Detection of Degenerative Changes on MR Images of the Lumbar Spine with a Convolutional Neural Network: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection and Expert Reading
2.2. MR Imaging Protocol
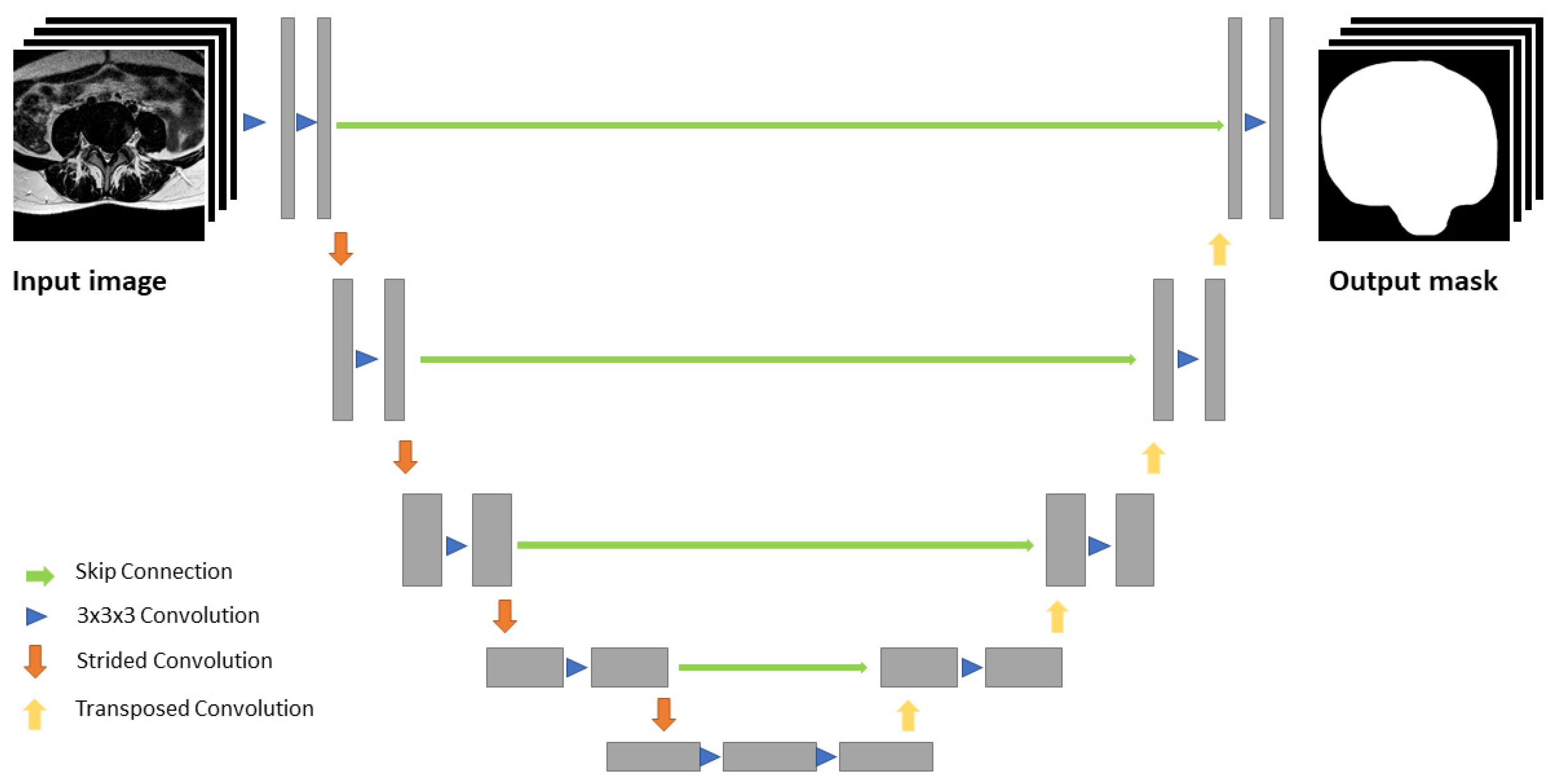
2.3. Machine Learning Algorithm and Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Cohort
3.2. CNN Diagnostic Performance
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Patel, N.D.; Broderick, D.F.; Burns, J.; Deshmukh, T.K.; Fries, I.B.; Harvey, H.B.; Holly, L.; Hunt, C.H.; Jagadeesan, B.D.; Kennedy, T.A.; et al. ACR appropriateness criteria low back pain. J. Am. Coll. Radiol. 2016, 13, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.; Scuderi, G.; Scuderi, C.; Grewal, R.; Sandhu, S.J. The use of imaging in management of patients with low back pain. J. Clin. Imaging Sci. 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; Schwartz, K.M.; Eckel, L.J.; Diehn, F.E.; Hunt, C.H.; Bartholmai, B.J.; Erickson, B.J.; Kallmes, D.F. The effects of changes in utilization and technological advancements of cross-sectional imaging on radiologist workload. Acad. Radiol. 2015, 22, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Jaremko, J.L.; Poncet, P.; Ronsky, J.; Harder, J.; Dansereau, J.; Labelle, H.; Zernicke, R.F. Estimation of spinal deformity in scoliosis from torso surface cross sections. Spine 2001, 26, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Galbusera, F.; Niemeyer, F.; Wilke, H.-J.; Bassani, T.; Casaroli, G.; Anania, C.; Costa, F.; Brayda-Bruno, M.; Sconfienza, L.M. Fully automated radiological analysis of spinal disorders and deformities: A deep learning approach. Eur. Spine J. 2019, 28, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Klinder, T.; Ostermann, J.; Ehm, M.; Franz, A.; Kneser, R.; Lorenz, C. Automated model-based vertebra detection, identification, and segmentation in CT images. Med. Image Anal. 2009, 13, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lu, L.; Zhan, Y.; Zhou, X.; Salganicoff, M.; Krishnan, A. Hierarchical segmentation and identification of thoracic vertebra using learning-based edge detection and coarse-to-fine deformable model. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2010; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2010; Volume 6361, pp. 19–27. [Google Scholar]
