 | 72.4 | 60.0 | 79.8 | 0.79 | 74.1 | 63.9 | 80.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elwali, A.; Moussavi, Z. Predicting Polysomnography Parameters from Anthropometric Features and Breathing Sounds Recorded during Wakefulness. Diagnostics 2021, 11, 905. https://doi.org/10.3390/diagnostics11050905
Elwali A, Moussavi Z. Predicting Polysomnography Parameters from Anthropometric Features and Breathing Sounds Recorded during Wakefulness. Diagnostics. 2021; 11(5):905. https://doi.org/10.3390/diagnostics11050905
Chicago/Turabian StyleElwali, Ahmed, and Zahra Moussavi. 2021. "Predicting Polysomnography Parameters from Anthropometric Features and Breathing Sounds Recorded during Wakefulness" Diagnostics 11, no. 5: 905. https://doi.org/10.3390/diagnostics11050905
APA StyleElwali, A., & Moussavi, Z. (2021). Predicting Polysomnography Parameters from Anthropometric Features and Breathing Sounds Recorded during Wakefulness. Diagnostics, 11(5), 905. https://doi.org/10.3390/diagnostics11050905







