Abstract
Despite the substantial changes resulting from the introduction of combination antiretroviral therapy (cART), the prevalence of HIV-associated neurocognitive disorders (HAND) remains substantial. Blood–brain barrier impairment (BBBi) is a frequent feature in people living with HIV (PLWH) and it may persist despite effective antiretroviral treatment. A cross-sectional study was performed in PLWH who underwent lumbar puncture for clinical reasons or research protocols and several cerebrospinal fluid biomarkers were studied. BBBi was defined as cerebrospinal fluid-to-serum albumin ratio (CSAR) >6.5 (<40 years) or >8 (>40 years). We included 464 participants: 147 cART-naïve and 317 on cART. Male sex was prevalent in both groups (72.1% and 72.2% respectively); median age was 44 (38–52) years in naïve and 49 (43–57) years in treated subjects. BBBi was observed in 35.4% naïve and in 22.7% treated participants; the use of integrase inhibitors was associated with a lower prevalence (18.3 vs. 30.9%, p = 0.050). At multivariate binary logistic regression (including age and sex) nadir CD4 cell count (p = 0.034), presence of central nervous system (CNS) opportunistic infections (p = 0.024) and cerebrospinal fluid (CSF) HIV RNA (p = 0.002) in naïve participants and male sex (p = 0.021), a history of CNS opportunistic infections (p = 0.001) and CSF HIV RNA (p = 0.034) in treated patients were independently associated with BBBi. CSF cells and neopterin were significantly higher in participants with BBBi. BBBi was prevalent in naïve and treated PLWH and it was associated with CSF HIV RNA and neopterin. Systemic control of viral replication seems to be essential for BBB integrity while sex and treatment influence need further studies.
1. Introduction
There are approximately 38 million people living with HIV worldwide [1], out of which 20–50% are estimated to develop a certain degree of cognitive impairment [2]. Despite combination antiretroviral therapy (cART) that has substantially contributed to reducing the HIV-related complications and mortality, HIV-associated neurocognitive disorders (HAND) continue to be relevant, especially considering their prevalence on a global scale [3]. Blood–brain barrier impairment (BBBi) may play a crucial role in the pathogenesis of HAND. In this regard, up to 22% of asymptomatic HIV-positive subjects, 50% of patients suffering from AIDS and up to 100% of patients with HIV-associated dementia (HAD) showed an increased BBB permeability [4].
Monocyte transit across the BBB is a pivotal process in HIV central nervous system (CNS) infection [5] and several mechanisms have been involved in the pathogenesis of HAND, including neuroinflammation, antiretroviral neurotoxicity, tight junction dysregulation and the role of Tat and gp120 as neurotoxic viral proteins able to induce dose-dependent oxidative stress directly damaging BBB integrity [6]. Moreover, since co-receptors that could be used by HIV to enter CD4+ T cells have been detected in human astrocytes, a key cell in maintaining BBB integrity [6], several studies support the idea that astrocytes play a key role in the pathogenesis of HAND and they should be considered as a target for therapy [7,8,9]. Indeed, it is evident that BBB impairment is associated with vascular damage, permeability alteration and the accumulation of toxins [6].
A number of neurodegenerative disorders, such as Alzheimer’s disease (AD), are associated with BBBi, microvascular and neurovascular degeneration [10]. Moreover, even though vascular dysfunction in AD has been usually ascribed to the accumulation of beta--amyloid and tau [11], a recent study suggests that neurovascular dysfunction associated with BBBi is an early biomarker of cognitive decline in AD regardless of beta-amyloid and tau abnormalities [12].
BBBi has been observed despite cART and it has been associated with neuronal damage biomarkers, such as increased levels of total tau and phosphorylated tau [7]. Several blood and cerebrospinal fluid (CSF) biomarkers and imaging investigations have been examined as potential HAND biomarkers, yet so far none of them has proved sufficient accuracy in diagnosing and monitoring patients affected by HAND [13].
The aim of this study was to describe demographic, clinical and therapeutic characteristics, risk factors, comorbidities, as well as CSF and plasma biomarkers in both naïve and treated PLWH, potentially associated with BBBi.