- Glocker, B.; Feulner, J.; Criminisi, A.; Haynor, D.R.; Konukoglu, E. Automatic localization and identification of vertebrae in arbitrary field-of-view CT scans. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2012; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2012; Volume 7512, pp. 590–598. [Google Scholar]
- Sekuboyina, A.; Rempfler, M.; Valentinitsch, A.; Menze, B.H.; Kirschke, J.S. Labeling vertebrae with two-dimensional reformations of multidetector CT images: An adversarial approach for incorporating prior knowledge of spine anatomy. Radiol. Artif. Intell. 2020, 2, e190074. [Google Scholar] [CrossRef]
- Peng, Z.; Zhong, J.; Wee, W.; Lee, J.-H. Automated vertebra detection and segmentation from the whole spine MR images. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2005, 2005, 2527–2530. [Google Scholar] [CrossRef]
- Han, Z.; Wei, B.; Mercado, A.; Leung, S.; Li, S. Spine-GAN: Semantic segmentation of multiple spinal structures. Med. Image Anal. 2018, 50, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, S.; Lee, Y.H.; Lee, S.H.; Lee, H.S.; Kim, S. Performance of the deep convolutional neural network based magnetic resonance image scoring algorithm for differentiating between tuberculous and pyogenic spondylitis. Sci. Rep. 2018, 8, 13124. [Google Scholar] [CrossRef]
- Kim, S.; Bae, W.C.; Masuda, K.; Chung, C.B.; Hwang, D. Fine-grain segmentation of the intervertebral discs from MR spine images using deep convolutional neural networks: BSU-Net. Appl. Sci. 2018, 8, 1656. [Google Scholar] [CrossRef] [PubMed]
- Gaonkar, B.; Beckett, J.; Villaroman, D.; Ahn, C.; Edwards, M.; Moran, S.; Attiah, M.; Babayan, D.; Ames, C.; Villablanca, J.P.; et al. Quantitative analysis of neural foramina in the lumbar spine: An imaging informatics and machine learning study. Radiol. Artif. Intell. 2019, 1, 180037. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, Y.; Chen, Q.; Gu, G.; Sui, X. Automatic lumbar MRI detection and identification based on deep learning. J. Digit. Imaging 2018, 32, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Jamaludin, A.; Lootus, M.; Kadir, T.; Zisserman, A.; Urban, J.; Battié, M.C.; Fairbank, J.; McCall, I. ISSLS Prize in Bioengineering Science 2017: Automation of reading of radiological features from magnetic resonance images (MRIs) of the lumbar spine without human intervention is comparable with an expert radiologist. Eur. Spine J. 2017, 26, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Lundervold, A.S.; Lundervold, A. An overview of deep learning in medical imaging focusing on MRI. Z. Med. Phys. 2019, 29, 102–127. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.; Jeong, Y.-S. Compact and accurate scene text detector. Appl. Sci. 2020, 10, 2096. [Google Scholar] [CrossRef]
