Recent Developments of ICG-Guided Sentinel Lymph Node Mapping in Oral Cancer
Abstract
1. Introduction
2. Conventional Sentinel Lymph Node Biopsy
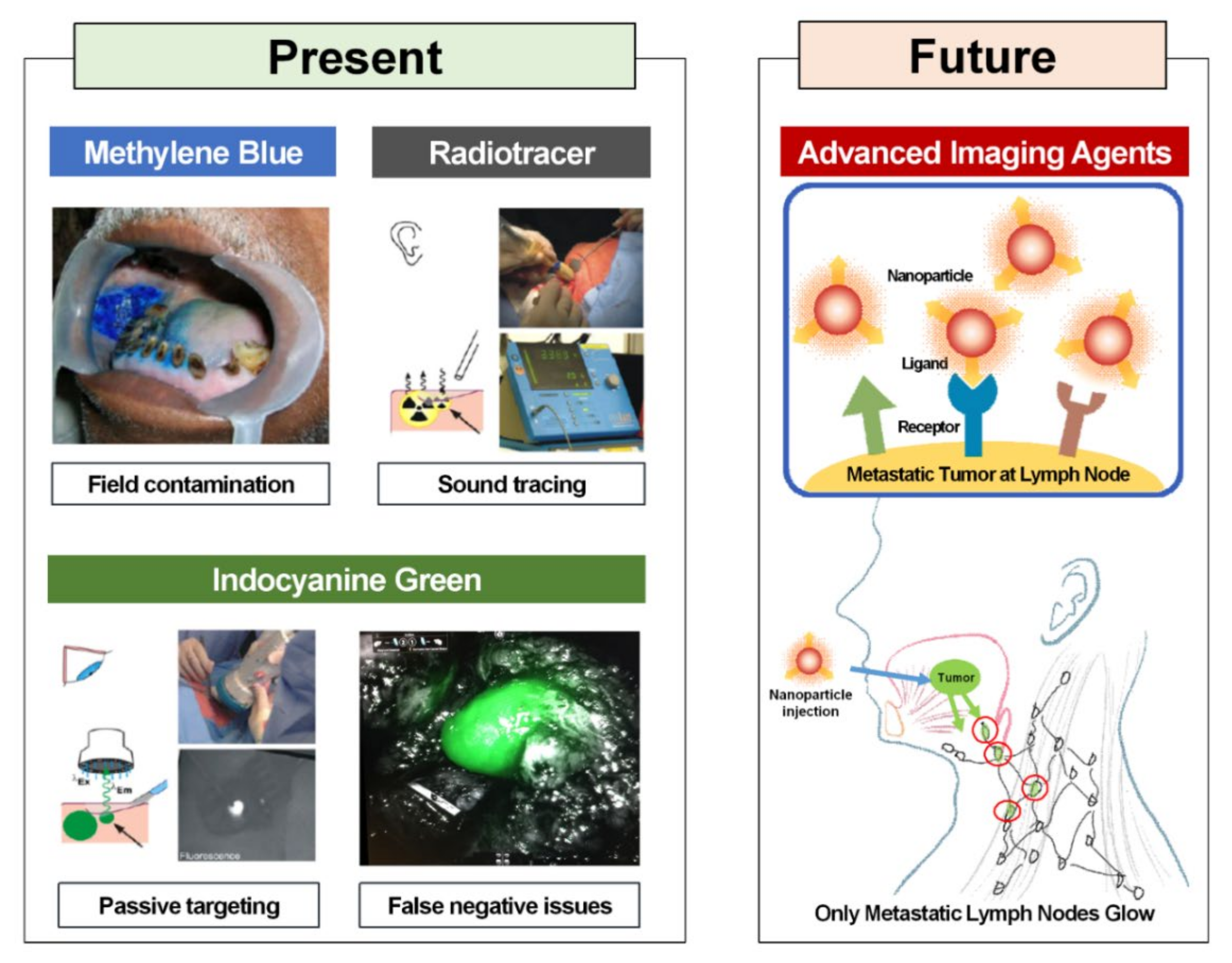
3. Currently Used Fluorophore for Sentinel Lymph Node Biopsy
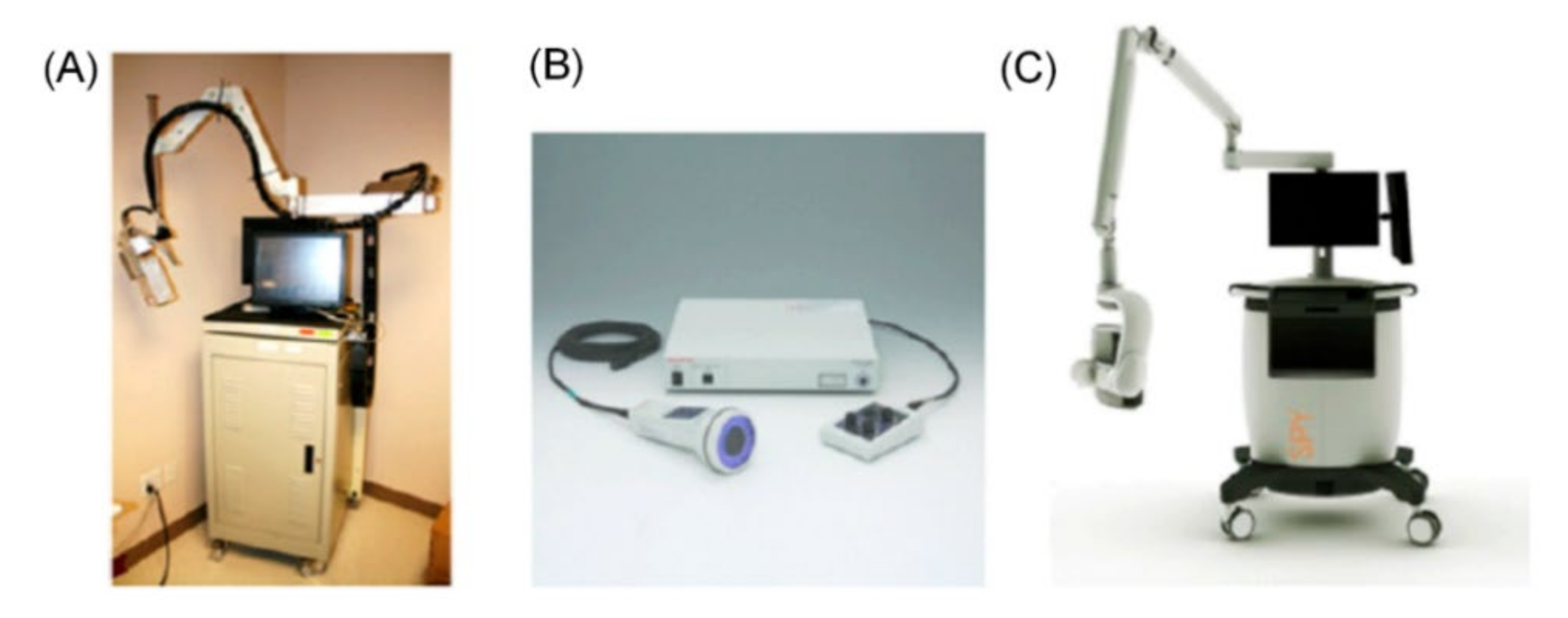
4. Near-Infrared Fluorescence Imaging
5. Recent Applications of ICG for Sentinel Lymph Node Biopsy in Oral Cancer
5.1. ICG Concentration and Dose
5.2. ICG Detection Timing
5.3. ICG Depth Penetration
5.4. ICG Versus Radiotracer
6. Advanced Imaging Contrast Agents
7. Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Pantel, K.; Brakenhoff, R.H. Dissecting the metastatic cascade. Nat. Rev. Cancer 2004, 4, 448–456. [Google Scholar] [CrossRef]
- Nathanson, S.D. Insights into the mechanisms of lymph node metastasis. Cancer 2003, 98, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA A Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; Forastiere, A.A. Head and Neck Cancer: Changing Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2008, 83, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, L.; Millán, I.; Torre, A.; Aragón, G.; Otero, J. Prognostic factors for survival and tumor control in cervical lymph node metastases from head and neck cancer: A multivariate study of 492 cases. Cancer 2010, 69, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, C.; Velten, M.; Jung, G.-M.; Bronner, G.; Flesch, H.; Borel, C. Prognostic indicators for survival in head and neck squamous cell carcinomas: Analysis of a series of 621 cases. Head Neck 2005, 27, 801–808. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, A.K.; Vaish, R.; Kapre, N.; Dandekar, M.; Gupta, S.; Hawaldar, R.; Agarwal, J.P.; Pantvaidya, G.; Chaukar, D.; Deshmukh, A.; et al. Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. N. Engl. J. Med. 2015, 373, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, S.J.; Yang, X.; Peng, H. Diagnostic Efficacy of Sentinel Lymph Node Biopsy in Early Oral Squamous Cell Carcinoma: A Meta-Analysis of 66 Studies. PLoS ONE 2017, 12, e0170322. [Google Scholar] [CrossRef]
- Crocetta, F.M.; Botti, C.; Pernice, C.; Murri, D.; Castellucci, A.; Menichetti, M.; Costantini, M.; Venturelli, F.; Bassi, M.C.; Ghidini, A. Sentinel node biopsy versus elective neck dissection in early-stage oral cancer: A systematic review. Eur. Arch. Otorhinolaryngol. 2020, 277, 3247–3260. [Google Scholar] [CrossRef]
- Thompson, C.F.; John, M.A.S.; Lawson, G.; Grogan, T.; Elashoff, D.; Mendelsohn, A.H. Diagnostic value of sentinel lymph node biopsy in head and neck cancer: A meta-analysis. Eur. Arch. Otorhinolaryngol. 2012, 270, 2115–2122. [Google Scholar] [CrossRef]
- Toom, I.J.D.; Heuveling, D.A.; Flach, G.B.; Van Weert, S.; Karagozoglu, K.H.; Van Schie, A.; Bloemena, E.; Leemans, C.R.; De Bree, R. Sentinel node biopsy for early-stage oral cavity cancer: The VU University Medical Center experience. Head Neck 2014, 37, 573–578. [Google Scholar] [CrossRef]
- Veronesi, U.; Paganelli, G.; Viale, G.; Luini, A.; Zurrida, S.; Galimberti, V.; Intra, M.; Veronesi, P.; Maisonneuve, P.; Gatti, G.; et al. Sentinel-lymph-node biopsy as a staging procedure in breast cancer: Update of a randomised controlled study. Lancet Oncol. 2006, 7, 983–990. [Google Scholar] [CrossRef]
- Li, H.; Kim, D.; Yao, Q.; Ge, H.; Chung, J.; Fan, J.; Wang, J.; Peng, X.; Yoon, J. Activity-Based NIR Enzyme Fluorescent Probes for the Diagnosis of Tumors and Image-Guided Surgery. Angew. Chem. 2020. [Google Scholar] [CrossRef]
- How, J.; Gotlieb, W.; Press, J.; Abitbol, J.; Pelmus, M.; Ferenczy, A.; Probst, S.; Gotlieb, R.; Brin, S.; Lau, S. Comparing indocyanine green, technetium, and blue dye for sentinel lymph node mapping in endometrial cancer. Gynecol. Oncol. 2015, 137, 436–442. [Google Scholar] [CrossRef]
- Plante, M.; Touhami, O.; Trinh, X.-B.; Renaud, M.-C.; Sebastianelli, A.; Grondin, K.; Gregoire, J. Sentinel node mapping with indocyanine green and endoscopic near-infrared fluorescence imaging in endometrial cancer. A pilot study and review of the literature. Gynecol. Oncol. 2015, 137, 443–447. [Google Scholar] [CrossRef]