2. Materials and Methods
A cross-sectional study was performed involving patients with confirmed HIV infection, who underwent lumbar puncture for clinical reasons or research protocols and who were capable to sign the informed consent. The trial was approved by Ethics Committee of the University of Turin (prospective study on predictors of neurocognitive decline in HIV-positive patients PRODIN, protocol code 103/2015 approved on 22 June 2015).
Demographic data, risk factors, co-infections, psychiatric comorbidities, therapeutic and immunovirological data were recorded. The cerebrospinal fluid-to-serum albumin ratio (CSAR), calculated as CSF albumin (mg/L)/serum albumin (g/L), was used to evaluate BBB integrity.
Blood–brain barrier damage definition was derived from age-adjusted Reibergrams (normal if below 6.5 in patients aged <40 years and below 8 in patients >40 years) [14].
CSF total tau (t–tau), phosphorylated tau (p–tau), and beta–amyloid1–42 (Abeta1–42) were measured by immunoenzymatic methods (Innogenetics, Ghent, Belgium, EU) with limits of detection, respectively, of 87, 15, and 87 pg/mL. Neopterin was measured through validated ELISA methods (DRG Diagnostics, Marnurg, Germany, EU). Reference values were as follows: t–tau < 300 pg/mL (in patients aged 21–50), <450 pg/mL (in patients aged 51–70), and <500 pg/mL in older patients; p–tau < 61 pg/mL; 1–42 beta-amyloid > 500 pg/mL; neopterin < 1.5 ng/mL HIV–RNA was quantified by the Roche Amplicor assay v2.0 (Hoffman–La Roche, Basel, Switzerland) with a lower limit of quantification of 20 copies/mL.
HAND was diagnosed according to the Frascati criteria [15]; the neurocognitive evaluation was based on 14 tests, assessing eight different cognitive domains; patients were categorized as having either asymptomatic (ANI) or mild neurocognitive impairment (MND), or HIV-associated dementia (HAD).
Data were analyzed using nonparametric statistical methods: variables were described with medians (interquartile ranges, IQR), absolute values (proportion) or ranges (minimum–maximum). Then, the associations between these variables with BBBi were assessed using Spearman’s test for continuous numerical variables, Mann–Whitney and Fisher’s exact/Chi2 test for categorical ones. A multivariate analysis was performed to select the determinants independently associated with BBBi: we used a binary logistic regression analysis using variables with a p-value <0.05 at bivariate comparisons. Data analysis was performed using SPSS software for Mac (version 22.0, IBM Corp, Armonk, NY, USA).
3. Results
3.1. Participants’ Demographic and Clinical Features
Four hundred sixty-four patients were included, of which 147 were cART-naïve (median age of 44 years) and 317 were cART-treated (median age of 49 years); their demographic features, risk factors and comorbidities are shown in Table 1.

Table 1.
Demographic features, risk factors and comorbidities of study participants.
3.2. Antiretroviral Naïve and Treated Participants
Both groups of patients consisted mostly of male subjects (72.1% of naïve and 72.2% of ART-treated subjects). With regard to the co-infections, HCV, past syphilis and toxoplasmosis were prevalent among ART-treated subjects (HCV-positive 28.2% vs. 16.8%; past syphilis 22.0% vs. 21.4% and toxoplasmosis 42% vs. 31.2% in cART-treated and cART naïve, respectively).
Clinical and cART-related features of the study participants are shown in Table 2. Clinical categories were represented by: HAND (asymptomatic or mild neurocognitive impairment, and HIV-associated dementia), primary HIV acute infections, late presenters, CNS opportunistic infections, HIV–encephalitis, CSF viral escape or rebound encephalitis, isolated white matter hyperintensities, leukoencephalopathy, syphilis or neurosyphilis, and other CNS disorders. HAND prevalence was 28.5% among treated subjects versus 10.1% in naïve patients.

Table 2.
Clinical and cART-related features of study participants.