- Vu, T.; Nguyen, C.V.; Pham, T.X.; Luu, T.M.; Yoo, C.D. Fast and efficient image quality enhancement via desubpixel convolutional neural networks. In Computer Vision—ECCV 2018 Workshops; Leal-Taixé, L., Roth, S., Eds.; Springer: Cham, Switzerland, 2019; pp. 243–259. ISBN 978-3-030-11020-8. [Google Scholar]
- Le Cun, Y.; Bottou, L.; Bengio, Y. Reading checks with multilayer graph transformer networks. In Proceedings of the 1997 IEEE International Conference on Acoustics, Speech, and Signal Processing, Munich, Germany, 21–24 April 1997; pp. 151–154, ISBN 0-8186-7919-0. [Google Scholar]
- Glorot, X.; Bengio, Y. Understanding the difficulty of training deep feed forward neural networks. J. Mach. Learn. Res. 2010, 9, 249–256. [Google Scholar]
- Pascanu, R.; Mikolov, T.; Bengio, Y. On the Difficulty of Training Recurrent Neural Networks. 2012. Available online: http://arxiv.org/pdf/1211.5063v2 (accessed on 10 February 2021).
- Up-to-Date Results of the IVDM3Seg Segmentation Challenge. Available online: https://ivdm3seg.weebly.com/results.html# (accessed on 19 December 2020).
- Fardon, D.F.; Williams, A.L.; Dohring, E.J.; Murtagh, F.R.; Rothman, S.L.G.; Sze, G.K. Lumbar disc nomenclature: Version 2.0. Spine J. 2014, 14, 2525–2545. [Google Scholar] [CrossRef]
- Koslosky, E.; Gendelberg, D. Classification in Brief: The Meyerding classification system of spondylolisthesis. Clin. Orthop. Relat. Res. 2020, 478, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Guen, Y.L.; Joon, W.L.; Hee, S.C.; Kyoung-Jin, O.; Heung, S.K.; Lee, G.Y.; Lee, J.W.; Choi, H.S.; Oh, K.-J.; Kang, H.S. A new grading system of lumbar central canal stenosis on MRI: An easy and reliable method. Skelet. Radiol. 2011, 40, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, N.; Asenov, A. Automatic segmentation of lumbar spine MRI using ensemble of 2D algorithms. In Computational Methods and Clinical Applications for Spine Imaging; Springer: Berlin/Heidelberg, Germany, 2019; pp. 154–162. ISBN 978-3-030-13735-9. [Google Scholar]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. 2015. Available online: http://arxiv.org/pdf/1505.04597v1 (accessed on 10 February 2021).