- Tajima, Y.; Murakami, M.; Yamazaki, K.; Masuda, Y.; Kato, M.; Sato, A.; Goto, S.; Otsuka, K.; Kato, T.; Kusano, M. Sentinel Node Mapping Guided by Indocyanine Green Fluorescence Imaging During Laparoscopic Surgery in Gastric Cancer. Ann. Surg. Oncol. 2010, 17, 1787–1793. [Google Scholar] [CrossRef]
- Siesto, G.; Romano, F.; Fiamengo, B.; Vitobello, D. Sentinel Node Mapping Using Indocyanine Green and Near-infrared Fluorescence Imaging Technology for Uterine Malignancies: Preliminary Experience with the Da Vinci Xi System. J. Minim. Invasive Gynecol. 2016, 23, 470–471. [Google Scholar] [CrossRef]
- Eriksson, A.G.Z.; Beavis, A.; Soslow, R.A.; Zhou, Q.; Abu-Rustum, N.R.; Gardner, G.J.; Zivanovic, O.; Roche, K.L.; Sonoda, Y.; Leitao, M.M.; et al. A Comparison of the Detection of Sentinel Lymph Nodes Using Indocyanine Green and Near-Infrared Fluorescence Imaging Versus Blue Dye During Robotic Surgery in Uterine Cancer. Int. J. Gynecol. Cancer 2017, 27, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.G.; Feber, T. Surgical treatment of cervical node metastases from squamous carcinoma of the upper aerodigestive tract: Evaluation of the evidence for modifications of neck dissection. Head Neck 2001, 23, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Bilde, A.; Von Buchwald, C.; Therkildsen, M.H.; Mortensen, J.; Kirkegaard, J.; Charabi, B.; Specht, L. Need for Intensive Histopathologic Analysis to Determine Lymph Node Metastases When Using Sentinel Node Biopsy in Oral Cancer. Laryngoscope 2008, 118, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.; Bilde, A.; Therkildsen, M.H.; Mortensen, J.; Charabi, B.; Kirkegaard, J.; Specht, L.; Von Buchwald, C. The prevalence of occult metastases in nonsentinel lymph nodes after step-serial sectioning and immunohistochemistry in cN0 oral squamous cell carcinoma. Laryngoscope 2011, 121, 294–298. [Google Scholar] [CrossRef]
- Werner, J.A.; Dünne, A.A.; Ramaswamy, A.; Dalchow, C.; Behr, T.; Moll, R.; Folz, B.J.; Davis, R.K. The sentinel node concept in head and neck cancer: Solution for the controversies in the n0 neck? Head Neck 2004, 26, 603–611. [Google Scholar] [CrossRef]
- Cabanas, R.M. An approach for the treatment of penile carcinoma. Cancer 1977, 39, 456–466. [Google Scholar] [CrossRef]
- Morton, D.L.; Wen, D.-R.; Wong, J.H.; Economou, J.S.; Cagle, L.A.; Storm, F.K.; Foshag, L.J.; Cochran, A.J. Technical Details of Intraoperative Lymphatic Mapping for Early Stage Melanoma. Arch. Surg. 1992, 127, 392–399. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Kirgan, D.M.; Guenther, J.M.; Morton, D.L. Lymphatic Mapping and Sentinel Lymphadenectomy for Breast Cancer. Ann. Surg. 1994, 220, 391–401. [Google Scholar] [CrossRef]
- Ross, G.L.; Soutar, D.S.; Macdonald, D.G.; Shoaib, T.; Camilleri, I.; Roberton, A.G.; Sorensen, J.A.; Thomsen, J.; Grupe, P.; Álvarez, J.; et al. Sentinel Node Biopsy in Head and Neck Cancer: Preliminary Results of a Multicenter Trial. Ann. Surg. Oncol. 2004, 11, 690–696. [Google Scholar] [CrossRef]
- Cousins, A.; Thompson, S.K.; Wedding, A.B.; Thierry, B. Clinical relevance of novel imaging technologies for sentinel lymph node identification and staging. Biotechnol. Adv. 2014, 32, 269–279. [Google Scholar] [CrossRef]
- Borgstein, P.J.; Pijpers, R.; Comans, E.F.; Van Diest, P.J.; Boom, R.P.; Meijer, S. Sentinel Lymph Node Biopsy in Breast Cancer: Guidelines and Pitfalls of Lymphoscintigraphy and Gamma Probe Detection. J. Am. Coll. Surg. 1998, 186, 275–283. [Google Scholar] [CrossRef]
- O’Hea, B.J.; Hill, A.D.; El-Shirbiny, A.M.; Yeh, S.D.; Rosen, P.P.; Coit, D.G.; Borgen, P.I.; Cody, H.S., 3rd. Sentinel lymph node biopsy in breast cancer: Initial experience at Memorial Sloan-Kettering Cancer Center. J. Am. Coll. Surg. 1998, 186, 423–427. [Google Scholar] [CrossRef]
- Cimmino, V.M.; Brown, A.C.; Szocik, J.F.; Pass, H.A.; Moline, S.; De, S.K.; Domino, E.F. Allergic reactions to isosulfan blue during sentinel node biopsy—A common event. Surgery 2001, 130, 439–442. [Google Scholar] [CrossRef]