Plasma and CSF characteristics and bivariate comparisons among naïve and treated subjects are shown in Table 3. Median CD4+ count nadir was higher in ART-treated patients (97 cell/mm3 versus 49 cell/mm3 of naïve patients), as well as current CD4+ T cell count (366 cell/mm3 in ART-treated subjects versus 57 CD4+/mm3 in naïve). With regard to CSF biomarkers, naïve subjects had higher neopterin (2.96 vs. 0.94 ng/mL, p <0.001), but lower tau (165 vs. 222 pg/mL, p = 0.045) and p–tau levels (33 vs. 37 pg/mL, p = 0.040); Beta42 (962 vs. 919 pg/mL, p = 0.901) and S100Beta (145 vs. 129 pg/mL, p = 0.758) were similar between the two groups.

Table 3.
Laboratory features and biomarkers according to treatment group. Variables were tested through Mann–Whitney (continuous variables) or Chi–square/Fisher’s exact test (binomial).
3.3. Blood–Brain Barrier Impairment and CSF Biomarkers
BBBi was observed in 35.4% of naïve patients and in 22.7% of ART-treated people; clinical, demographic, and therapeutic variables stratified according to treatment group and presence/absence of BBBi are shown in Table 4 and depicted in Figure 1.

Table 4.
Demographic and clinical features, plasma, and cerebrospinal fluid biomarkers in patients with normal and impaired blood–brain barrier. P values are calculated separately in naïve (first column) and treated (second column) participants.
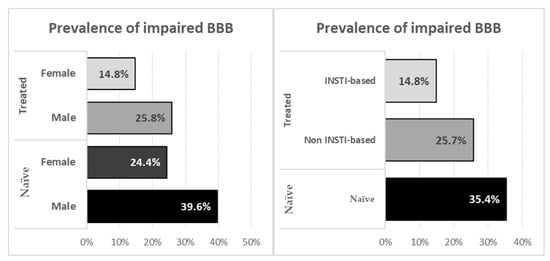
Figure 1.
Prevalence of impaired blood–brain barrier stratified according to sex (left panel) and to treatments (right panel).
Given the potentially different mechanisms underlying BBBi in naïve and cART receiving participants, we stratified analysis on predictors and biomarkers according to treatment status.
In naïve participants we observed that BBBi was associated with younger age (41 vs. 46 years, p = 0.034), higher CSF HIV RNA (4.36 vs. 3.71 Log10 copies/mL, p = 0.002) and with the presence of CNS opportunistic infections (25 vs. 6.3%, p <0.002). In treated participants BBBi was associated with male sex (30.6 vs. 18.1%, p = 0.037), higher CSF HIV RNA (1.53 vs. <1.28 Log10 copies/mL, p = 0.029), a history of CNS opportunistic infections (22.2 vs. 7.3%, p <0.001) and with non INSTI based regimens (30.9 vs. 18.3%, p = 0.050).
Of note, demographic, clinical and immunovirological features were not statistically different among INSTI and other-ARV recipients with the exception of a longer time since first positive HIV serology (167 vs. 124 months, p = 0.046) in INSTI–receivers.
JCV, CMV and EBV DNA were detected more commonly in participants with BBBi with statistically significant differences for CMV DNA (in naïve subjects) and EBV DNA (in treated individuals) (Figure 2).
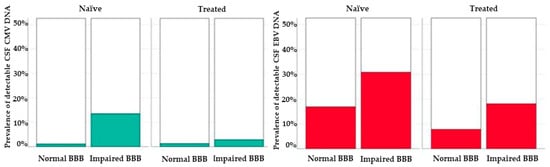
Figure 2.
Prevalence of detectable CMV (left, green bars) and EBV (right, red bars). DNA in the cerebrospinal fluid of study participants according to blood–brain barrier integrity and treatment status.
Besides higher CSF HIV RNA, we observed significantly higher levels of CSF neopterin in participants with BBBi (Figure 3).