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778, ISBN 978-1-4673-8851-1. [Google Scholar]
- Lin, T.-Y.; Maire, M.; Belongie, S.; Bourdev, L.; Girshick, R.; Hays, J.; Perona, P.; Ramanan, D.; Zitnick, C.L.; Dollár, P. Microsoft COCO: Common Objects in Context. 2014. Available online: http://arxiv.org/pdf/1405.0312v3 (accessed on 10 February 2021).
- Kuhn, M. Building predictive models in R using the Caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Cai, Y.; Osman, S.; Sharma, M.; Landis, M.; Li, S. Multi-modality vertebra recognition in arbitrary views using 3D deformable hierarchical model. IEEE Trans. Med. Imaging 2015, 34, 1676–1693. [Google Scholar] [CrossRef]
- Law, M.W.; Tay, K.; Leung, A.; Garvin, G.J.; Li, S. Intervertebral disc segmentation in MR images using anisotropic oriented flux. Med. Image Anal. 2013, 17, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Kelm, B.M.; Wels, M.; Zhou, S.K.; Seifert, S.; Suehling, M.; Zheng, Y.; Comaniciu, D. Spine detection in CT and MR using iterated marginal space learning. Med. Image Anal. 2013, 17, 1283–1292. [Google Scholar] [CrossRef]
- Cai, Y.; Leung, S.; Warrington, J.; Pandey, S.; Shmuilovich, O.; Li, S. Direct spondylolisthesis identification and measurement in MR/CT using detectors trained by articulated parameterized spine model. In Medical Imaging 2017: Image Processing; SPIE: Bellingham, WA, USA, 2017; p. 1013319. [Google Scholar]