- Juzeniene, A.; Juzenas, P.; Ma, L.-W.; Iani, V.; Moan, J. Topical application of 5-aminolaevulinic acid, methyl 5-aminolaevulinate and hexyl 5-aminolaevulinate on normal human skin. Br. J. Dermatol. 2006, 155, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Nour, A. Efficacy of Methylene Blue Dye in Localization of Sentinel Lymph Node in Breast Cancer Patients. Breast J. 2004, 10, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Thongvitokomarn, S.; Polchai, N. Indocyanine Green Fluorescence Versus Blue Dye or Radioisotope Regarding Detection Rate of Sentinel Lymph Node Biopsy and Nodes Removed in Breast Cancer: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2020, 21, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Brahma, B.; Putri, R.I.; Karsono, R.; Andinata, B.; Gautama, W.; Sari, L.; Haryono, S.J. The predictive value of methylene blue dye as a single technique in breast cancer sentinel node biopsy: A study from Dharmais Cancer Hospital. World J. Surg. Oncol. 2017, 15, 1–7. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef]
- Chance, B. Near-Infrared Images Using Continuous, Phase-Modulated, and Pulsed Light with Quantitation of Blood and Blood Oxygenationa. Ann. N. Y. Acad. Sci. 1998, 838, 29–45. [Google Scholar] [CrossRef]
- Vahrmeijer, A.L.; Hutteman, M.; Van Der Vorst, J.R.; Van De Velde, C.J.H.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013, 10, 507–518. [Google Scholar] [CrossRef]
- Schaafsma, B.E.; Mieog, J.S.; Hutteman, M.; van der Vorst, J.R.; Kuppen, P.J.; Löwik, C.W.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided onco-logic surgery. J. Surg. Oncol. 2011, 104, 323–332. [Google Scholar] [CrossRef]
- Zhang, R.R.; Schroeder, A.B.; Grudzinski, J.J.; Rosenthal, E.L.; Warram, J.M.; Pinchuk, A.N.; Eliceiri, K.W.; Kuo, J.S.; Weichert, J.P. Beyond the margins: Real-time detection of cancer using targeted fluorophores. Nat. Rev. Clin. Oncol. 2017, 14, 347–364. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Tsien, R.Y. Fluorescence-guided surgery with live molecular navigation—A new cutting edge. Nat. Rev. Cancer 2013, 13, 653–662. [Google Scholar] [CrossRef]
- Crane, L.M.; Themelis, G.; Pleijhuis, R.G.; Harlaar, N.J.; Sarantopoulos, A.; Arts, H.J.; van der Zee, A.G.; Ntziachristos, V.; van Dam, G.M. Intraoperative multispectral fluorescence imaging for the detection of the sentinel lymph node in cervical cancer: A novel concept. Mol. Imaging Biol. 2011, 13, 1043–1049. [Google Scholar] [CrossRef]
- Zelken, J.A.; Tufaro, A.P. Current Trends and Emerging Future of Indocyanine Green Usage in Surgery and Oncology: An Update. Ann. Surg. Oncol. 2015, 22, 1271–1283. [Google Scholar] [CrossRef]
- Chi, C.; Du, Y.; Ye, J.; Kou, D.; Qiu, J.; Wang, J.; Tian, J.; Chen, X. Intraoperative Imaging-Guided Cancer Surgery: From Current Fluorescence Molecular Imaging Methods to Future Multi-Modality Imaging Technology. Theranostics 2014, 4, 1072–1084. [Google Scholar] [CrossRef]
- Moody, E.D.; Viskari, P.J.; Colyer, C.L. Non-covalent labeling of human serum albumin with indocyanine green: A study by capillary electrophoresis with diode laser-induced fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 1999, 729, 55–64. [Google Scholar] [CrossRef]
- Ogawa, M.; Kosaka, N.; Choyke, P.L.; Kobayashi, H. In vivo Molecular Imaging of Cancer with a Quenching Near-Infrared Fluorescent Probe Using Conjugates of Monoclonal Antibodies and Indocyanine Green. Cancer Res. 2009, 69, 1268–1272. [Google Scholar] [CrossRef]
- Landsman, M.L.; Kwant, G.; Mook, G.A.; Zijlstra, W.G. Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J. Appl. Physiol. 1976, 40, 575–583. [Google Scholar] [CrossRef]
- Alford, R.; Simpson, H.M.; Duberman, J.; Hill, G.C.; Ogawa, M.; Regino, C.; Kobayashi, H.; Choyke, P.L. Toxicity of Organic Fluorophores Used in Molecular Imaging: Literature Review. Mol. Imaging 2009, 8, 341–354. [Google Scholar] [CrossRef]
- Shimizu, S.; Kamiike, W.; Hatanaka, N.; Yoshida, Y.; Tagawa, K.; Miyata, M.; Matsuda, H. New method for measuring ICG Rmax with a clearance meter. World J. Surg. 1995, 19, 113–118. [Google Scholar] [CrossRef]
- Speich, R.; Saesseli, B.; Hoffmann, U.; Neftel, K.A.; Reichen, J. Anaphylactoid Reactions after Indocyanine-Green Administration. Ann. Intern. Med. 1988, 109, 345–346. [Google Scholar] [CrossRef]