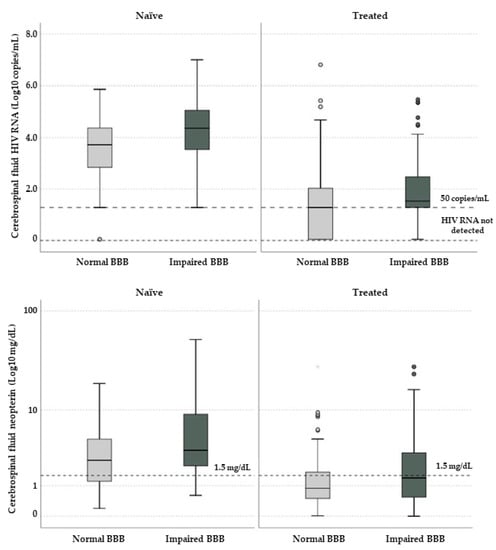
Figure 3.
Cerebrospinal fluid HIV RNA (above) and neopterin (below) in study participants according to blood–brain barrier integrity and treatment status. Horizontal lines and boxes represent median values and interquartile ranges; whiskers show 10th and 90th percentiles while circles and stars are outliers and extreme outliers. In the graph above dotted horizontal lines represent 50 copies/mL and “target not detected” values; in the one below the horizontal dotted line represents the proposed threshold for cerebrospinal fluid neopterin (1.5 mg/dL).
At multivariate binary logistic regression (including age and sex) we identified nadir CD4 cell count (p = 0.034, for 100 cells/uL increase aOR 1.401, 95% CI 1.026–1.912), presence of CNS opportunistic infections (p = 0.024, aOR 4.193, 95% CI 1.207–14.565) and CSF HIV RNA (p = 0.002, aOR for 1 Log10 increase 1.798, 95% CI 1.245–2.595) in naïve participants.
Aside from the aforementioned factors, we included the use of INSTI in the multivariate model for cART-treated participants: male sex (p = 0.021, aOR 3.230, 95% CI 1.191–8.755), a history of CNS opportunistic infections (p = 0.001, aOR 5.439, 95% CI 2.054–14.405) and CSF HIV RNA (p = 0.034, aOR for 1 Log10 increase 1.336, 95% CI 1.022–1.747) were independently associated with BBBi.
4. Discussion
We studied the prevalence of BBBi and a large set of variables in order to identify what may predict this event. We observed a prevalence of BBB impairment of 35.4% in ART-naïve and of 22.7% in cART-treated PLWH supporting the evidence that BBB alterations may persist despite antiretroviral therapy.
We have also identified female sex and cART therapy as independent protective factors for BBBi. In particular, male participants showed a higher prevalence of BBB alteration, both in ART-treated and naïve subjects. Furthermore at multivariate analysis female sex was an independent predictor of BBB integrity.
This difference could be ascribed to a greater prevalence of risk factors in males, such as hepatitis B (HBV), whose rate of infection is higher in men than in women in all Mediterranean countries [16]. Furthermore, male sex plays a key role in the progression of severe forms of chronic liver diseases [17].
It is worth noting that a major problem of the literature is the clear inequality of female representation in the study populations. Women constitute 51% of HIV-positive subjects worldwide and sex differences in HIV infection have been highlighted in several studies conducted both before and after the introduction of cART [18]. Nevertheless, several studies investigate sex differences and the prevalence of neurocognitive disorders in the female population, but the conclusions are not unequivocal. Some authors, as Maki and Martin–Thormeyer, highlight that there could be a higher risk of cognitive disorder in HIV-infected women [19]. Other studies reached similar conclusions, in particular suggesting that this difference is higher in some cognitive domains, such as memory and learning [20]. By contrast, in a recent study, Namagga and colleagues show higher prevalence and risk factors of HAND in men compared with women [21]. Finally, another evidence indicates that there are no substantial differences in cognitive impairment based on sex, except a higher risk of cognitive deficiency in the psychomotor domain for women [22]. These conflicting results suggest that further studies that specifically investigate the female population are needed especially considering the sex differences concerning the immune response, the pathogenesis of HIV infection, the pharmacological and pharmacodynamic responses [18], as well as the alteration of the blood–brain barrier [23].