- Won, D.; Lee, H.-J.; Lee, S.-J.; Park, S.H. Spinal stenosis grading in magnetic resonance imaging using deep convolutional neural networks. Spine 2020, 45, 804–812. [Google Scholar] [CrossRef]
- Park, S.H.; Han, K. Methodologic guide for evaluating clinical performance and effect of artificial intelligence technology for medical diagnosis and prediction. Radiology 2018, 286, 800–809. [Google Scholar] [CrossRef]
- England, J.R.; Cheng, P. Artificial intelligence for medical image analysis: A guide for authors and reviewers. Am. J. Roentgenol. 2019, 212, 513–519. [Google Scholar] [CrossRef]
- Ting, D.S.W.; Cheung, C.Y.-L.; Lim, G.; Tan, G.S.W.; Quang, N.D.; Gan, A.; Hamzah, H.; Garcia-Franco, R.; Yeo, I.Y.S.; Lee, S.Y.; et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. J. Am. Med. Assoc. 2017, 318, 2211–2223. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Jang, H.Y.; Kim, K.W.; Shin, Y.; Park, S.H. Design characteristics of studies reporting the performance of artificial intelligence algorithms for diagnostic analysis of medical images: Results from recently published papers. Korean J. Radiol. 2019, 20, 405–410. [Google Scholar] [CrossRef]
- Del Giudice, F.; Barchetti, G.; De Berardinis, E.; Pecoraro, M.; Salvo, V.; Simone, G.; Sciarra, A.; Leonardo, C.; Gallucci, M.; Catalano, C.; et al. Prospective assessment of vesical imaging reporting and data system (VI-RADS) and its clinical impact on the