- Vahrmeijer, A.L.; Frangioni, J.V. Seeing the invisible during surgery. BJS 2011, 98, 749–750. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Orihashi, K.; Nishimori, H.; Wariishi, S.; Fukutomi, T.; Kondo, N.; Kihara, K.; Sato, T.; Sasaguri, S. Indocyanine green angiography for intra-operative assessment in vascular surgery. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2012, 43, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Keereweer, S.; Van Driel, P.; Snoeks, T.; Kerrebijn, J.; De Jong, R.; Robert, J.B.; Vahrmeijer, A.; Sterenborg, H.J.; Löwik, C. Optical Image-Guided Cancer Surgery: Challenges and Limitations. Clin. Cancer Res. 2013, 19, 3745–3754. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Sevick-Muraca, E.M. A review of performance of near-infrared fluorescence imaging devices used in clinical studies. Br. J. Radiol. 2015, 88, 20140547. [Google Scholar] [CrossRef]
- Tummers, Q.R.J.G.; Hoogstins, C.E.S.; Peters, A.A.W.; De Kroon, C.D.; Trimbos, J.B.M.Z.; Van De Velde, C.J.H.; Frangioni, J.V.; Vahrmeijer, A.L.; Gaarenstroom, K.N. The Value of Intraoperative Near-Infrared Fluorescence Imaging Based on Enhanced Permeability and Retention of Indocyanine Green: Feasibility and False-Positives in Ovarian Cancer. PLoS ONE 2015, 10, e0129766. [Google Scholar] [CrossRef]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P.A. Review of Indocyanine Green Fluorescent Imaging in Surgery. Int. J. Biomed. Imaging 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Ohnishi, S.; Lomnes, S.J.; Laurence, R.G.; Gogbashian, A.; Mariani, G.; Frangioni, J.V. Organic Alternatives to Quantum Dots for Intraoperative Near-Infrared Fluorescent Sentinel Lymph Node Mapping. Mol. Imaging 2005, 4, 172–181. [Google Scholar] [CrossRef]
- Kong, S.-H.; Marchegiani, F.; Soares, R.; Liu, Y.-Y.; Suh, Y.-S.; Lee, H.-J.; Dallemagne, B.; Yang, H.-K.; Marescaux, J.; Diana, M. Fluorescence lymphangiography-guided full-thickness oncologic gastric resection. Surg. Endosc. 2018, 33, 620–632. [Google Scholar] [CrossRef]
- Kong, S.-H.; Noh, Y.-W.; Suh, Y.-S.; Park, H.S.; Lee, H.-J.; Kang, K.W.; Kim, H.C.; Lim, Y.T.; Yang, H.-K. Evaluation of the novel near-infrared fluorescence tracers pullulan polymer nanogel and indocyanine green/γ-glutamic acid complex for sentinel lymph node navigation surgery in large animal models. Gastric Cancer 2015, 18, 55–64. [Google Scholar] [CrossRef]
- Mieog, J.S.D.; Troyan, S.L.; Hutteman, M.; Donohoe, K.J.; Van Der Vorst, J.R.; Stockdale, A.; Liefers, G.-J.; Choi, H.S.; Gibbs-Strauss, S.L.; Putter, H.; et al. Toward Optimization of Imaging System and Lymphatic Tracer for Near-Infrared Fluorescent Sentinel Lymph Node Mapping in Breast Cancer. Ann. Surg. Oncol. 2011, 18, 2483–2491. [Google Scholar] [CrossRef]
- Cho, S.S.; Salinas, R.; Lee, J.Y.K. Indocyanine-Green for Fluorescence-Guided Surgery of Brain Tumors: Evidence, Techniques, and Practical Experience. Front. Surg. 2019, 6, 11. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, D.; Wang, Z.; Zhang, X.; Zhang, Q.; Newton, A.D.; Singhal, S.; Cai, H.; Wang, Y.; Lu, Q.; et al. Kinetics of indocyanine green: Optimizing tumor to normal tissue fluorescence in image-guided oral cancer surgery applications. Head Neck 2019, 41, 1032–1038. [Google Scholar] [CrossRef]
- Al-Dam, A.; Precht, C.; Barbe, A.; Kohlmeier, C.; Hanken, H.; Wikner, J.; Schön, G.; Heiland, M.; Assaf, A.T. Sensitivity and specificity of sentinel lymph node biopsy in patients with oral squamous cell carcinomas using indocyanine green fluorescence imaging. J. Cranio-Maxillofac. Surg. 2018, 46, 1379–1384. [Google Scholar] [CrossRef]
- Grischke, E.-M.; Röhm, C.; Hahn, M.; Helms, G.; Brucker, S.; Wallwiener, D. ICG Fluorescence Technique for the Detection of Sentinel Lymph Nodes in Breast Cancer: Results of a Prospective Open-label Clinical Trial. Geburtshilfe und Frauenheilkd 2015, 75, 935–940. [Google Scholar] [CrossRef]
- Kinami, S.; Oonishi, T.; Fujita, J.; Tomita, Y.; Funaki, H.; Fujita, H.; Nakano, Y.; Ueda, N.; Kosaka, T. Optimal settings and accuracy of indocyanine green fluorescence imaging for sentinel node biopsy in early gastric cancer. Oncol. Lett. 2016, 11, 4055–4062. [Google Scholar] [CrossRef][Green Version]