In this study cART therapy resulted as an independent protective factor of BBBi. Interestingly, the effect of antiretroviral therapy on BBB has not been clearly demonstrated in literature despite similar analytical methods. Indeed, in their study, Abdulle and colleagues did not find a significant correlation between cART treatment and the reduction of BBB permeability [24]. Furthermore, a recent study highlights that despite CSF and plasma HIV–RNA suppression, neurofilament light chain (NFL) level and albumin ratio did not change after cART therapy [25]. Among the studied biomarkers, we observed a significant reduction in CSF 179 neopterin (suggesting the reduction in CNS inflammation and immune activation) and no change in 42–beta amyloid and S–100 prompting towards no effect of amyloid metabolism and astrocytosis. Interestingly, we noted higher total tau and phosphorylated tau in treated individuals suggesting either a detrimental effect of treatment on axonal integrity or the potential for age-associated damage potentially in common with Alzheimer’s dementia [26]. Despite years of research, a clear benefit of specific ARVs or combinations in improving CNS virological control and patients’ neurocognitive performance is unclear.
Our univariate analysis shows a lower prevalence of BBB alteration among participants treated with integrase inhibitors (INSTI), compared with patients treated with other classes of antiviral drugs (protease inhibitors or non–nucleoside reverse transcriptase inhibitors). Integrase inhibitors (Raltegravir, Elvitegravir, Dolutegravir and Bictegravir) are commonly used as first-line cART regimen, in association with other classes of antivirals, such as two nucleoside reverse transcriptase inhibitors [27]. Moreover, other elements can influence the therapy response, such as HIV DNA, substance abuse, pharmacogenetics, adherence to antiretrovirals and demographic factors, e.g., age and ethnicity [28]. Considering that, the current study shows two limitations worth mentioning: firstly, in the multivariate analysis not all of these factors have been considered as they were not available; secondly, a wider range of patients was treated with INSTI compared to those treated with other classes of drugs (28%, versus 17.4% NNRTI treated patients, 12.9% treated with PI and 5% with Maraviroc).
The association found between BBBi and CSF viral load, both among ART-naïve and treated patients, confirms the evidence observing an increase in CSF HIV–RNA among patients affected by neurological symptoms which were associated with BBBi [28]: a direct or indirect pathogenic mechanism linking uncontrolled HIV replication and BBB integrity seem reasonable. A persistent compartmental HIV replication (and potentially of other persistent viruses) may increase cell trafficking and immune activation. Inflammation seems to be a key factor in influencing BBB function and integrity since pleocytosis and abnormally high CSF neopterin were more prevalent in participants with BBBi. Although in recent studies neopterin was not associated with BBBi [7], the results of the current analysis support this concept. Indeed, a recent study has demonstrated that neopterin levels are elevated in CSF of untreated HIV patients, then its concentrations decrease as cART is started, and eventually reaches a plateau persisting at higher levels despite ART-therapy compared to HIV-negative controls [29].
In our analysis, EBV and CMV infections were more common among ART-naïve patients. The incidence of CMV infection has significantly decreased with cART introduction [30], despite that in their study Perello et al. [31] reported a higher incidence of acute CMV infection in the last years than in the early period of cART. In this regard, as in pre-cART era CMV coinfection caused a major risk of progression to AIDS, several studies show that even in cART epoch the presence of CMV in the blood is associated with a worse prognosis, cause of the increased risk of CMV disease progression, AIDS-defining diagnosis and death [32]. As reported in literature, our results confirm the less prevalence of CMV infection in cART treated patients, although CMV remains a relevant comorbidity even in the cART era.
EBV infects more than 90% of the worldwide population and it is associated with several cancers in PLWH, including Hodgkin lymphomas, non-Hodgkin lymphomas and Burkitt lymphomas [33]. Dehee and colleagues found a similar rate of EBV detection among 227 HIV-positive subjects and controls [34], but higher viral loads in the former ones: despite EBV replication may just represent a less controlling immune system studying EBV may be useful for understanding chronic immune activation and some HIV-associated non-infectious comorbidities [35].
Finally, the mechanism that underlies BBB impairment and HAND is not totally clear. HAND could be a direct consequence of the BBB breakdown by different mechanisms, such as an increased permeability, that allow an increasing entry of virus to CNS [36], or a higher neurotoxicity, caused by an increased drug concentration [37]; furthermore BBB disruption may reflect astrocytes and neurons alteration [8,28]. This is beyond the aim of the current study since we did not specifically analyze the link between BBB integrity and neurocognition.