management of high-risk non–muscle-invasive bladder cancer patients candidate for repeated transurethral resection. Eur. Urol. 2020, 77, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, F.; Leonardo, C.; Simone, G.; Pecoraro, M.; De Berardinis, E.; Cipollari, S.; Flammia, S.; Bicchetti, M.; Busetto, G.M.; Chung, B.I.; et al. Preoperative detection of vesical imaging-reporting and data system (VI-RADS) score 5 reliably identifies extravesical extension of urothelial carcinoma of the urinary bladder and predicts significant delayed time to cystectomy: Time to reconsider the need for primary deep transurethral resection of bladder tumour in cases of locally advanced disease? BJU Int. 2020, 126, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, F.; Pecoraro, M.; Vargas, H.A.; Cipollari, S.; De Berardinis, E.; Bicchetti, M.; Chung, B.I.; Catalano, C.; Narumi, Y.; Catto, J.W.F.; et al. Systematic review and meta-analysis of vesical imaging-reporting and data system (VI-RADS) inter-observer reliability: An added value for muscle invasive bladder cancer detection. Cancers 2020, 12, 2994. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.C.; Buerba, R.A.; Long, W.D.; Blizzard, D.J.; Lischuk, A.W.; Haims, A.H.; Grauer, J.N. Interrater and intrarater agreements of magnetic resonance imaging findings in the lumbar spine: Significant variability across degenerative conditions. Spine J. 2014, 14, 2442–2448. [Google Scholar] [CrossRef] [PubMed]