- van der Vorst, J.R.; Schaafsma, B.E.; Verbeek, F.P.; Keereweer, S.; Jansen, J.C.; van der Velden, L.-A.; Langeveld, A.P.; Hutteman, M.; Löwik, C.W.; van de Velde, C.J.; et al. Near-infrared fluorescence sentinel lymph node mapping of the oral cavity in head and neck cancer patients. Oral Oncol. 2013, 49, 15–19. [Google Scholar] [CrossRef]
- Kim, J.H.; Byeon, H.K.; Kim, D.H.; Kim, S.-H.; Choi, E.C.; Koh, Y.W. ICG-Guided Sentinel Lymph Node Sampling during Robotic Retroauricular Neck Dissection in cN0 Oral Cancer. Otolaryngol. Neck Surg. 2020, 162, 410–413. [Google Scholar] [CrossRef]
- Peng, H.; Wang, S.J.; Niu, X.; Yang, X.; Chi, C.; Zhang, G. Sentinel node biopsy using indocyanine green in oral/oropharyngeal cancer. World J. Surg. Oncol. 2015, 13, 1–7. [Google Scholar] [CrossRef]
- Nakamura, T.; Kogashiwa, Y.; Nagafuji, H.; Yamauchi, K.; Kohno, N. Validity of sentinel lymph node biopsy by ICG fluo-rescence for early head and neck cancer. Anticancer. Res. 2015, 35, 1669–1674. [Google Scholar]
- Christensen, A.; Juhl, K.; Charabi, B.; Mortensen, J.; Kiss, K.; Kjær, A.; Von Buchwald, C. Feasibility of Real-Time Near-Infrared Fluorescence Tracer Imaging in Sentinel Node Biopsy for Oral Cavity Cancer Patients. Ann. Surg. Oncol. 2015, 23, 565–572. [Google Scholar] [CrossRef]
- Bredell, M.G. Sentinel lymph node mapping by indocyanin green fluorescence imaging in oropharyngeal cancer—Preliminary experience. Head Neck Oncol. 2010, 2, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Berg, N.S.V.D.; Brouwer, O.R.; Klop, W.M.C.; Karakullukcu, B.; Zuur, C.L.; Tan, I.B.; Balm, A.J.M.; Brekel, M.W.M.V.D.; Olmos, R.A.V.; Van Leeuwen, F.W.B. Concomitant radio- and fluorescence-guided sentinel lymph node biopsy in squamous cell carcinoma of the oral cavity using ICG-99mTc-nanocolloid. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Iwai, T.; Maegawa, J.; Hirota, M.; Tohnai, I. Sentinel lymph node biopsy using a new indocyanine green fluorescence imaging system with a colour charged couple device camera for oral cancer. Br. J. Oral Maxillofac. Surg. 2013, 51, e26–e28. [Google Scholar] [CrossRef] [PubMed]
- Borbón-Arce, M.; Brouwer, O.; Berg, N.V.D.; Mathéron, H.; Klop, W.; Balm, A.; Van Leeuwen, F.; Valdés-Olmos, R. An innovative multimodality approach for sentinel node mapping and biopsy in head and neck malignancies. Revista Española de Medicina Nuclear e Imagen Molecular 2014, 33, 274–279. [Google Scholar] [CrossRef]
- Murase, R.; Tanaka, H.; Hamakawa, T.; Goda, H.; Tano, T.; Ishikawa, A.; Hino, S.; Sumida, T.; Nakashiro, K.; Hamakawa, H. Double sentinel lymph node mapping with indocyanine green and 99m-technetium–tin colloid in oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2015, 44, 1212–1217. [Google Scholar] [CrossRef]
- Honda, K.; Ishiyama, K.; Suzuki, S.; Kawasaki, Y.; Saito, H.; Horii, A. Sentinel Lymph Node Biopsy Using Preoperative Computed Tomographic Lymphography and Intraoperative Indocyanine Green Fluorescence Imaging in Patients with Localized Tongue Cancer. JAMA Otolaryngol. Neck Surg. 2019, 145, 735–740. [Google Scholar] [CrossRef]
- Yokoyama, J.; Hasegawa, Y.; Sugasawa, M.; Shiotani, A.; Murakami, Y.; Ohba, S.; Kohno, N. Long term-follow-up multicenter feasibility study of ICG fluorescence-navigated sentinel node biopsy in oral cancer. Mol. Clin. Oncol. 2020, 13, 1. [Google Scholar] [CrossRef]
- Nakamura, Y.; Fujisawa, Y.; Nakamura, Y.; Maruyama, H.; Furuta, J.-I.; Kawachi, Y.; Otsuka, F. Improvement of the sentinel lymph node detection rate of cervical sentinel lymph node biopsy using real-time fluorescence navigation with indocyanine green in head and neck skin cancer. J. Dermatol. 2013, 40, 453–457. [Google Scholar] [CrossRef]
- Stoffels, I.; Leyh, J.; Pöppel, T.; Schadendorf, D.; Klode, J. Evaluation of a radioactive and fluorescent hybrid tracer for sentinel lymph node biopsy in head and neck malignancies: Prospective randomized clinical trial to compare ICG-99mTc-nanocolloid hybrid tracer versus 99mTc-nanocolloid. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1631–1638. [Google Scholar] [CrossRef]