5. Conclusions
BBB impairment is a common finding in PLWH affected by neurological or neurocognitive disorders either with or without suppressive cART. Indeed, BBB plays a critical role in CNS physiology and in neurological disorders, such as neurodegenerative disorders.
We observed higher levels of HIV RNA and neopterin in the CSF of study participants with BBBi, highlighting the significant role of inflammation in the pathogenesis of BBBi. Aside from virological control, female sex was found to be protective: further studies are needed to confirm this finding and to understand thelong–term consequences of an impaired BBB in PLWH.
Author Contributions
A.C., M.T. and G.C. performed data curation; A.C. analysed the data; M.T., V.G., J.C., M.N., C.A., A.C. contributed materials; D.I., S.B., G.D.P. supervised the study; G.C. wrote the paper; M.T. and A.C. reviewed the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding specifically dedicated to the Department of Medical Sciences from the Italian Ministry for Education, University and Research (Ministero dell’Istruzione, dell’Università e della Ricerca—MIUR) under the program “Dipartimenti di Eccellenza 2018–2022”.Project no D15D18000410001.
Institutional Review Board Statement
The trial was approved by Ethics Committee of the University of Turin (protocol code 103/2015 approved on 22 June 2015). Written informed consent was obtained from all participants.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Calcagno reports grants from VIIV, GILEAD, personal fees from VIIV, GILEAD, JANSSEN-CILAG, INSMED, MSD, outside the submitted work. Prof. Bonora and Prof. Di Perri report grants from VIIV, GILEAD, personal fees from VIIV, GILEAD, JANSSEN-CILAG, MSD, outside the submitted work. The remaining authors have no conflict of interest to declare.
References
- World Health Organization. Global Health Observatory (GHO) Data. Available online: https://www.who.int/gho/hiv/en (accessed on 30 March 2021).
- Trunfio, M.; Vai, D.; Montrucchio, C.; Alcantarini, C.; Livelli, A.; Tettoni, M.; Orofino, G.; Audagnotto, S.; Imperiale, D.; Bonora, S.; et al. Diagnostic accuracy of new and old cognitive screening tools for HIV–associated neurocognitive disorders. HIV Med. 2018, 19, 455–464. [Google Scholar] [CrossRef]
- Saloner, R.; Cysique, L.A. HIV–Associated Neurocognitive Disorders: A Global Perspective. J. Int. Neuropsychol. Soc. 2017, 23, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, A.; Alberione, M.C.; Romito, A.; Imperiale, D.; Ghisetti, V.; Audagnotto, S.; Lipani, F.; Raviolo, F.; Di Perri, G.; Bonora, S. Prevalence and predictors of blood–brain barrier damage in the HAART era. J. Neurovirol. 2014, 20, 521–525. [Google Scholar] [CrossRef]
- Strazza, M.; Pirrone, V.; Wigdahl, B.; Nonnemacher, M.R. Breaking down the barrier: The effects of HIV–1 on the blood–brain barrier. Brain Res. 2011, 1399, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Ivey, N.S.; MacLean, A.G.; Lackner, A.A. Acquired immunodeficiency syndrome and the blood–brain barrier. J. Neurovirol. 2009, 15, 111–122. [Google Scholar] [CrossRef]
- Calcagno, A.; Atzori, C.; Romito, A.; Vai, D.; Audagnotto, S.; Stella, M.L.; Montrucchio, C.; Imperiale, D.; Di Perri, G.; Bonora, S. Blood brain barrier impairment is associated with cerebrospinal fluid markers of neuronal damage in HIV–positive patients. J. Neurovirol. 2016, 22, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Eugenin, E.A.; Clements, J.E.; Zink, M.C.; Berman, J.W. Human Immunodeficiency Virus Infection of Human Astrocytes Disrupts Blood–Brain Barrier Integrity by a Gap Junction–Dependent Mechanism. J. Neurosci. 2011, 31, 9456–9465. [Google Scholar] [CrossRef]