- Pacilè, S.; Lopez, J.; Chone, P.; Bertinotti, T.; Grouin, J.M.; Fillard, P. improving breast cancer detection accuracy of mammography with the concurrent use of an artificial intelligence tool. Radiol. Artif. Intell. 2020, 2, e190208. [Google Scholar] [CrossRef] [PubMed]
- Cummins, J.; Lurie, J.D.; Tosteson, T.D.; Hanscom, B.; Abdu, W.A.; Birkmeyer, N.J.O.; Herkowitz, H.; Weinstein, J. Descriptive epidemiology and prior healthcare utilization of patients in the spine patient outcomes research trial’s (SPORT) three observational cohorts: Disc herniation, spinal stenosis, and degenerative spondylolisthesis. Spine 2006, 31, 806–814. [Google Scholar] [CrossRef] [PubMed]

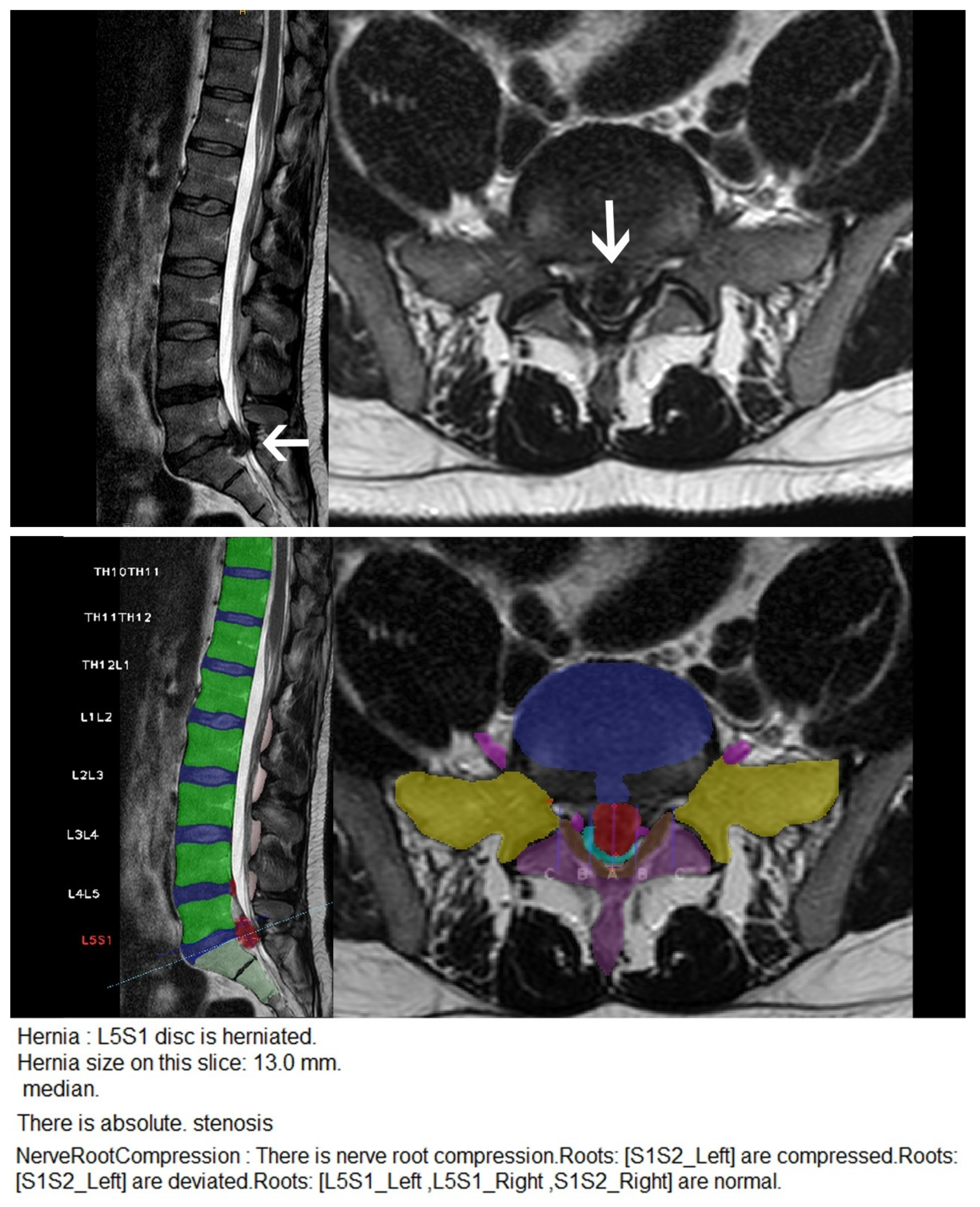
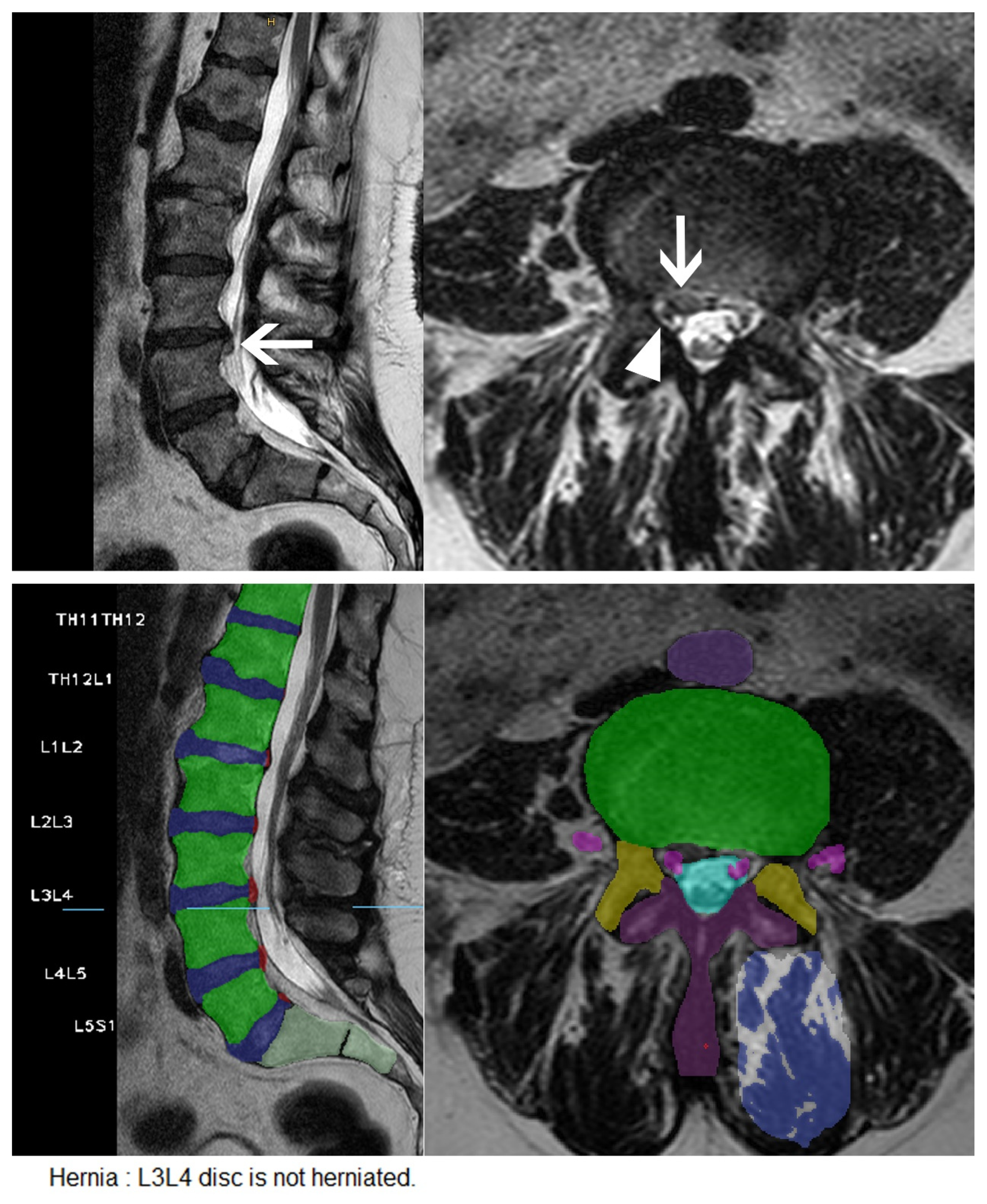
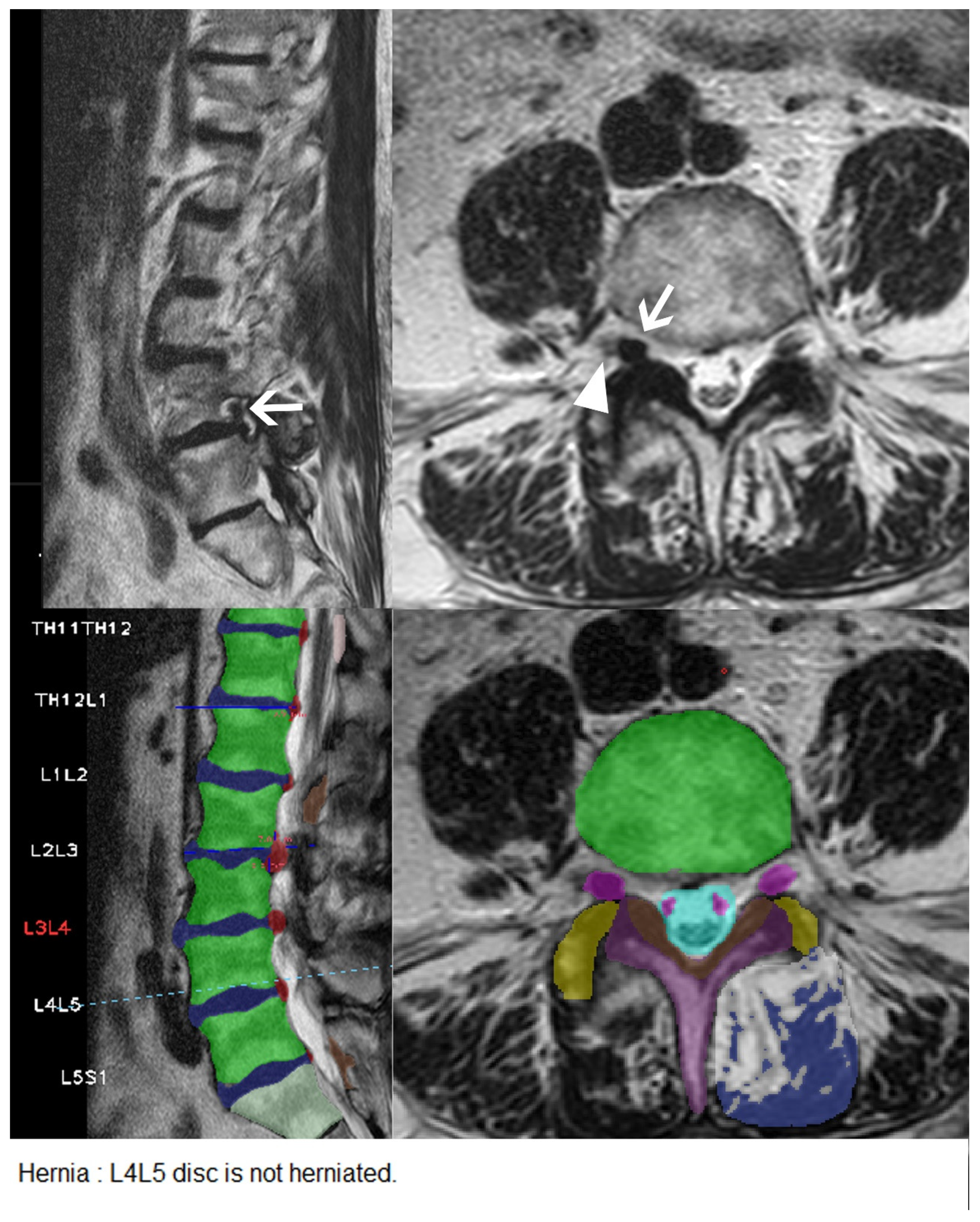
| Characteristic | Herniation | Extrusion | Stenosis | Bulging | Nerve Root Compression | Spondylolisthesis |
|---|---|---|---|---|---|---|
| n | 77 (8.67%) | 46 (5.18%) | 35 (3.94%) | 133 (14.98%) | 59 (5.70%) | 20 (2.25%) |
| TP | 58 | 41 | 27 | 69 | 42 | 16 |
| TN | 713 | 727 | 844 | 602 | 896 | 762 |
| FP | 98 | 115 | 9 | 153 | 81 | 106 |
| FN | 19 | 5 | 8 | 64 | 17 | 4 |
| Sensitivity | 75.33% | 89.13% | 77.14% | 51.88% | 71.19% | 80.00% |
| Specificity | 87.92% | 86.34% | 98.95% | 79.74% | 91.71% | 87.79% |
| Accuracy | 86.82% | 86.49% | 98.09% | 75.56% | 90.54% | 87.61% |