- Schilling, C.; Stoeckli, S.J.; Vigili, M.G.; De Bree, R.; Lai, S.Y.; Alvarez, J.; Christensen, A.; Cognetti, D.M.; D’Cruz, A.K.; Frerich, B.; et al. Surgical consensus guidelines on sentinel node biopsy (SNB) in patients with oral cancer. Head Neck 2019, 41, 2655–2664. [Google Scholar] [CrossRef]
- Desmettre, T.; Devoisselle, J.M.; Mordon, S. Fluorescence properties and metabolic features of indocyanine green (ICG) as related to angiography. Surv. Ophthalmol. 2000, 45, 15–27. [Google Scholar] [CrossRef]
- Noh, Y.-W.; Kong, S.-H.; Choi, D.-Y.; Park, H.S.; Yang, H.-K.; Lee, H.-J.; Kim, H.C.; Kang, K.W.; Sung, M.-H.; Lim, Y.T. Near-Infrared Emitting Polymer Nanogels for Efficient Sentinel Lymph Node Mapping. ACS Nano 2012, 6, 7820–7831. [Google Scholar] [CrossRef]
- Noh, Y.-W.; Park, H.S.; Sung, M.-H.; Lim, Y.T. Enhancement of the photostability and retention time of indocyanine green in sentinel lymph node mapping by anionic polyelectrolytes. Biomaterials 2011, 32, 6551–6557. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Fu, Y.-Y.; Peng, Q.; Guo, S.-S.; Liu, G.; Li, J.; Yang, H.-H.; Chen, G.-N. Dye-enhanced graphene oxide for photothermal therapy and photoacoustic imaging. J. Mater. Chem. B 2013, 1, 5762–5767. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumor-itropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Khullar, O.; Frangioni, J.V.; Grinstaff, M.; Colson, Y.L. Image-Guided Sentinel Lymph Node Mapping and Nanotechnology-Based Nodal Treatment in Lung Cancer Using Invisible Near-Infrared Fluorescent Light. Semin. Thorac. Cardiovasc. Surg. 2010, 21, 309–315. [Google Scholar] [CrossRef]
- Tsuchimochi, M.; Hayama, K.; Toyama, M.; Sasagawa, I.; Tsubokawa, N. Dual-modality imaging with 99mTc and fluorescent indocyanine green using surface-modified silica nanoparticles for biopsy of the sentinel lymph node: An animal study. EJNMMI Res. 2013, 3, 33. [Google Scholar] [CrossRef]
- Mok, H.; Jeong, H.; Kim, S.-J.; Chung, B.H. Indocyanine green encapsulated nanogels for hyaluronidase activatable and selective near infrared imaging of tumors and lymph nodes. Chem. Commun. 2012, 48, 8628–8630. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Tse, B.W.-C.; Yang, H.; Thorling, C.A.; Liu, Y.; Touraud, M.; Chouane, J.B.; Liu, X.; Roberts, M.S.; et al. Indocyanine green-incorporating nanoparticles for cancer theranostics. Theranostics 2018, 8, 1227–1242. [Google Scholar] [CrossRef]
- Riaz, A.; Shreedhar, B.; Kamboj, M.; Natarajan, S. Methylene blue as an early diagnostic marker for oral precancer and cancer. SpringerPlus 2013, 2, 95. [Google Scholar] [CrossRef]
- Van Dam, G.M.; Themelis, G.; Crane, L.M.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; De Jong, J.S.; Arts, H.J.G.; Van Der Zee, A.G.J.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef]
- De Boer, E.; Warram, J.M.; Tucker, M.D.; Hartman, Y.E.; Moore, L.S.; De Jong, J.S.; Chung, T.K.; Korb, M.L.; Zinn, K.R.; Van Dam, G.M.; et al. In Vivo Fluorescence Immunohistochemistry: Localization of Fluorescently Labeled Cetuximab in Squamous Cell Carcinomas. Sci. Rep. 2015, 5, 10169. [Google Scholar] [CrossRef]
- Moore, L.S.; Rosenthal, E.L.; Chung, T.K.; De Boer, E.; Patel, N.; Prince, A.C.; Korb, M.L.; Walsh, E.M.; Young, E.S.; Stevens, T.M.; et al. Characterizing the Utility and Limitations of Repurposing an Open-Field Optical Imaging Device for Fluorescence-Guided Surgery in Head and Neck Cancer Patients. J. Nucl. Med. 2016, 58, 246–251. [Google Scholar] [CrossRef]
- Rosenthal, E.L.; Moore, L.S.; Tipirneni, K.; De Boer, E.; Stevens, T.M.; Hartman, Y.E.; Carroll, W.R.; Zinn, K.R.; Warram, J.M. Sensitivity and Specificity of Cetuximab-IRDye800CW to Identify Regional Metastatic Disease in Head and Neck Cancer. Clin. Cancer Res. 2017, 23, 4744–4752. [Google Scholar] [CrossRef]
- Gao, R.W.; Teraphongphom, N.; De Boer, E.; Berg, N.S.V.D.; Divi, V.; Kaplan, M.J.; Oberhelman, N.J.; Hong, S.S.; Capes, E.; Colevas, A.D.; et al. Safety of panitumumab-IRDye800CW and cetuximab-IRDye800CW for fluorescence-guided surgical navigation in head and neck cancers. Theranostics 2018, 8, 2488–2495. [Google Scholar] [CrossRef]