- Gonzalez, H.; Podany, A.; Al–Harthi, L.; Wallace, J. The far–reaching HAND of cART: cART effects on astrocytes. J. Neuroimmune Pharmacol. 2021, 16, 144–158. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood–Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Govindpani, K.; McNamara, L.G.; Smith, N.R.; Vinnakota, C.; Waldvogel, H.J.; Faull, R.L.; Kwakowsky, A. Vascular Dysfunction in Alzheimer’s Disease: A Prelude to the Pathological Process or a Consequence of It? J. Clin. Med. 2019, 8, 651. [Google Scholar] [CrossRef]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef]
- Smail, R.C.; Brew, B.J. HIV–associated neurocognitive disorder. Handb. Clin. Neurol. 2018, 152, 75–97. [Google Scholar]
- Reiber, H. External quality assessment in clinical neurochemistry: Survey of analysis for cerebrospinal fluid (CSF) proteins based on CSF/serum quotients. Clin. Chem. 1995, 41, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Antinori, A.; Arendt, G.; Becker, J.T.; Brew, B.J.; Byrd, D.A.; Cherner, M.; Clifford, D.B.; Cinque, P.; Epstein, L.G.; Goodkin, K.; et al. Updated research nosology for HIV–associated neurocognitive disorders. Neurology 2007, 69, 1789–1799. [Google Scholar] [CrossRef]
- Madihi, S.; Syed, H.; Lazar, F.; Zyad, A.; Benani, A. A Systematic Review of the Current Hepatitis B Viral Infection and Hepatocellular Carcinoma Situation in Mediterranean Countries. BioMed Res. Int. 2020, 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sagnelli, E.; Stroffolini, T.; Sagnelli, C.; Pirisi, M.; Babudieri, S.; Colloredo, G.; Russello, M.; Coppola, N.; Gaeta, G.B.; Cacopardo, B.; et al. Gender differences in chronic liver diseases in two cohorts of 2001 and 2014 in Italy. Infection 2018, 46, 93–101. [Google Scholar] [CrossRef]
- Scully, E.P. Sex Differences in HIV Infection. Curr. HIV/AIDS Rep. 2018, 15, 136–146. [Google Scholar] [CrossRef]
- Maki, P.M.; Martin-Thormeyer, E. HIV, Cognition and Women. Neuropsychol. Rev. 2009, 19, 204–214. [Google Scholar] [CrossRef]
- Rubin, L.H.; Neigh, G.N.; Sundermann, E.E.; Xu, Y.; Scully, E.P.; Maki, P.M. Sex Differences in Neurocognitive Function in Adults with HIV: Patterns, Predictors, and Mechanisms. Curr. Psychiatry Rep. 2019, 21, 94. [Google Scholar] [CrossRef] [PubMed]
- Namagga, J.K.; Rukundo, G.Z.; Voss, J.G. Prevalence and Risk Factors of HIV–Associated Neurocognitive Disorders in Rural Southwestern Uganda. J. Assoc. Nurses AIDS Care 2019, 30, 31–38. [Google Scholar] [CrossRef]
- Burlacu, R.; Umlauf, A.; Luca, A.; Gianella, S.; Radoi, R.; Ruta, S.M.; Marcotte, T.D.; Ene, L.; Achim, C.L. Sex–based differences in neurocognitive functioning in HIV–infected young adults. AIDS 2018, 32, 217–225. [Google Scholar] [CrossRef]
- Erickson, M.A.; Liang, W.S.; Fernandez, E.G.; Bullock, K.M.; Thysell, J.A.; Banks, W.A. Genetics and sex influence peripheral and central innate immune responses and blood–brain barrier integrity. PLoS ONE 2018, 13, e0205769. [Google Scholar] [CrossRef]
- Abdulle, S.; Hagberg, L.; Gisslen, M. Effects of antiretroviral treatment on blood–brain barrier integrity and intrathecal immunoglobulin production in neuroasymptomatic HIV–1—infected patients. HIV Med. 2005, 6, 164–169. [Google Scholar] [CrossRef]