| PPV | 37.18% | 26.28% | 75.00% | 31.08% | 34.15% | 13.11% |
| NPV | 97.40% | 99.32% | 99.06% | 90.39% | 98.14% | 99.48% |
| p | <0.001 | <0.001 | 1 | <0.001 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehnen, N.C.; Haase, R.; Faber, J.; Rüber, T.; Vatter, H.; Radbruch, A.; Schmeel, F.C. Detection of Degenerative Changes on MR Images of the Lumbar Spine with a Convolutional Neural Network: A Feasibility Study. Diagnostics 2021, 11, 902. https://doi.org/10.3390/diagnostics11050902
Lehnen NC, Haase R, Faber J, Rüber T, Vatter H, Radbruch A, Schmeel FC. Detection of Degenerative Changes on MR Images of the Lumbar Spine with a Convolutional Neural Network: A Feasibility Study. Diagnostics. 2021; 11(5):902. https://doi.org/10.3390/diagnostics11050902
Chicago/Turabian StyleLehnen, Nils Christian, Robert Haase, Jennifer Faber, Theodor Rüber, Hartmut Vatter, Alexander Radbruch, and Frederic Carsten Schmeel. 2021. "Detection of Degenerative Changes on MR Images of the Lumbar Spine with a Convolutional Neural Network: A Feasibility Study" Diagnostics 11, no. 5: 902. https://doi.org/10.3390/diagnostics11050902
APA StyleLehnen, N. C., Haase, R., Faber, J., Rüber, T., Vatter, H., Radbruch, A., & Schmeel, F. C. (2021). Detection of Degenerative Changes on MR Images of the Lumbar Spine with a Convolutional Neural Network: A Feasibility Study. Diagnostics, 11(5), 902. https://doi.org/10.3390/diagnostics11050902