- Gao, R.W.; Teraphongphom, N.T.; Berg, N.S.V.D.; Martin, B.A.; Oberhelman, N.J.; Divi, V.; Kaplan, M.J.; Hong, S.S.; Lu, G.; Ertsey, R.; et al. Determination of Tumor Margins with Surgical Specimen Mapping Using Near-Infrared Fluorescence. Cancer Res. 2018, 78, 5144–5154. [Google Scholar] [CrossRef]
- van Keulen, S.; Berg, N.S.V.D.; Nishio, N.; Birkeland, A.; Zhou, Q.; Lu, G.; Wang, H.-W.; Middendorf, L.; Forouzanfar, T.; Martin, B.A.; et al. Rapid, non-invasive fluorescence margin assessment: Optical specimen mapping in oral squamous cell carcinoma. Oral Oncol. 2019, 88, 58–65. [Google Scholar] [CrossRef]
- Van Keulen, S.; Nishio, N.; Fakurnejad, S.; Birkeland, A.; Martin, B.A.; Lu, G.; Zhou, Q.; Chirita, S.U.; Forouzanfar, T.; Colevas, A.D.; et al. The Clinical Application of Fluorescence-Guided Surgery in Head and Neck Cancer. J. Nucl. Med. 2019, 60, 758–763. [Google Scholar] [CrossRef]
| Author | Stage | Population | NIR Imaging Device | Tracer | ICG Dilution Solvent | ICG Concen-Tration (mg/mL) | ICG Dose (ml) | Injection Route | ICG Injection Time | Time to SLN Identification after Injection |
|---|---|---|---|---|---|---|---|---|---|---|
| Bredell (2010) [71] | TxN0 | 8 (5: OSCC, 3: Maxilla) | PDE | ICG alone | sterile water | 10 | ICG: 1 | Peritumoral at least 5P | After induction of anesthesia | 30 min initially, down to 5 min or less in the latter cases |
| van den Berg (2012) [72] | T1-2N0 | 14 | HEMS | ICG-99m Tc nanocolloid | sterile water | 5 | total 0.4 mL of median of 77 (range 67–94) MBq hybrid tracer | Peritumoral 3-4P | 3–19 h before surgery | NA |
| Iwai (2012) [73] | TxN0 | 1 | HEMS | ICG alone | NA | 5 | ICG: 0.5–1 | Peritumoral 4P | After induction of anesthesia | Within several minutes |
| van der Vorst (2013) [66] | T1-2N0 | 10 (8: OSCC, 2: OPC) | Mini-FLARE | ICG:HSA | sterile water | 2.5 | 1.6-mL of 500 μM ICG:HSA | Peritumoral 4P | After flap elevation | 5, 10, 15, 20, 25, 30, 45 and 60 min |
| Borbón-Arce (2014) [74] | T1-2N0 | 25 (9: OSCC, 16: Melanoma) | PDE | ICG-99m Tc nanocolloid | sterile water | 5 | total 0.4 mL median of 85 MBq (range 66–158 MBq) hybrid tracer | Peritumoral 3-4P | 3–24 h before surgery | NA |
| Murase (2015) [75] | T1-2N0 | 16 | PDE | ICG+ 99m Tc-tincolloid | sterile water | 5 | ICG: 0.4 (0.4ml of 74MBq 99mTc–tin colloid) | Peritumoral | After induction of anesthesia | NA |
| Peng (2015) [68] | T1-2N0 | 26 (19: OSCC, 7: OPC) | OMIONS | ICG + MB | NA | 5 (MB: 10) | ICG: 1 (MB: 1.5) | Peritumoral 4P | Before skin incision | NA |
| Nakamura (2015) [69] | T1-2N0 | 19 (15: OSCC, 2: OPC, 2:HPC) | HEMS | 99mTc-tin colloid (n = 13), ICG + 99mTc-tin colloid (n = 4), ICG (n = 2) | sterile water | 2.5 | ICG: 0.5 (1.0 mL of 99m Tc-tin colloid) | Peritumoral 4P | SLN detection at 15 min after ICG injection | ICG or ICG + RI: 19.8 ± 12.6 min RI alone: 30.6 ± 11.6 minutes |
| Christensen (2016) [70] | T1-2N0 | 30 | Fluobeam 800 | ICG-99m Tc nanocoll | sterile water | 5 | total 0.2 mL of hybrid tracer (55 MBq at same day, 110 MBq at day before surgery) | Peritumoral 4P | NA | from skin incision to skin closure: average 39 min |
| Al-Dam (2018) [63] | T1-2N0 | 20 | PDE | ICG | sterile water | higher | 0.5 mg/kg in 2 mL | Peritumoralat least 5P | After flap elevation | 8.1 min (range 1–22) |
| Honda (2019) [76] | T1-2N0 | 18 | HEMS/ PDE | ICG | sterile water | 5 | ICG: 2mL | Peritumoral | After flap elevation | 1 or 2 min after injection |
| Kim (2020) [67] | T1-2N0 | 9 | Da Vinci Robotic system Firefly | ICG | sterile water | 2.5 | ICG: 2 mL | Peritumoral 4P | 12 h before surgery | NA |
| Yokohama (2020) [77] | T2-3N0 | 18 | PDE | ICG | NA | 2.5 | NA | Peritumoral 4P | During surgery | 10 min after injection, transcutaneous SLN detection |
| Author | Preoperative Imaging Modality | Number of Preoperative Localized SLNs | Number of Intraoperative Radioactive SLNs | Number of Intraoperative Fluorescent SLNs | Number of Patient with Detected SLNS | Number of Patient with Metastatic SLNs | Recurrence (Number of Patients) | Type of Surgical Procedure | Number of Patient with False Negative |
|---|---|---|---|---|---|---|---|---|---|