- Rahimy, E.; Li, F.Y.; Hagberg, L.; Fuchs, D.; Robertson, K.; Meyerhoff, D.J.; Zetterberg, H.; Price, R.W.; Gisslén, M.; Spudich, S. Blood–Brain Barrier Disruption Is Initiated During Primary HIV Infection and Not Rapidly Altered by Antiretroviral Therapy. J. Infect. Dis. 2017, 215, 1132–1140. [Google Scholar] [CrossRef]
- Calcagno, A.; Celani, L.; Trunfio, M.; Orofino, G.; Imperiale, D.; Atzori, C.; Arena, V.; D’Ettorre, G.; Guaraldi, G.; Gisslen, M.; et al. Alzheimer’s Dementia in People Living With HIV. Neurol. Clin. Pract. 2021. [Google Scholar] [CrossRef]
- Bougea, A.; Spantideas, N.; Galanis, P.; Gkekas, G.; Thomaides, T. Optimal treatment of HIV–associated neurocognitive disorders: Myths and reality. A critical review. Ther. Adv. Infect. Dis. 2019, 6, 204993611983822. [Google Scholar] [CrossRef]
- Calcagno, A.; Romito, A.; Atzori, C.; Ghisetti, V.; Cardellino, C.; Audagnotto, S.; Scarvaglieri, E.; Lipani, F.; Imperiale, D.; Di Perri, G.; et al. Blood Brain Barrier Impairment in HIV–Positive Naïve and Effectively Treated Patients: Immune Activation Versus Astrocytosis. J. Neuroimmune Pharmacol. 2017, 12, 187–193. [Google Scholar] [CrossRef]
- Rahimian, P.; He, J.J. HIV/neuroAIDS biomarkers. Prog. Neurobiol. 2017, 157, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Lurain, N.S.; Hanson, B.A.; Hotton, A.L.; Weber, K.M.; Cohen, M.H.; Landay, A.L. The Association of Human Cytomegalovirus with Biomarkers of Inflammation and Immune Activation in HIV–1–Infected Women. AIDS Res. Hum. Retrovir. 2016, 32, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Perello, R.; Vergara, A.; Monclus, E.; Jimenez, S.; Montero, M.; Saubi, N.; Moreno, A.; Eto, Y.; Inciarte, A.; Mallolas, J.; et al. Cytomegalovirus infection in HIV–infected patients in the era of combination antiretroviral therapy. BMC Infect. Dis. 2019, 19, 1030. [Google Scholar] [CrossRef] [PubMed]
- Deayton, J.R.; Sabin, C.A.; Johnson, M.A.; Emery, V.C.; Wilson, P.; Griffiths, P.D. Importance of cytomegalovirus viraemia in risk of disease progression and death in HIV–infected patients receiving highly active antiretroviral therapy. Lancet 2004, 363, 2116–2121. [Google Scholar] [CrossRef]
- Fellner, M.D.; Durand, K.; Correa, R.M.; Redini, L.; Yampolsky, C.; Colobraro, A.; Sevlever, G.; Teyssié, A.R.; Benetucci, J.; Picconi, M.A. Circulating Epstein–Barr virus (EBV) in HIV–infected patients and its relation with primary brain lymphoma. Int. J. Infect. Dis. 2007, 11, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Dehee, A.; Asselot, C.; Piolot, T.; Jacomet, C.; Rozenbaum, W.; Vidaud, M.; Garbarg-Chenon, A.; Nicolas, J.C. Quantification of Epstein–Barr virus load in peripheral blood of human immunodeficiency virus–infected patients using real–time PCR. J. Med. Virol. 2001, 65, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Lupia, T.; Milia, M.G.; Atzori, C.; Gianella, S.; Audagnotto, S.; Imperiale, D.; Mighetto, L.; Pirriatore, V.; Gregori, G.; Lipani, F.; et al. Presence of Epstein–Barr virus DNA in cerebrospinal fluid is associated with greater HIV RNA and inflammation. AIDS 2020, 34, 373–380. [Google Scholar] [CrossRef]
- Berger, J.R. The blood brain barrier in HIV infection. Front. Biosci. 2004, 9, 2680. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bertrand, L.; Nair, M.; Toborek, M. Solving the Blood–Brain Barrier Challenge for the Effective Treatment of HIV Replication in the Central Nervous System. Curr. Pharm. Des. 2016, 22, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).