| Bredell (2010) [71] | NA | NA | NA | 1–5 per patient (average 3) | 8/8 (100%) | 1 (12.5%) | NA | Biopsy | NA |
| van den Berg (2012) [72] | LSG followed by SPECT/CT | 41 | 43 | 47 | 14/14 (100%) | 1 (7.1%) | NA | Biopsy | NA |
| Iwai (2012) [73] | CT lymphography | NA | NA | NA | NA | NA | NA | Biopsy | NA |
| van der Vorst (2013) [66] | NA | NA | NA | 17 (average 1.7 ± 0.8 per patient) | 10/10 (100%) | 3 (30%) | NA | Planned neck dissection | 1 |
| Borbón-Arce (2014) [74] | LSG followed by SPECT/CT | 67 | 87 | 86 | 25/25 (100%) | 6 (24%) | NA | Biopsy | 0 |
| Murase (2015) [75] | LSG followed by SPECT/CT | 25 | 28 | 35 | 16/16 (100%) | 2 (12.5%) | 1 (in positive SLN): DOD-11 months, 1 (in negative SLN): DOC-24 months (3 years follow up) | Biopsy | 0 |
| Peng (2015) [68] | NA | NA | NA | 88 (average 3.4 per patient) | 26/26 (100%) | 4 (15.4%) | NA | Planned neck dissection | 0 |
| Nakamura (2015) [69] | LSG | 31 | 31 | ICG alone: total 3 LNs, ICG + RI: average 3 LNs, RI alone: average 2 LNs | 19/19 (100%) | 2 (10.5%) ICG alone: none ICG + RI: 1 RI alone: 1 | 1 (RI-alone): nodal recurrence 1 year later | Biopsy(+)-> neck dissection | 1 |
| Christensen (2016) [70] | LSG followed by SPECT/CT | 68 | 83 | 94 | 30/30 (100%) | 6 (20%) | NA | Biopsy | 0 |
| Al-Dam (2018) [63] | NA | NA | NA | 39 (average 1.95 per patient) | 20/20 (100%) | 8 (40%) | 4: regional relapse (2.4 years follow up) | Planned neck dissection | 4 |
| Honda (2019) [76] | CT lymphography | 25 (16/18 pts) | NA | 29 | 16/16 (100%) | 5 (31.3%) | 2 | T1-2: Biopsy(+)-> neck dissection, Advanced T2: Planned neck dissection | 2 |
| Kim (2020) [67] | NA | NA | NA | 31 | 9/9 (100%) | 2 (22.2%) | None (4 years follow up) | Planned neck dissection | 0 |
| Yokohama (2020) [77] | LSG with or without SPECT/CT | NA | 63 | 67 | 18/18(100%) | 5/18 (27.7%) | 5 | Biopsy(+)-> neck dissection | 0 |
| ClinicalTrials.Gov Identifier | Start | No. of Patients | Target | Timing | Dose | Primary and Secondary Outcome | Country |
|---|---|---|---|---|---|---|---|
| NCT02027831 | 2013 | 10 | All patients requiring neck dissection with or without resection of the primary head and neck cancer | Intravenous injection before the surgery | 0.25 mg/kg | distribution of ICG in the normal and pathological lymph nodes | Belgium |
| NCT02640170 | 2015 | 500 | Resectable solid tumors (lung, breast, kidney, parathyroid, prostate, stomach, head and neck etc.) | NA | NA | monitor the rate of recurrence in patients who undergo cancer surgery. (prospective design) | USA |
| NCT02920216 | 2016 | 10 | Salvage surgery for recurrence of head and neck cancer in irradiated area | Intravenous injection before the surgery | 0.25 mg/kg | Sensitivity of ICG in irradiated area and surgical margins | France |
| NCT02997553 | 2017 | 744 | ICG guided SLN biopsy compared with the 99mTc guided SLN biopsy in patients with cancers and subjected to surgery. (breast, head and neck, melanoma, cervix, rectum etc.) | Intravenous injection | 2.5 mg/mL | Non-inferiority of ICG guided SLN biopsy | France |
| NCT03745690 | 2018 | 20 | Head and neck cancer | Intravenous injection the day before surgery | NA | safety profile of high-dose ICG, the efficacy of high-dose ICG to identify cancer compared to surrounding normal tissue | USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Ku, M.; Yang, J.; Byeon, H.K. Recent Developments of ICG-Guided Sentinel Lymph Node Mapping in Oral Cancer. Diagnostics 2021, 11, 891. https://doi.org/10.3390/diagnostics11050891
Kim J-H, Ku M, Yang J, Byeon HK. Recent Developments of ICG-Guided Sentinel Lymph Node Mapping in Oral Cancer. Diagnostics. 2021; 11(5):891. https://doi.org/10.3390/diagnostics11050891
Chicago/Turabian StyleKim, Ji-Hoon, Minhee Ku, Jaemoon Yang, and Hyung Kwon Byeon. 2021. "Recent Developments of ICG-Guided Sentinel Lymph Node Mapping in Oral Cancer" Diagnostics 11, no. 5: 891. https://doi.org/10.3390/diagnostics11050891
APA StyleKim, J.-H., Ku, M., Yang, J., & Byeon, H. K. (2021). Recent Developments of ICG-Guided Sentinel Lymph Node Mapping in Oral Cancer. Diagnostics, 11(5), 891. https://doi.org/10.3390/diagnostics11050891







